Abstract
Background and objectives: The treatment of idiopathic nephrotic syndrome is often complicated by a refractory and relapsing course, with risk of drug toxicity and progressive renal failure. We report the efficacy and safety of rituximab in patients with steroid-resistant (SRNS) and steroid-dependent nephrotic syndrome (SDNS) refractory to standard therapy.
Design, setting, participants, & measurements: This was a cohort study in academic, tertiary care centers in India and the United States. Patients with SRNS or SDNS, not responding to medications or showing calcineurin inhibitor toxicity, treated with two to four doses of intravenous rituximab, and followed ≥12 months were included. Remission was termed as complete, partial, or no response.
Results: Thirty-three patients with SRNS (24 initial, 9 late resistance) and 24 with SDNS, with mean ages of 12.7 ± 9.1 and 11.7 ± 2.9 years, respectively, were included. Six months after rituximab therapy, 9 (27.2%) patients with SRNS showed complete remission, 7 (21.2%) had partial remission, and 17 (51.5%) had no response. At 21.5 ± 11.5 months, remission was sustained in 15 (complete: 7, partial: 8) patients. Of 24 patients with SDNS, remission was sustained in 20 (83.3%) at 12 months and in 17 (71%) at follow-up of 16.8 ± 5.9 months. The mean difference in relapses before and 12 months after treatment with rituximab was 3.9 episodes/patient per year.
Conclusions: Therapy with rituximab was safe and effective in inducing and maintaining remission in a significant proportion of patients with difficult SRNS and SDNS.
Although many patients with idiopathic nephrotic syndrome have a satisfactory long-term course, 40% show steroid dependence (SDNS) and 10 to 15% are steroid resistant (SRNS) (1,2). The former are at risk of steroid toxicity, whereas the latter show a complicated course and may progress to end-stage renal disease (3,4). Therapeutic options are limited in patients with SRNS who fail to respond to calcineurin inhibitors and alkylating agents (5,6). Thus, patients with difficult nephrotic syndrome are prone to complications of the disease, prolonged immunosuppressive therapy, and are at risk for progressive renal injury. Management of these patients poses a therapeutic challenge, justifying the need for a therapeutic alternative.
Rituximab, a chimeric monoclonal antibody directed against the CD20 cell surface receptor expressed on B cells, is approved for the treatment of patients with non-Hodgkin lymphoma (7). Other conditions where this agent has been used successfully include rheumatoid arthritis, systemic lupus erythematosus, vasculitis, and nephrotic syndrome (8–10). Evidence is emerging that B lymphocyte–targeted treatments may be useful in selected patients with minimal change disease (MCD) or idiopathic focal segmental glomerulosclerosis (FSGS) not responding to standard therapy (6,10–12). However, most reports emphasize the immediate outcome of therapy, and data on long-term follow-up of these patients are limited.
We present our experience in 57 patients with SRNS and SDNS who were followed for at least 12 months after therapy with rituximab. The short-term outcome of five of these patients has been previously reported (13).
Materials and Methods
Records of patients with idiopathic SRNS (initial or late) or SDNS who were treated with rituximab between January 2006 and February 2009 at the All India Institute of Medical Sciences (New Delhi), Children's National Medical Center (Washington, DC), and Cedars Sinai Medical Center (Los Angeles, CA) and followed for a minimum period of 12 months were reviewed. Therapy with rituximab was initiated after approvals from the ethics committee and the Drug Controller General of India. Parents were provided detailed information about limited data on the efficacy and off-label use and the potential side effects of rituximab therapy.
Definitions and Indications of Therapy
SRNS was defined as lack of remission (urine albumin nil/trace by dipstick for 3 consecutive days) despite therapy with prednisone at 2 mg/kg per day for 4 weeks. Initial resistance was defined as resistance at the onset of nephrotic syndrome, and the term late resistance was used for subsequent nonresponders. Patients with initial resistance were screened for mutations in NPHS1 and NPHS2 genes using conformation-sensitive gel electrophoresis, followed by sequencing. Patients were steroid dependent if they relapsed on two occasions while receiving prednisone on alternate days or within 14 days of its discontinuation.
