Abstract
Background and objectives: Children with chronic kidney disease (CKD) have an increased risk of progression to ESRD. There is a need to identify treatments to slow the progression of CKD, yet there are limited data regarding clinical risk factors that may be suitable targets to slow progression.
Design, setting, participants, & measurements: We performed a retrospective cohort study using the North American Pediatric Renal Trials and Cooperative Studies CKD database. There were 4166 pediatric subjects with CKD stages II to IV. Disease progression was defined as a GFR on follow-up of <15 ml/min per 1.73 m2 or termination in the registry because of dialysis or transplantation. We used Kaplan-Meier and Cox proportional hazards methods to describe progression rates and determine factors associated with CKD progression.
Results: In the univariate analysis, CKD progression was associated with age, gender, race, primary disease, CKD stage, registration year, hematocrit, albumin, corrected calcium, corrected phosphorus, and use of certain medications. Factors that remained significant in the multivariate analysis were age, primary disease, CKD stage, registration year, hypertension, corrected phosphorus, corrected calcium, albumin, hematocrit, and medication proxies for anemia and short stature.
Conclusions: There are multiple risk factors associated with disease progression in the pediatric CKD population. Factors that may be amenable to intervention include anemia, hypoalbuminemia, hyperphosphatemia, hypocalcemia, hypertension, and short stature. Because of the retrospective nature of our study, confirmation of our results from ongoing prospective studies is warranted before recommending prospective interventional trials.
There is an urgent need to identify risk factors and develop new methods to halt chronic kidney disease (CKD) progression in children. Progression to ESRD at a young age results in lifelong disability and significant reduction in lifespan. The expected remaining lifetime after ESRD in the 0- to 14-year age group is 30 years (1). Among survivors of childhood ESRD, health-related quality of life, rates of vocational placement, and independence from parents are lower compared with peers in the general population (2,3). Although the dominant causes of CKD in adults are diabetic nephropathy and hypertension, nearly 60 to 70% of children affected with CKD have congenital or inherited kidney disorders. Compared with adults with CKD, pediatric patients with CKD require greater amount of resources, specialized care, and care coordination to achieve optimal outcomes (4).
Significant research emphasis has been placed on early identification of modifiable factors related to CKD progression. Within the pediatric CKD population, hypertension and proteinuria have been shown to be strong clinical risk factors for renal progression (5–10). Recently, Furth et al. (11) reported that anemia and hypoalbuminemia were associated with an accelerated decline of GFR among 23 adolescents with CKD. Additionally, the recently published, prospective Effect of Strict Blood Pressure Control and ACE-Inhibition on Progression of Chronic Renal Failure in Pediatric Patients (ESCAPE) trial found that optimal BP control can slow the progression of CKD (10). The pediatric studies to date have been either limited in range of clinical measures surveyed or sample size. Although the literature is growing, these data within the pediatric CKD population remain sparse, and the reported associations need further validation.
Using the North American Renal Trials and Cooperative Studies (NAPRTCS) Chronic Kidney Disease Registry, we report on multiple clinical factors associated with disease progression in a large pediatric cohort of patients with CKD. Particular attention was paid to potentially modifiable factors that may be targets for future clinical trials to slow CKD progression in children.
Materials and Methods
Study Population
Details of the methods used for this analysis are published elsewhere (12). Briefly, patients are eligible for enrollment in the voluntary NAPRTCS database through their 20th birthday. Patients were classified by disease severity into one of five stages using the Kidney Disease Outcomes Quality Initiative (KDOQI) CKD staging system (13). Estimated GFR (eGFR) was determined using the Schwartz equation (14). The original Schwartz equation was used in this analysis because the data analysis was complete before the publication of the revised equation. Disease progression was defined as an eGFR at any follow-up visit of <15 ml/min per 1.73 m2 or registry termination because of initiation of dialysis or transplantation (cumulatively referred to as CKD stage V). All patients with CKD stages II to IV at registry entry and any available follow-up data were included in the analysis. Subjects excluded from the study were those with a history of prior kidney transplant or dialysis or those who were <2 years of age, because the KDOQI staging system is not applicable to children <2 years old (13).
