Abstract
Background and objectives: We assessed whether nonalcoholic steatohepatitis (NASH) diagnosed by liver biopsy is associated with decreased kidney function and whether such association is independent of insulin resistance and features of the metabolic syndrome.
Design, settings, participants, & measurements: We enrolled 80 consecutive overweight patients with biopsy-proven NASH and 80 nonsteatotic control subjects who were matched for age, gender, and body mass index. Chronic kidney disease (CKD) was defined as the presence of estimated GFR (eGFR) of ≤60 ml/min per 1.73 m2 and/or abnormal albuminuria (i.e., urinary albumin/creatinine ratio ≥30 mg/g).
Results: NASH patients had significantly (P < 0.001) lower eGFR (75.3 ± 12 versus 87.5 ± 6 ml/min per 1.73 m2) and a greater frequency of abnormal albuminuria (14 versus 2.5%) and CKD (25 versus 3.7%) than control subjects. The significant differences in eGFR, albuminuria, and CKD that were observed between the two groups were only slightly weakened after adjustment for age, gender, body mass index, smoking status, insulin resistance (by homeostasis model assessment), and components of the metabolic syndrome. Notably, histologic severity of NASH (i.e., fibrosis stage) was strongly associated with either decreasing eGFR or increasing albuminuria (P < 0.01 or less), independently of potential confounding factors.
Conclusions: Our findings suggest that patients with biopsy-proven NASH have moderately decreased eGFR and a higher frequency of abnormal albuminuria and CKD than matched control subjects and that the severity of NASH histology is associated with decreased kidney function, independently of traditional risk factors, insulin resistance, and components of the metabolic syndrome.
Nonalcoholic fatty liver disease (NAFLD) is the most frequent cause of chronic liver disease among adults in Western countries (1–4). It comprises a disease spectrum ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis. The prevalence of NAFLD has been estimated to be between 20 and 30% in the general adult population, but this value is much higher in people with type 2 diabetes or obesity (i.e., approximately 70 and 90%, respectively) (1–4).
In recent years, the increasing recognition of the importance of NAFLD/NASH and its strong association with the metabolic syndrome has stimulated an interest in the putative role of NAFLD/NASH in the development and progression of cardiovascular disease (5). Similarly, the possible link between NAFLD/NASH and chronic kidney disease (CKD) has also attracted scientific interest. Although there is now growing evidence to suggest that NAFLD/NASH is closely linked to an increased risk of cardiovascular morbidity and mortality (2,5), the available information on the association between NAFLD/NASH and kidney disease is quantitatively limited.
A number of epidemiologic studies have recently shown that NAFLD/NASH is strongly associated with an increased prevalence (6–10) and incidence (11–14) of CKD in both nondiabetic and diabetic individuals. However, in all these studies, the diagnosis of NAFLD/NASH was based on either serum liver enzymes or ultrasound imaging, but was not confirmed by a liver biopsy, which is the gold standard for diagnosing NAFLD/NASH (1,2).
Thus, the aim of this study was to evaluate whether patients with histologically defined NASH have a greater frequency of CKD than nonsteatotic healthy controls and whether there is a significant association between decreased kidney function and the histologic features of NASH. Clarification of this research question may help to clarify the underlying biologic mechanisms and may be of clinical importance in planning preventive and therapeutic strategies.
Materials and Methods
We enrolled 80 consecutive overweight subjects with biopsy-proven NASH (i.e., cases) and 80 subjects with normal serum liver enzymes and without hepatic steatosis on ultrasound (i.e., controls), who were selected in 1:1 ratio with the cases to be matched for age, gender, and body mass index (BMI). Some of these participants have been included in a previous study (15).
No participants had any clinical evidence of cancer, cirrhosis, advanced kidney disease, or cardiovascular events. All patients with NASH had chronically elevated serum liver enzymes and hepatic steatosis on ultrasound. Ten subjects with NASH had pre-existing type 2 diabetes: seven managed their diabetes with diet alone, and three were taking metformin. None of the control subjects had diagnosed diabetes. The local Ethics Committee approved the study. All participants gave their informed consent.