Rituximab was administered to patients with SRNS if there was lack of remission despite therapy with intravenous cyclophosphamide (500 mg/m2 monthly for 6 months) and/or calcineurin inhibitors (cyclosporine 5 to 6 mg/kg per day; tacrolimus 0.1 to 0.15 mg/kg per day for 6 months), disease recurrence after stopping prolonged (>3-yr) calcineurin inhibitor therapy, or presence of nephrotoxicity (striped pattern of interstitial fibrosis or tubular atrophy and/or arteriolar medial hyalinosis) (14). In patients with SDNS, the medication was used if there was lack of steroid sparing effect (inability to sustain remission at a prednisone dose of 0.5 mg/kg every other day) or presence of steroid toxicity (cataract, or body mass index >95th percentile for age) (15) despite treatment with oral cyclophosphamide (2 mg/kg per day for 12 weeks), levamisole (2.5 mg/kg for 6 months), mycophenolate mofetil (MMF; 1000 mg/m2 for 6 months), and calcineurin inhibitors. Patients with relapses after prolonged (>3 years) therapy with calcineurin inhibitors or those showing nephrotoxicity were also included.
Patients were not eligible for therapy with rituximab if they showed (1) estimated GFR <60 ml/min per 1.73 m2, (2) infantile onset of nephrotic syndrome, (3) underlying hepatitis B or HIV positivity, systemic lupus erythematosus, amyloidosis, Henoch Schonlein purpura, IgA nephropathy, membranoproliferative glomerulonephritis or membranous nephropathy, (4) more than one episode of serious infections (e.g., peritonitis, lower respiratory infection, cellulitis) in the past 12 months, or (5) current or previous therapy for tuberculosis.
Rituximab Therapy
Treatment was administered at a dose of 375 mg/m2 once every week for four doses in patients with SRNS and two doses for those with SDNS. The medication, dissolved in normal saline (concentration 2 mg/ml), was infused over 3 to 4 hours after 30-minute premedication with oral acetaminophen (15 mg/kg) and diphenhydramine (0.5 mg/kg); intravenous hydrocortisone (4 mg/kg) was given before the first dose. Whereas in patients with SDNS, rituximab was given during corticosteroid-induced remission, this was not possible in patients with SRNS. Patients were monitored for infusion-related reactions and screened at each visit for infections.
Concomitant Medications
Oral prednisone was given every other day at a dose of 1.5 mg/kg for 2 weeks, 1 mg/kg for 4 weeks, 0.75 mg/kg for another 4 weeks, and then 0.5 mg/kg. In patients receiving a calcineurin inhibitor, its dose was reduced to 50% at 3 months, with cessation, if possible, at 6 months. Therapy with other immunosuppressive agents was discontinued before treatment with rituximab, except in two adult patients with SRNS who received long-term therapy with MMF. Enalapril was given at 0.5 mg/kg per day in all patients with SRNS and in SDNS with hypertension. Furosemide was used for control of edema, if indicated. Patients with SRNS and blood LDL-cholesterol >130 mg/dl were treated with atorvastatin.
Follow-Up
Patients or caretakers were advised to perform daily urine albumin testing with a dipstick. Spot urine protein/creatinine ratio (Up/Uc) in the first morning sample was done at 1, 3, 6, and 12 months after the last dose of rituximab. Complete blood counts and levels of creatinine, albumin, and cholesterol were obtained at baseline and every 3 months thereafter.
Statistical Analyses
Continuous data are represented as mean ± SD (95% confidence interval [CI]). Paired data were compared using one-way ANOVA and ordinate variables using the χ2 test; P < 0.05 was considered significant. Multivariate logistic regression was done to define factors determining response to treatment. Results were analyzed using STATA 9.0 version software.