Statistical Analyses
Variables examined in the univariate analysis included age, gender, race, primary diagnosis, CKD registry year (before 2000 and after), erythropoietin use, growth hormone use, iron supplementation, hypertension (systolic or diastolic BP >95 percentile for age, gender, and height), anti-hypertensive medication use, parathyroid hormone (PTH), CKD stage, anemia (hematocrit < 33%), height z-score, weight z-score, body mass index z-score, corrected calcium (low, normal, high), age-adjusted phosphorus (normal, high), and albumin (as a dichotomous variable ≤4 or >4 g/dl). Serum calcium was adjusted for serum albumin as follows: measured Ca (mg/dl) + 0.8 × [4 − serum albumin (g/dl)]. We defined a calcium value of 8.5 to 10.0 mg/dl as normal. Calcium values <8.5 mg/dl were classified as low, and values >10.0 mg/dl as high. The definition of hyperphosphatemia was adjusted for age in the following manner: ≥6.5 mg/dl for 2 to 5 years; ≥5.8 mg/dl for 6 to 12 years; ≥4.5 mg/dl for 13 to 20 years.
Patients with any missing data were excluded from the multivariate analysis (although included in the univariate analysis for the data that were available). Additionally excluded from the multivariate analysis were height, weight, and body mass index z-scores because they were not significant in the univariate analysis. PTH was not included multivariate analysis because of >1800 missing values. All other covariates were included in the multivariate analysis.
Univariate analysis using Kaplan-Meier time-to-event curves and log-rank tests were used to identify individual covariates associated with disease progression. All significant factors were included in a multivariate Cox proportional hazards regression model. Model selection used backward elimination methods, with final covariates significant at the 0.05 level. The analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC).
Results
Population Demographics
A total of 6133 of the available patients enrolled in the NAPRTCS CKD database had baseline eGFR and hematocrit data available. Of those, 4166 were at least 2 years old, had one set of follow-up data, and had CKD stages II to IV at registry initiation. These patients were included in the initial analysis.
Demographic data for included patients is presented in Table 1. At the time of registration, 79.8% were 6 years of age or older, 60.9% were white, 61.5% were male, and the most common diagnosis was obstructive uropathy (20.1%). Although 37.9% of patients were anemic (hematocrit < 33%), only 13.4% were receiving erythropoietin and 23.9% supplemental iron. Additionally, 6.7% reported using growth hormone. Almost one half (46.9%) of the patients were hypertensive, whereas 46.3% reported using anti-hypertensive medications. Regarding other laboratory values at registration, 5.1% had a low serum calcium, 28.3% had elevated serum phosphorus, 20.5% had a PTH value greater than two times the upper limit, and 46.9% had a serum albumin ≤4.0 g/dl. The majority of patients had CKD stage III at registry initiation (51.9%), followed by stage IV (28.2%) and stage II (19.9%).
Table 1.