Liver ultrasonography scanning was performed in all participants by an experienced radiologist, who was blinded to participants' details. The diagnosis of hepatic steatosis was made on the basis of characteristic sonographic features, i.e., evidence of diffuse hyperechogenicity of liver relative to kidneys, ultrasound beam attenuation, and poor visualization of intrahepatic structures (1–4).
The diagnosis of NASH was based on liver biopsy and exclusion of other secondary causes of chronic liver disease (alcohol abuse, viral hepatitis, autoimmune hepatitis, hemochromatosis, or use of potentially hepato-toxic medications). An experienced hepato-pathologist blinded to subjects' details scored liver biopsy specimens using the semiquantitative classification of Brunt et al. (16). NASH was defined as the presence of steatosis plus lobular inflammation plus hepatocellular ballooning or steatosis plus any stage of fibrosis.
BMI was calculated by dividing weight in kilograms by height in meters squared. Waist circumference was measured at the level of the umbilicus. BP was measured with a standard mercury manometer.
Venous blood was drawn in the morning after an overnight fast. Serum liver enzymes, creatinine (measured using a Jaffé rate-blanked and compensated assay), and other biochemical blood measurements were determined by standard laboratory procedures (DAX 96; Bayer Diagnostics, Milan, Italy). Normal ranges for aspartate aminotransferase, alanine aminotransferase, and γ-glutamyltransferase, in our laboratory, were 10 to 35 U/L for women and 10 to 50 U/L for men. LDL cholesterol was calculated by the Friedewald's equation. All participants had negative serology for viral hepatitis B and C. Insulin resistance was estimated by the homeostasis model assessment (HOMA-IR score) (17). Hemoglobin A1c (HbA1c) was measured using an HPLC analyser (HA-8140; Menarini Diagnostics, Florence, Italy) only in the subgroup of NASH patients with diagnosed diabetes (n = 10); their mean HbA1c was 6.6 ± 0.5% (SD).
GFR was estimated from the four-variable Modification of Diet in Renal Disease (MDRD) study equation as follows: estimated GFR (eGFR) = 175 × (serum creatinine−1.154) × (age−0.203) × 1.212 (if black) × 0.742 (if female) (18). Urinary albumin excretion was measured from an early morning urine sample as the albumin/creatinine ratio by an immuno-nephelometric method. Abnormal albuminuria was defined as an albumin/creatinine ratio ≥30 mg/g. CKD was defined as the presence of estimated GFR ≤60 ml/min per 1.73 m2 and/or abnormal albuminuria (18).
The metabolic syndrome was diagnosed by a modified Adult Treatment Panel III definition that was recently proposed by the American Heart Association and the National Heart, Lung, and Blood Institute (19). In accordance with this definition, a person was classified as having the metabolic syndrome if he/she had at least three of the following risk determinants: (1) waist circumference >102 cm in men or >88 cm in women, (2) fasting glucose ≥5.6 mmol/L or drug treatment, (3) triglycerides ≥1.70 mmol/L or drug treatment, (4) HDL <1.0 mmol/L in men and <1.29 mmol/L in women or drug treatment, and (5) BP ≥130/85 mmHg or drug treatment.
Statistical Analyses
Data are expressed as means ± SD or percentages. Skewed variables (serum liver enzymes, triglycerides, and HOMA-IR score) were logarithmically transformed to improve normality before analysis and then back-transformed to their natural units for presentation in tables. Statistical analyses included one-way ANOVA, χ2 test with Yates's correction for continuity (for categorical variables), and analysis of covariance. Nonparametric statistical tests were also used, but because the results were identical to those obtained by parametric procedures, only the latter are presented. The independence of the associations of variables with CKD, considered as the dependent variable, was also assessed by multivariate logistic regression analysis and expressed as odds ratio (OR). In the fully adjusted logistic regression model, along with the presence/absence of NASH, gender, age, BMI, smoking history, HOMA-IR score, and the metabolic syndrome (considered as a single clinical entity) were included as covariates. Separate regression models were also tested with the individual components of the metabolic syndrome that were simultaneously included as either continuous or categorical variables in the same equation. The above-mentioned covariates were chosen because of their biologic plausibility or statistical association with CKD. The existence of multicollinearity in the logistic regression model was excluded by using appropriate collinearity diagnostic statistics. P < 0.05 was considered statistically significant.