Remission was defined as complete (Up/Uc < 0.2) or partial (Up/Uc between 0.2 and 2, serum albumin > 2.5 g/dl, and no edema). No response was the presence of nephrotic range proteinuria (Up/Uc > 2), serum albumin <2.5 g/dl, or edema. Relapse was defined as urine albumin 3 to 4+ for 3 consecutive days in a patient previously in remission. The proportion of patients with SRNS having complete or partial remission at 6 months and last follow-up was recorded. For patients with SDNS, we compared relapse rates 12 months prior to and after therapy with rituximab, the time to first relapse, and the proportion of patients with sustained remission.
Results
Of 57 patients treated with rituximab and followed for at least 12 months, 33 (including 3 adults) had SRNS and 24 had SDNS.
SRNS
The baseline features are shown in Table 1. Of 24 patients with initial steroid resistance, 17 did not show mutations in the NPHS1 or NPHS2 genes. Two patients were heterozygous for p.R229Q in the NPHS2 gene. Genetic testing was not done for two children and three adult patients. The mean duration of nephrotic syndrome before treatment with rituximab was 6.4 ± 4.7 years (range, 1 to 15 years). The duration between onset of nephrotic syndrome and initial renal biopsy was 0.6 ± 0.1 years and that between commencement of therapy with a calcineurin inhibitor and repeat biopsy was 3.1 ± 1.1 years. All patients had previously received treatment with one or more of the following: long-term alternate-day prednisone, intravenous steroids, oral or intravenous cyclophosphamide, calcineurin inhibitors, levamisole, MMF, and vincristine.
Table 1.
Baseline clinical and histologic characteristics of patients with SRNS or SDNS
| Characteristic | SRNSa (n = 33) | SDNS (n = 24) |
|---|---|---|
| Age at onset (years) | 6.3 ± 4.8 (1 to 41) | 2.8 ± 0.9 (1 to 5) |
| Age at rituximab therapy (years) | 12.7 ± 9.1 (2 to 41) | 11.7 ± 2.9 (5 to 17) |
| Boys | 17 | 19 |
| Type of resistance (initial/late) | 24/9 | — |
| Renal histology | n = 33 | n = 16 |
| MCD | 17 | 12 |
| FSGS | 16 | 2 |
| mesangial hypercellularity | — | 2 |
| Previous immunosuppressive therapy | 33 | 24 |
| long-term alternate day prednisolone | 33 | 24 |
| intravenous methylprednisolone | 9 | 2 |
| levamisole | 2 | 16 |
| mycophenolate mofetil | 3 | 15 |
| cyclophosphamide | 20 (23 courses) | 22 (24 courses) |
| calcineurin inhibitor | 24 | 15 |
| vincristine | 2 | 0 |
| Duration of CNI therapy (months) | 22.7 ± 17.1 (4 to 56) | 20.7 ± 10.2 (8 to 36) |
| CNI toxicity | 11 | 5 |
| Cushingoid features | 2 | 24 |
| Cataract | 0 | 8 |
Data shown as mean ± SD (range). CNI, calcineurin inhibitor.
Includes three adult patients, 35, 36, and 41 years old, with initial steroid resistance.
Rituximab was administered at a mean dose of 410 ± 26.6 mg/wk for 4 weeks in all but five patients (one dose in three adults and one child; two doses in one child). Four patients received concomitant therapy with cyclosporine/tacrolimus, and two adult patients received therapy with MMF. Cotrimoxazole prophylaxis for Pneumocystis carinii was given to the first 16 patients for 6 months. Because we did not find an increased risk of infections and patients had normal levels of IgG, this was later discontinued.
Response at 6 Months and Follow-Up
At 6-month follow-up, 9 patients achieved complete remission, 7 patients had partial remission and no response was seen in 17 patients (Table 2). The median time to response was 32 days (range, 8 to 60 days) after the last dose of rituximab. Therapy with prednisone was continued in all patients at 0.5 mg/kg every other day, calcineurin inhibitors were stopped in two patients, and calcineurin inhibitor dose was tapered to 50% in two patients. At 6 months, 11 of 24 (45.8%) patients with initial SRNS and 5 of 9 (55.5%) patients with late SRNS were in complete or partial remission (P = 0.7; Table 2). Remission was seen in 11 of 17 (64.7%) patients with MCD and 5 of 16 (31.2%) patients with FSGS (P = 0.08).