Patient characteristics
| N (%) | |
|---|---|
| Total cases (≥2 years) | 4166 (100) |
| Age (years) | |
| 2 to 5 | 836 (20.1) |
| 6 to 12 | 1681 (40.4) |
| >12 | 1649 (39.6) |
| Gender | |
| male | 2562 (61.5) |
| female | 1604 (38.5) |
| Race | |
| white | 2539 (60.9) |
| African American | 786 (18.9) |
| Hispanic | 577 (13.9) |
| other | 257 (6.2) |
| Primary diagnosis | |
| obstructive uropathy | 837 (20.1) |
| focal segmental glomerulosclerosis | 478 (11.5) |
| renal dysplasia | 529 (12.7) |
| reflux nephropathy | 389 (9.3) |
| other | 1833 (44.0) |
| Anemia | |
| hematocrit ≥33% | 2589 (62.1) |
| hematocrit <33% | 1577 (37.9) |
| CKD stage | |
| stage II | 828 (19.9) |
| stage III | 2162 (51.9) |
| stage IV | 1176 (28.2) |
| CRI registry year | |
| before 2000 | 2795 (67.1) |
| 2000 and after | 1371 (32.9) |
| Erythropoietin use | |
| yes | 557 (13.4) |
| no | 3606 (86.6) |
| Growth hormone use | |
| yes | 281 (6.7) |
| no | 3878 (93.1) |
| Calcium (corrected) | |
| low | 214 (5.1) |
| normal | 2728 (65.5) |
| high | 632 (15.2) |
| Phosphorus | |
| normal | 2654 (63.7) |
| elevated | 1177 (28.3) |
| Parathyroid hormone | |
| <2× upper limit | 1477 (35.5) |
| >2× upper limit | 853 (20.5) |
| unknown | 1836 (44.1) |
| Iron supplementation | |
| yes | 997 (23.9) |
| no | 3157 (75.8) |
| Anti-hypertensive medication use | |
| yes | 1928 (46.3) |
| no | 2230 (53.5) |
| Albumin | |
| ≤4.0 g/dl | 1954 (46.9) |
| >4.0 g/dl | 1674 (40.2) |
| Hypertension | |
| yes | 1954 (46.9) |
| no | 2154 (51.7) |
Percents may not total 100% because of missing values.
As described previously, the prevalence of anemia (hematocrit < 33%) was 21% in CKD stage II patients and increased to 59% in CKD stage IV patients (data not shown) (12).
Univariate Progression Rates
The median time to progression for all patients (n = 4166) was 53.7 months from registry initiation. Table 2 provides the Kaplan-Meier progression rates (the percent of patients who progress to CKD stage V) at 1 and 3 years from the time of registration, as well as the median months to reach CKD stage V (number of months when 50% of the cases have progressed to CKD stage V). African-American and Hispanic patients had a higher rate of progression compared with white CKD patients (median time to progression, 43.9 and 47.1 versus 60.3, respectively). Patients diagnosed with focal segmental glomerulosclerosis had a significantly shorter median time to progression (22.9 months) than did those with obstructive uropathy (67.2 months), reflux nephropathy (88.5 months), renal dysplasia (54.0 months), and other diagnoses (52.3 months).
Table 2.
Time to progression: Kaplan-Meier rates
| Patient Variables | 1-Year Progression Rate (%) | 3-Year Progression Rate (%) | Median Months to Progression |
|---|---|---|---|
| All cases | 17.3 | 39.4 | 53.7 |
| Age (years) | |||
| 2 to 5 | 11.8 | 28.6 | 78.6 |
| 6 to 12 | 16.0 | 38.4 | 52.8 |
| >12 | 21.6 | 46.9 | 40.9 |
| Gender | |||
| male | 16.5 | 37.9 | 57.5 |
| female | 18.6 | 42.0 | 48.0 |
| Race | |||
| white | 16.3 | 37.5 | 60.3 |
| African American | 19.5 | 44.1 | 43.9 |
| Hispanic | 17.3 | 41.2 | 47.1 |
| other | 21.0 | 41.5 | 51.8 |