Results
Among patients with NASH, the liver histopathology results showed NASH with fibrosis stage of 0 in 26 subjects, NASH/fibrosis stage 1 in 27 subjects, NASH/fibrosis stage 2 in 16 subjects, and NASH/fibrosis stage 3 in 11 subjects. None had cirrhosis (NASH/fibrosis stage 4).
The clinical and biochemical characteristics of participants stratified by NASH status are shown in Table 1. Because of the study design, case and control subjects were almost identical in terms of age, gender, and BMI. The metabolic syndrome and all its individual components occurred more frequently in patients with NASH. They also had higher serum liver enzymes and HOMA-IR score. Compared with nonsteatotic control subjects, those with NASH had moderately decreased eGFR and a greater frequency of abnormal albuminuria or CKD (i.e., defined as eGFR ≤60 ml/min per 1.73 m2 and/or abnormal albuminuria). Among the NASH patients, 20% of them had stage 3 CKD (irrespective of albuminuria), whereas no patients had stage 4 CKD. When albuminuria was considered as a continuous measure, NASH patients had significantly higher mean (SD) values of albuminuria than control subjects (19.0 ± 58 versus 9.5 ± 23 mg/g; P < 0.01).
Table 1.
Clinical and biochemical characteristics of NASH patients and control subjects
| NASH Patients (n = 80) | Control Subjects (n = 80) | P | |
|---|---|---|---|
| Sex (% male) | 63 | 63 | NSa |
| Age (years) | 51 ± 2 | 50 ± 2 | NSa |
| Body mass index (kg/m2) | 27.2 ± 2.3 | 27.0 ± 2.1 | NSa |
| Current smokers (%) | 20 | 23 | 0.73 |
| Waist circumference (cm) | 98 ± 5 | 92 ± 3 | <0.05 |
| Systolic BP (mmHg) | 130 ± 9 | 124 ± 4 | <0.05 |
| Diastolic BP (mmHg) | 84 ± 5 | 81 ± 3 | 0.23 |
| Fasting glucose (mmol/L) | 6.4 ± 1.0 | 5.5 ± 0.3 | <0.05 |
| HOMA insulin resistance score | 4.12 ± 2.0 | 2.78 ± 1.1 | <0.001 |
| Triglycerides (mmol/L) | 1.49 ± 0.8 | 1.15 ± 0.4 | <0.001 |
| HDL cholesterol (mmol/L) | 1.33 ± 0.4 | 1.44 ± 0.3 | <0.05 |
| LDL cholesterol (mmol/L) | 3.17 ± 0.3 | 3.19 ± 0.3 | 0.61 |
| ALT (U/L) | 98 ± 20 | 24 ± 3 | <0.001 |
| GGT (U/L) | 61 ± 20 | 32 ± 4 | <0.001 |
| Metabolic syndrome (%) | 56 | 1.2 | <0.001 |
| Diabetes (%) | 12.5 | 0 | <0.001 |
| Estimated GFR (ml/min per 1.73 m2) | 75.3 ± 12 | 87.5 ± 6 | <0.001 |
| Abnormal albuminuria (%) | 14 | 2.5 | <0.001 |
| Chronic kidney disease (%)b | 25 | 3.7 | <0.001 |
Data are expressed as means ± SD or percentages. ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; NS, not significant.
Matched variables.
Chronic kidney disease was defined as abnormal albuminuria (i.e., albumin/creatinine ratio ≥ 30 mg/g) and/or estimated GFR ≤ 60 ml/min per 1.73 m2.
Notably, the marked differences in eGFR, abnormal albuminuria, and CKD that were observed between the two groups were only slightly weakened after adjustment for age, gender, BMI, smoking status, HOMA-IR score, and presence of the metabolic syndrome (P < 0.01 for albuminuria and P < 0.001 for both eGFR and CKD, by analysis of covariance). Almost identical results were also obtained when we adjusted for the individual components of the metabolic syndrome (instead of the metabolic syndrome considered as a single clinical entity) or when NASH patients with diagnosed diabetes (n = 10) were removed from analysis (data not shown).