Table 2.
Response rates at 6 months in patients with SRNS according to type of resistance and renal histology
| IR (n = 24) | LR (n = 9) | MCD (n = 17) | FSGS (n = 16) | Total (n = 33) | |
|---|---|---|---|---|---|
| Complete remission (%) | 5a (20.8) | 4 (44.5) | 7 (41.2) | 2 (12.5) | 9 (27.2) |
| Partial remission (%) | 6a (25) | 1 (11.1) | 4 (23.5) | 3 (18.7) | 7 (21.2) |
| Non response (%) | 13 (54.1) | 4 (44.5) | 6 (35.2) | 11 (68.7) | 17 (51.5) |
| P | 0.7 | 0.08 | |||
IR, initial resistance; LR, late resistance.
Includes two adult patients with complete remission (both with MCD) and one with partial remission (FSGS).
At a mean follow-up of 21.5 ± 11.5 months (range, 12 to 48 months), remission was sustained in 15 patients (complete in 7 and partial in 8); no response was seen in 18 patients. Nine patients (all nonresponders) had an estimated GFR <60 ml/min per 1.73 m2 at 12 months, including five with CKD stage 5. On multivariate logistic regression, none of the following were associated with response to rituximab therapy: duration of nephrotic syndrome; initial or late resistance; renal histology, tubulointerstitial damage and calcineurin inhibitor nephrotoxicity; and prior response and duration of treatment with calcineurin inhibitors.
Retreatment
A repeat course (two doses each) was given to three patients, two patients with complete and one patient with partial remission, 12 to 24 months after the first course. At a subsequent follow-up of 9 to 18 months, two patients showed partial remission and one had no response.
SDNS
The mean (range) duration of nephrotic syndrome before rituximab therapy was 8.9 ± 2.9 years (range, 2.4 to 14 years) years (Table 1). The duration between onset of nephrotic syndrome and renal biopsy was 2.2 ± 0.4 years, and from start of therapy with calcineurin inhibitors and repeat biopsy was 2.8 ± 0.6 years. All patients had previously received therapy with the following: long-term alternate-day prednisone, levamisole, cyclophosphamide, MMF, and calcineurin inhibitors without sustained response. Previous therapy with 12 weeks of oral cyclophosphamide had resulted in mean remission of 4.2 ± 1.2 months (range, 2 to 18 months).
After steroid-induced remission, rituximab was given at a mean dose of 400 ± 20.7 mg/wk for 2 weeks; four patients received concomitant therapy with calcineurin inhibitors.
Relapse Rates at 12 Months
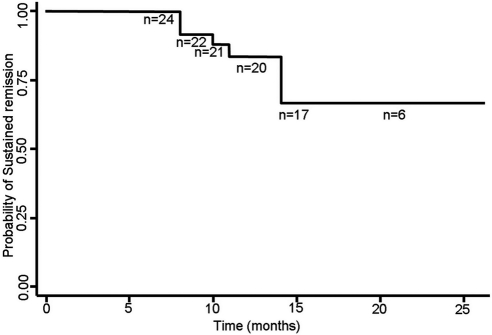
Twelve months after rituximab therapy, remission was sustained in 20 (83.3%) patients. The mean number of relapses before therapy was 4.0 ± 0.4 episodes/patient per year, which reduced significantly to 0.2 ± 0.3 episodes/patient per year in the next 12 months (mean difference, 3.9; 95% CI, 3.6, 4.1; P = 0.000, ANOVA). The time to first relapse was 11.2 ± 2.7 months (range, 8 to 14 months). At a mean follow-up of 16.8 ± 5.9 months (range, 12 to 38 months), remission was sustained in 17 (71%) patients (Figure 1). One or more immunosuppressive medications were withdrawn in 12 patients. In eight additional patients, the dose of prednisone could be tapered to 0.3 to 0.5 mg/kg every other day. One patient had multiple relapses 11 months after the first course of treatment. He received two additional doses of rituximab and is in remission 10 months later.