| Primary diagnosis | |||
| obstructive uropathy | 10.8 | 29.4 | 67.2 |
| focal segmental glomerulosclerosis | 31.7 | 61.7 | 22.9 |
| renal dysplasia | 14.3 | 39.3 | 54.0 |
| reflux nephropathy | 10.5 | 28.4 | 88.5 |
| other | 18.2 | 39.7 | 52.3 |
| CKD stage | |||
| stage II | 3.5 | 15.4 | 137.7 |
| stage III | 8.6 | 28.8 | 67.8 |
| stage IV | 43.0 | 74.5 | 15.5 |
| Hypertension | |||
| yes | 21.0 | 44.8 | 43.8 |
| no | 14.2 | 34.5 | 63.7 |
| Laboratory values at registration | |||
| anemia | |||
| HCT ≥33% | 10.3 | 28.2 | 77.3 |
| HCT <33% | 29.0 | 57.6 | 26.2 |
| calcium | |||
| low | 33.5 | 53.3 | 29.1 |
| normal | 16.9 | 39.3 | 52.7 |
| high | 17.1 | 40.3 | 55.6 |
| phosphorus | |||
| normal | 10.9 | 29.8 | 70.1 |
| high | 32.3 | 62.5 | 23.6 |
| albumin | |||
| ≤4.0 g/dl | 24.5 | 52.2 | 32.2 |
| >4.0 g/dl | 10.4 | 27.6 | 74.6 |
| Medication use at registration | |||
| anti-hypertensive medication use | |||
| yes | 21.5 | 45.7 | 42.7 |
| no | 13.7 | 34.1 | 64.0 |
| erythropoietin use | |||
| yes | 40.9 | 68.8 | 17.7 |
| no | 13.7 | 34.8 | 61.2 |
| iron supplementation | |||
| yes | 29.2 | 56.3 | 28.8 |
| no | 13.5 | 34.0 | 63.7 |
Laboratory values collected at the time of registration showed anemia, low serum calcium, high serum phosphorus, and low serum albumin were associated with faster rate of progression. Anemic patients progressed appreciably more rapidly than did nonanemic patients (26.2 versus 77.3 months). CKD patients with low serum-corrected calcium had a median time to CKD stage V of 29.1 months compared with patients with normal or high serum calcium (respectively, 52.7 and 55.6 months); those with high serum phosphorus levels had a median time to progression of 23.6 months compared with those with normal serum phosphorus, who had a median time of 70.1 months. Hypoalbuminemic patients progressed more rapidly compared with those with a normal serum albumin (respectively, 32.2 and 74.6 months).
Hypertensive patients progressed to CKD stage V almost 2 years sooner than did nonhypertensive patients (respectively, 43.8 and 63.7 months). Additionally, use of erythropoietin and iron (likely indicating anemia) were associated with more rapid progression to CKD stage V compared with nonuse of these medications.
In the univariate analysis, all variables reported in Table 2 were significantly associated with progression to CKD stage V, with severity of CKD and primary diagnosis showing the greatest association. The z-scores for height, weight, and body mass index were not associated with renal progression.
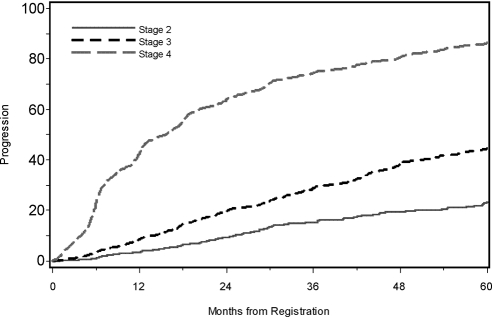
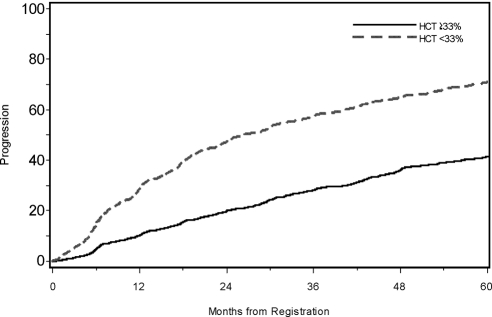
Kaplan-Meier curves for disease progression associated with CKD stage and anemia are presented in Figures 1 and 2. At 5 years, 23.2% of those with CKD stage II at registry initiation had progressed, whereas 86.4% of those with CKD stage IV at initiation had reached stage V. Also, 71.0% of anemic patients had progressed to stage V by 5 years, whereas only 41.6% of nonanemic patients had progressed.
Figure 1.
Time to progression by CKD stage at registration.
Figure 2.
Time to progression by anemia (defined by hematocrit %) at registration.