Similarly, in logistic regression analysis, the presence of NASH was strongly associated with an increased prevalence of CKD (OR, 8.31; 95% confidence interval, 2.4 to 16.8; P < 0.001). This association was only slightly attenuated after adjustment for age, gender, BMI, waist circumference, smoking, systolic BP, HOMA-IR score, and plasma triglycerides (adjusted OR, 6.14; 95% confidence interval, 1.6 to 12.8; P < 0.001). Other independent predictors of CKD were older age, male gender, higher BMI, and higher systolic BP.
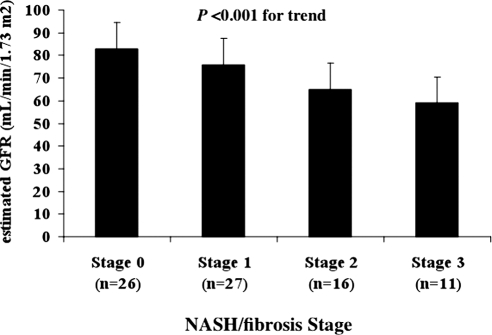
As shown in Figure 1, the histologic severity of NASH (i.e., NASH/fibrosis stage that was included as categorical variable) was associated with decreasing mean (SD) values of eGFR even after adjustment for age, gender, waist circumference, HOMA-IR score, systolic BP, and plasma triglycerides (P < 0.001 for the trend by analysis of covariance). Results remained essentially unchanged after additional adjustment for albuminuria or when patients with diagnosed diabetes (n = 10) were removed from analysis (data not shown). Similar significant and independent trends were also found for albuminuria (P < 0.01) and CKD (P < 0.001), respectively.
Figure 1.
Adjusted means (±SD) of eGFR in relation to the histologic severity of nonalcoholic steatohepatitis (i.e., NASH/fibrosis stage increasing from 0 to 3) in patients with histologically defined NASH. P value for the trend is assessed by analysis of covariance.
Discussion
This study has shown, for the first time, that patients with histologically defined NASH have moderately decreased eGFR and higher frequencies of abnormal albuminuria and CKD than matched nonsteatotic controls and that the severity of NASH histology (i.e., fibrosis stage) is associated with decreased kidney function, independently of several potential confounding factors, including the metabolic syndrome, an atherogenic condition that is closely associated with NASH and CKD.
These findings confirm and extend a recent work by Yilmaz et al. (20), showing that microalbuminuria is independently associated with the histologic features of NAFLD (i.e., fibrosis stage) in a selected sample of nondiabetic patients with biopsy-proven NAFLD; however, in this study, both patients with simple steatosis and those with borderline or definitive NASH were included and, more importantly, a control group was lacking (20). Our findings are also corroborated by recent large observational studies showing that the presence of NAFLD as detected by either serum liver enzymes (6,7,11,12) or liver ultrasound (8–10,13,14) (and should therefore be interpreted with caution) is strongly associated with an increased prevalence and incidence of CKD in both nondiabetic and diabetic individuals.
Given the cross-sectional design of this study, we are unable to draw conclusions about causality of the relationship between NASH and decreased kidney function. The most obvious explanation for our findings is that the decreased kidney function in NASH patients may simply reflect the coexistence of underlying cardiometabolic risk factors, such as overweight/obesity, hypertension, and the metabolic syndrome. Several recent epidemiologic studies have shown that obesity and the metabolic syndrome are independent predictors of CKD (21,22) and NASH (1,2). Both entities share many cardiometabolic risk factors and some pathophysiological mechanisms (1,2,5,23,24). However, because in our study the participants with and without NASH were comparable for age, gender, and BMI, and the presence of NASH was significantly associated with lower eGFR, higher albuminuria, and higher CKD even after adjusting for the clinical traits of the metabolic syndrome, HOMA-estimated insulin resistance, and smoking, it is also possible to speculate that NASH is not only associated with CKD as a consequence of the shared cardiometabolic risk factors but that NASH itself might at least in part contribute to the development of CKD independently of shared risk factors. Nevertheless, the possibility of a reverse causality (i.e., CKD causing the development of NASH) cannot be definitely excluded from our study. However, the evidence from recent prospective studies suggests that ultrasound-diagnosed NAFLD independently predicts incident CKD (13,14).
The underlying mechanisms putatively responsible for the observed association between NASH and kidney disease are not fully understood. The close intercorrelations between NASH, abdominal obesity, insulin resistance, and the metabolic syndrome make it extremely difficult to dissect the precise causal relationships responsible for the increased risk of CKD observed in patients with NASH. Currently, however, there is mounting evidence to suggest that NAFLD, especially in its necro-inflammatory form (NASH), is not only a marker of kidney damage but also may be involved in its pathogenesis, possibly through the systemic release of pathogenic mediators from the steatotic and inflamed liver, including increased reactive oxygen species, advanced glycation end products, C-reactive protein (CRP), plasminogen activator inhibitor-1 (PAI-1), TNF-α, TGF-β, and other proinflammatory cytokines (5,13). Importantly, several studies have shown that these plasma inflammatory and hemostatic factors and oxidative stress markers are remarkably higher in patients with NAFLD/NASH than in those without those conditions (25,26) and are also thought to play a role in the development and progression of CKD (27,28). Additionally, some experimental studies reported a strong positive relationship between intrahepatic mRNA expression of IL-6, CRP, or PAI-1 and the severity of NASH histology (29–31). Recently, we also reported that overweight patients with biopsy-proven NASH had significantly higher plasma CRP, fibrinogen, and PAI-1 activity levels and lower adiponectin levels than nonsteatotic overweight patients with comparable values of visceral adiposity and that there were strong, graded relationships between these proinflammatory/hemostatic factors and the histologic features of NASH, independently of visceral adiposity, insulin resistance, and other potentially confounding factors (15). Consistent with the hypothesis that liver inflammation (or other liver-derived factors) in NAFLD/NASH may play a role in CKD pathogenesis, Cheng et al. (32) reported that, in a large cohort of individuals with type 2 diabetes, those with chronic hepatitis B virus infection were more likely to develop ESRD than those who were not infected with hepatitis B virus. Further research is needed to uncover other potentially causative mechanisms by which NAFLD/NASH may contribute to CKD pathogenesis. As reported above, however, it is also important to underline that other pathophysiologic mechanisms that are not strictly related to liver inflammation may contribute to the development of CKD in patients with NAFLD/NASH.
This study has some important limitations such as its cross-sectional design (that precludes the establishment of causal or temporal relationships between NASH and kidney dysfunction) and the use of eGFR (i.e., the four-variable MDRD study equation) instead of a directly measured GFR to define CKD. It is known that the MDRD study equation underestimates renal function in obese subjects and has greater inaccuracy in populations without known CKD than in those with kidney disease (18,33,34). Nonetheless, current GFR estimates facilitate the detection, evaluation, and management of CKD, and many organizations recommend that the MDRD study equation be used to estimate kidney function in epidemiologic studies and in clinical practice (18). Finally, although our results have been adjusted for HOMA-IR score, a reliable method for estimating insulin resistance in vivo (17), we did not directly measure insulin sensitivity by euglycemic hyperinsulinemic clamp. An accurate assessment of insulin resistance during an euglycemic clamp would be particularly important to better understand whether the relationship between NAFLD/NASH and CKD is affected by insulin resistance.
Despite these limitations, our study has several strengths, including the relatively large number of participants, the complete nature of the data set, the ability to adjust for multiple potential confounders, and the histologic diagnosis of NASH for all patients. In addition, our patients were free of diagnosed cardiovascular disease and cirrhosis (thus excluding also patients with hepato-renal syndrome); the evaluation of patients with such complications would almost certainly have confounded interpretation of the data.