Multivariate Analysis for Renal Progression
To adjust for differences in CKD stage, primary diagnosis, and other potential confounders, we performed a multivariate proportional hazards regression analysis to evaluate independent risk factors for renal progression (Table 3). Results showed younger age at time of registration was associated with a lower risk of progression compared with older age (hazard ratio [HR], 0.72; 95% confidence interval [CI], 0.61 to 0.84). CKD stages III and IV were associated with higher risk of disease progression compared with stage II (HR, 2.0 and 6.68; 95% CI, 1.64 to 2.42 and 5.46 to 8.18, respectively). Using obstructive uropathy as the referent group, focal segmental glomerulosclerosis was the only diagnosis that was independently associated with a risk of disease progression (HR, 1.77; 95% CI, 1.48 to 2.12).
Table 3.
Multivariate Cox regression analysis of factors associated with CKD progression
| Demographic Characteristics | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| Age (years) | ||
| 2 to 5 | 0.72 | 0.61 to 0.84a |
| 6 to 12 | 0.97 | 0.85 to 1.10 |
| >12 | 1.00 | — |
| CKD stage | ||
| stage II | 1.00 | — |
| stage III | 2.00 | 1.64 to 2.42a |
| stage IV | 6.68 | 5.46 to 8.18a |
| Primary diagnosis | ||
| dysplasia | 1.15 | 0.96 to 1.37 |
| reflux nephropathy | 0.85 | 0.68 to 1.06 |
| focal segmental glomerulosclerosis | 1.77 | 1.48 to 2.12a |
| other | 1.13 | 0.98 to 1.30 |
| obstructive uropathy | 1.00 | — |
| Cohort year | ||
| 1994 to 1999 | 0.78 | 0.69 to 0.88a |
| 2000 to 2008 | 1.00 | — |
| Hypertension | ||
| yes | 1.26 | 1.14 to 1.40a |
| no | 1.00 | — |
| Laboratory data at registration | ||
| anemia | ||
| hematocrit <33% | 1.52 | 1.36 to 1.69a |
| hematocrit >33% | 1.00 | — |
| calcium | ||
| <8.5 mg/dl | 1.29 | 1.06 to 1.58b |
| ≥8.5 mg/dl | 1.00 | — |
| phosphorus (high) | ||
| high | 1.41 | 1.25 to 1.59a |
| normal | 1.00 | — |
| albumin | ||
| ≤4.0 g/dl | 1.67 | 1.49 to 1.86a |
| >4.0 g/dl | 1.00 | — |
| Medication use at registration | ||
| erythropoietin use | ||
| yes | 1.30 | 1.14 to 1.49c |
| no | 1.00 | — |
| growth hormone use | ||
| yes | 1.24 | 1.04 to 1.48b |
| no | 1.00 | — |
Factors in the multivariate analysis include age, gender, race, hematocrit, albumin, CKD stage, primary diagnosis, cohort year, calcium, phosphorus, anemia, hypertension, iron supplementation, growth hormone use, and erythropoietin use.
P < 0.0001.
P < 0.05.
P < 0.005.
Independent of the other factors, anemia, hypocalcemia, hyperphosphatemia, and hypoalbuminemia each remained significantly associated with a higher risk for renal progression. We estimate that those with anemia at registration have an associated 52% increase in risk of disease progression compared with the nonanemic patient (HR, 1.52; 95% CI, 1.36 to 1.69). Compared with patients with a normal serum calcium at time of registration, those with hypocalcemia have a 29% higher risk for renal progression independent of other factors (HR, 1.29; 95% CI, 1.06 to 1.58). Furthermore, those with hyperphosphatemia have a 41% increase in risk for renal progression compared with those with normal serum phosphorus (HR, 1.41; 95% CI, 1.25 to 1.59). In the multivariate model, hypoalbuminemia was associated with a 67% higher risk for renal progression (HR, 1.67; 95% CI, 1.49 to 1.86).