In conclusion, our results suggest that patients with histologically confirmed NASH have moderately decreased eGFR and a greater frequency of abnormal albuminuria and CKD than matched nonsteatotic control subjects and that the severity of NASH histology is associated with kidney dysfunction, independently of classical risk factors, insulin resistance, and metabolic syndrome components. These cross-sectional findings, although not definitive, are sufficiently provocative and hypothesis-generating to warrant further study. Future experimental and follow-up studies using liver biopsies are needed to confirm these findings and to elucidate the underlying biologic mechanisms before causality can be firmly established.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1. de Alwis NMW, Day CP: Non-alcoholic fatty liver disease: The mist gradually clears. J Hepatol 48[Suppl 1]: S104–S112, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Vuppalanchi R, Chalasani N: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology 49: 306–317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G: Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30: 1212–1218, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Marchesini G, Moscatiello S, Di Domizio S, Forlani G: Obesity-associated liver disease. J Clin Endocrinol Metab 93[Suppl 1]: S74–S80, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Targher G, Marra F, Marchesini G: Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia 51: 1947–1953, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Targher G, Kendrick J, Smits G, Chonchol M: Relationship between elevated serum gamma-glutamyltransferase concentrations and chronic kidney disease in the United States population. Findings from the National Health and Nutrition Examination Survey 2001–2006. Nutr Metab Cardiovasc Dis August 20, 2009. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7. Targher G, Bosworth C, Kendrick J, Smits G, Lippi G, Chonchol M: Relationship of serum bilirubin concentrations to kidney function and albuminuria in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001–2006. Clin Chem Lab Med 47: 1055–1062, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M: Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 51: 444–450, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Targher G, Bertolini L, Chonchol M, Rodella S, Zoppini G, Lippi G, Zenari L, Bonora E: Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia 53: 1341–1348, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Hwang ST, Cho YK, Yun JW, Park JH, Kim HJ, Park DI, Sohn CI, Jeon WK, Kim BI, Rhee EJ, Oh KW, Lee WY, Jin W: Impact of NAFLD on microalbuminuria in patients with pre-diabetes and diabetes. Intern Med J 40: 437–442, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Lee DH, Jacobs DR, Gross M, Steffes M: Serum gamma-glutamyltransferase was differently associated with microalbuminuria by status of hypertension and diabetes: The Coronary Artery Risk development in Young Adults (CARDIA) study. Clin Chem 51: 1185–1191, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Ryu S, Chang Y, Kim DI, Kim WS, Suh BS: Gamma-glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem 53: 71–77, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Targher G, Chonchol M, Bertolini L, Rodella S, Zenari L, Lippi G, Franchini M, Zoppini G, Muggeo M: Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol 19: 1564–1570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang Y, Ryu S, Sung E, Woo HY, Oh E, Cha K, Jung E, Kim WS: Nonalcoholic fatty liver disease predicts chronic kidney disease in non-hypertensive and non-diabetic Korean men. Metabolism 57: 569–576, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G, Muggeo M, Day CP: NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring) 16: 1394–1399, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR: Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467–2474, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M: Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23: 57–63, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function: Measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute: Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112: 2735–2752, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Yilmaz Y, Alahdab YO, Yonal O, Kurt R, Kedrah AE, Celikel CA, Ozdogan O, Duman D, Imeryuz N, Avsar E, Kalayci C: Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: Association with liver fibrosis. Metabolism January 21, 2010. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21. Kurella M, Lo JC, Chertow GM: Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol 16: 2134–2140, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Bagby SP: Obesity-initiated metabolic syndrome and the kidney: A recipe for chronic kidney disease? J Am Soc Nephrol 15: 2775–2791, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Wahba IM, Mak RH: Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2: 550–562, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, Muggeo M: Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Haemost 35: 277–287, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Tilg H, Moschen AR: Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab 19: 371–379, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Vlagopoulos PT, Sarnak MJ: Traditional and non-traditional cardiovascular risk factors in chronic kidney disease. Med Clin North Am 89: 587–611, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kendrick J, Chonchol MB: Non-traditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol 4: 672–681, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE: Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008; 103: 1372–1379, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Abe Y, Kubota K, Saito S, Iwasaki T, Terauchi Y, Togo S, Maeyama S, Nakajima A: High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol 42: 573–582, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Königsrainer A, Maier KP, Bischoff SC, Bergheim I: Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 138: 1452–1455, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Cheng AY, Kong AP, Wong VW, So WY, Chan HL, Ho CS, Lam CW, Tam JS, Chow CC, Cockram CS, Chan JC, Tong PC: Chronic hepatitis B viral infection independently predicts renal outcome in type 2 diabetic patients. Diabetologia 49: 1777–1784, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A: Estimation of renal function in subjects with normal serum creatinine levels: Influence of age and body mass index. Am J Kidney Dis 46: 233–241, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Chudleigh RA, Dunseath G, Peter R, Harvey JN, Ollerton RL, Luzio S, Owens DR: Influence of body weight on the performance of glomerular filtration rate estimators in subjects with type 2 diabetes. Diabetes Care 31: 47–49, 2008 [DOI] [PubMed] [Google Scholar]