Use of erythropoietin and use of growth hormone were considered surrogate indicators of anemia and short stature. The surrogate indicators were associated with renal progression. Anti-hypertensive medication use was not associated with renal progression when hypertensive status is included in the multivariate model (Table 3).
To fully evaluate the relationships between the laboratory measure of anemia and erythropoietin use, we evaluated two interaction terms to identify any possible effect modification: (1) erythropoietin use and anemia and (2) CKD stage and anemia. Although we did find an increased risk of disease progression for those with anemia not on erythropoietin compared with those with anemia using erythropoietin (HR, 2.38 versus 1.60), when the term was added to the multivariate regression model, it became nonsignificant (P = 0.97; data not shown). Assessment for interaction between anemia and CKD stage with modeling of interactions terms was similarly not significant in the multivariate setting (P = 0.11).
Discussion
Although there has recently been significant emphasis on factors associated with disease progression in adult CKD populations, little is known about the pediatric population. Our review of a large pediatric CKD cohort identified a number of clinical factors associated with renal progression, several of which are potentially modifiable and provide potential targets for interventional trials aimed at slowing CKD progression. The Kaplan-Meier–based estimates regarding time to progression (Table 2) provide benchmark progression rates for future studies and are potentially useful to provide anticipatory guidance for families affected by childhood CKD. In our multivariate analysis, we found that age at registration, CKD stage at registration, cause of CKD, hypertension, anemia, hypoalbuminemia, hyperphosphatemia, hypocalcemia, and reported medications used to treat anemia and short stature were associated with CKD progression in children.
In adults with CKD, Hunsicker et al. (15) identified that renal progression was associated with race, degree of proteinuria, mean arterial pressure, HDL, and underlying diagnosis using data from the Modification of Diet in Renal Disease Study in 1997. Only mean arterial pressure, HDL cholesterol levels, and possibly degree of proteinuria were associated factors found in the study of Hunsicker et al. that might be amenable to correction. Similarly, Jungers et al. (16) identified primary disease and degree of proteinuria as major determinates of renal progression in their adult population of CKD patients without diabetic kidney disease. Although these associated factors may be helpful in predicting the rate of CKD progression for patients, only modifiable factors have the potential of slowing the rate of progression.
Our finding of increased risk of disease progression in patients with hypertension (HR, 1.26) supports the findings of prior studies using the NAPRTCS data. Mitsnefes et al. (6) used the NAPRTCS database to evaluate the association of hypertension and disease progression. In their evaluation of 3834 patients with CKD, they found that systolic hypertension was a significant independent risk factor for disease progression with a relative risk of 1.2. Recently additional prospective data from the ESCAPE trial has confirmed that optimal BP control can significantly retard the progression of CKD (10). The ESCAPE study provided firm evidence that higher BP and furthermore high levels of proteinuria increases the rate of renal progression in children. The NAPRTCS database does not collect data on proteinuria, and hence this was not evaluated in our analysis.
Our study confirmed findings of two other recent pediatric studies focused on associated risk factors for renal progression. Soares et al. (9) performed a retrospective review of 107 pediatric patients aimed to identify predictive factors associated with CKD progression. Our study confirmed their findings that primary cause of CKD and initial CKD stage were associated with renal progression in children. Although the findings from Soares et al. are valuable to the literature, their study was limited in sample size and lacked variables for modifiable risk factors, such as those identified in our study. Similar to what has been reported by Furth et al. (11), we found that anemia and hypoalbuminemia were associated with pediatric CKD progression. Although the study by Furth et al. was a multicenter prospective study, it was limited in size (only to 23 subjects) and was limited to adolescents and is thus not generalizable to younger CKD patients. Although our study was retrospective, our data included >4000 pediatric patients, with 60% of our population being 12 years of age or younger. Furthermore, Furth et al. (11) defined anemia as a hematocrit <36%. Although this may be one definition of anemia, the KDOQI anemia guidelines specify a hematocrit of <33% as the cut-off for anemia (17), which was the defined anemia threshold for our study.
In addition to anemia and hypoalbuminemia, we also identified that hypocalcemia and hyperphosphatemia were independent risk factors for renal progression in these children with CKD. Voormolen et al. (18) have previously found an association between hyperphosphatemia and a more rapid decline in renal function (as well as mortality) in the predialysis CKD population. However, their study included only adults with an eGFR <20 ml/min per 1.73 m2.
The use of medication in this cohort identified patients who were at risk for renal progression. It is notable that we found an increased rate of progression in patients using erythropoietin compared with those not using erythropoietin (HR, 1.29; 95% CI, 1.13 to 1.48). This association is likely not caused by the use of erythropoietin itself but rather that this variable served as a surrogate marker for the condition of anemia that is not fully explained by hematocrit alone. Because a very small proportion of patients with anemia are being treated with erythropoietin in our cohort (13%), it may be that those with more significant anemia (possible correlating with more significant disease) are those treated with erythropoietin, leading to the association of erythropoietin use and disease progression. Although subjects with anemia had an associated faster rate of progression, we could not detect a difference in the rate of progression with those treated with erythropoietin, when we modeled the relationship with an interaction term. Additionally, we did see that those with anemia who were not treated with erythropoietin had an increased risk of disease progression in the univariate analysis, suggesting that it is the anemia and not the use of erythropoietin that contributes to disease progression.
Furthermore, growth hormone as a surrogate measure for short stature was associated with a higher risk for renal progression independent of other factors in the analysis. Short stature in the pediatric CKD population is associated with a lower quality of life, increased rate of hospitalization, and death. Although the reported association between the use of growth hormone and renal progression is intriguing, a further analysis of the association with height was beyond the scope of our analysis but deserves further study.
We feel it important to acknowledge the limitations of our study. First, the NAPRTCS database, while being the largest database of its kind, is a voluntary registry. Thus, there may be a significant number of pediatric CKD patients who are not enrolled. Additionally, there is known to be a significant amount of missing data, which led to the exclusion of numerous patients in the multivariate analysis. Moreover, the available data are limited to information that is routinely collected in the database at 6-months intervals, not allowing for more complete or detailed information. Most importantly, it did not allow for the assessment of an association between chronic inflammatory state or degree of proteinuria and risk of disease progression, both areas of significant interest of late (19).
In these data, there is evidence of survivorship bias with evidence of a small but significant difference in progression rates between cohort years. Those with less severe disease and therefore slower progression may remain in the CKD cohort for longer and contribute more time at risk, leading the older cohort of patients to seem to have a lower risk of progression. Adjusting the analysis for cohort year will address this bias. However, as with any observational study, there exists the possibility of other sources of unmeasured bias that we were not able to address.
The eGFRs (and therefore CKD stages) were calculated using the original Schwartz equation (14). Our data analysis predated the revision of the recently published pediatric GFR estimating equation (20). The revised estimating equation for GFR was used for serum creatinine concentrations by enzymatic methods, whereas the original Schwartz formula is appropriate for serum creatinine concentrations performed by the Jaffe method (21). We acknowledge that having no indication of type of creatinine assay is a limitation of the database. We applied the original Schwartz formula to estimate GFRs for this analysis because of concern that applying the updated estimating equation would tend to exaggerate the rate of progression and overestimate the strength of association, especially for those in earlier cohort years when the Jaffe method for creatinine determination was more commonly used.
It is important to emphasize that this study showed only association between identifiable risk factors and disease progression and did not show evidence of cause and effect. Although we controlled for many potentially confounding variables, it is possible that identified risk factors may be acting as surrogate markers for other conditions that were not collected as part of the NAPRTCS database. The ongoing prospective cohort study of CKD in children (22) addresses many of the limitations of this study and will provide an appropriate platform to replicate the findings from our retrospective cohort study.
Conclusions
Our retrospective cohort study of >4000 pediatric patients with CKD identified multiple risk factors associated with CKD progression. Our findings underline the importance of early detection and management of pediatric CKD in an attempt to delay CKD progression. Although not modifiable, the CKD risk factors of primary cause of CKD, CKD stage, and age allow identification of high-risk groups for intensive monitoring and attentive management. We were able to identify several clinical risk factors associated with renal progression in children, including anemia, hyperphosphatemia, hypocalcemia, hypertension, and short stature. These may represent potential targets for improved management of pediatric CKD patients that have the potential to impact the rates of disease progression. Given the limitations of our study design, our findings need to be confirmed by ongoing prospective studies of pediatric CKD before recommending definitive guidelines or interventions.
Disclosures
None.
Acknowledgments
This study was presented in part at the American Society of Nephrology Annual meeting in San Diego, CA, November 14 through 19, 2006. A substantial portion of this work was performed while the first author, A.S., was receiving postdoctoral research support at the University of Washington from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease Pediatric Nephrology Research Training Program Grant (T32 DK007662).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. United States Renal Data System: USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 2. Groothoff JW, Cransberg K, Offringa M, van de Kar NJ, Lilien MR, Davin JC, Heymans HS: Long-term follow-up of renal transplantation in children: A Dutch cohort study. Transplantation 78:453–460, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Rosenkranz J, Reichwald-Klugger E, Oh J, Turzer M, Mehls O, Schaefer F: Psychosocial rehabilitation and satisfaction with life in adults with childhood-onset of end-stage renal disease. Pediatr Nephrol 20:1288–1294, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Stapleton FB, Andreoli S, Ettenger R, Kamil E, Sedman A, Chesney R: Future workforce needs for Pediatric Nephrology: An analysis of the nephrology workforce and training requirements by the workforce committee for the American Society of Pediatric Nephrology. J Am Soc Nephrol 8: S5, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Wingen AM, Fabian-Bach C, Schaefer F, Mehls O: Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet 349: 1117–1123, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Mitsnefes M, Ho PL, McEnery PT: Hypertension and progression of chronic renal insufficiency in children: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). J Am Soc Nephrol 14: 2618–2622, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Ardissino G, Testa S, Dacco V, Vigano S, Taioli E, Claris-Appiani A, Procaccio M, Avolio L, Ciofani A, Dello Strologo L, Montini G: Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatr Nephrol 19: 172–177, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Litwin M: Risk factors for renal failure in children with non-glomerular nephropathies. Pediatr Nephrol 19: 178–186, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Soares CM, Diniz JS, Lima EM, Oliveira GR, Canhestro MR, Colosimo EA, Simoes e Silva AC, Oliveira EA: Predictive factors of progression to chronic kidney disease stage 5 in a predialysis interdisciplinary programme. Nephrol Dial Transplant 24: 848–855, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Moller K, Wigger M, Peruzzi L, Mehls O, Schaefer F: Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Furth SL, Cole SR, Fadrowski JJ, Gerson A, Pierce CB, Chandra M, Weiss R, Kaskel F: The association of anemia and hypoalbuminemia with accelerated decline in GFR among adolescents with chronic kidney disease. Pediatr Nephrol 22: 265–271, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Staples AO, Wong CS, Smith JM, Gipson DS, Filler G, Warady BA, Martz K, Greenbaum LA: Anemia and risk of hospitalization in pediatric chronic kidney disease. Clin J Am Soc Nephrol 4: 48–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 14. Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 15. Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 51: 1908–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Jungers P, Hannedouche T, Itakura Y, Albouze G, Descamps-Latscha B, Man NK: Progression rate to end-stage renal failure in non-diabetic kidney diseases: A multivariate analysis of determinant factors. Nephrol Dial Transplant 10: 1353–1360, 1995 [PubMed] [Google Scholar]
- 17. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 50: 471–530, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW: High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Okamura DM, Himmelfarb J: Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr Nephrol 24: 2309–2319, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staples A, Wong C: Risk factors for progression of chronic kidney disease. Curr Opin Pediatr 22: 161–169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]