Figure 1.
Kaplan-Meier curve showing the probability of sustained remission in patients with SDNS. The number of patients at risk (n) is also shown.
B-Cell (CD19) Depletion
Data on B-cell depletion was available in 14 patients (5 SRNS and 9 SDNS). The CD19 counts at baseline were 12.6 ± 3.4% of the leukocyte count. After rituximab therapy, CD19 levels were 0.2 ± 0.1 and 0.3 ± 0.2% after two and four doses in patients with SDNS and SRNS, respectively.
Side Effects
Three patients with SRNS and one with SDNS had mild infusion-related reactions (three patients had chills and one patient had myalgia). There were no differences in total leukocyte count at baseline or 6 and 12 months after therapy. None of the patients had peritonitis, cellulitis, thrombosis, or any other serious infection or adverse event on follow-up.
Discussion
These observations confirm the efficacy of rituximab in patients with difficult nephrotic syndrome; remission was achieved in 48.5% patients with SRNS and a 95% reduction in relapse frequency was achieved in patients with SDNS. The patients reported here include mostly children with SRNS or SDNS who had either responded unsatisfactorily to treatment with multiple immunosuppressive agents including cyclophosphamide, levamisole, MMF, cyclosporine, and tacrolimus or had features suggestive of medication-related nephrotoxicity. Because occurrence of remission is considered an important predictor of long-term outcome (4), the demonstration of complete or partial remission in 16 of 33 patients with SRNS, who were otherwise refractory to all proven therapies, is valuable. Although similar outcomes were seen in patients with initial and late resistance, there was a trend toward better response rates in patients with steroid-resistant MCD compared with FSGS (Table 2).
We also found favorable long-term results in patients with difficult SDNS, with sustained remission being achieved in 83.3% and a steroid-sparing effect seen in a substantial proportion (75%) of patients at 12 months. These impressive results emphasize the potential role of therapy with rituximab in patients with difficult SDNS.
Multiple case reports (summarized by Haffner and Fischer) (10) support the efficacy of rituximab in inducing and/or sustaining remission of nephrotic syndrome. Similarly, data from three case series confirm the benefits of this medication in difficult nephrotic syndrome of childhood. An international, multicenter report comprising 28 patients with SDNS, 27 patients with SRNS, and 15 patients with post-transplant recurrence of nephrotic syndrome provides satisfactory data on efficacy and safety of rituximab (11). Patients with nephrotic syndrome of variable severity were reported, and there was heterogeneity between the centers regarding treatment regimens and the dose of rituximab. At a median follow-up of 5 months, 44% of patients with refractory SRNS showed reduction of proteinuria with normalization of serum albumin. There was no difference in response rates based on the underlying renal histology. Although the authors found satisfactory initial response in 82% patients with SDNS, only 40% had sustained remission at a follow-up of 4.5 months.
Guigonis et al. (16), in a multicenter report (from France), examined the efficacy of rituximab in 22 patients with SDNS or SRNS. The study included a heterogeneous group of patients receiving concomitant treatment with prednisone, calcineurin inhibitors, or MMF. At a median follow-up of 9.5 months, 19 (86.3%) patients had a beneficial effect with sustained remission or reduction of proteinuria. This study documented complete B-cell depletion in all patients at doses similar to that used in our study. Another report from France on 22 patients of SDNS (17) included patients with prolonged duration of nephrotic syndrome and diverse ethnicity who previously had received therapy with multiple agents. After one to four weekly infusions of rituximab, complete B-cell depletion was documented in all patients with reduction or stoppage of other immunosuppressive agents; 19 patients received at least one repeat course of the medication. At last follow-up, remission was sustained in 19 patients (86.3%), confirming a beneficial effect of therapy in patients with difficult SDNS.
This report, despite including ethnically different populations, had relatively uniform inclusion and exclusion criteria. Therapies for patients with SRNS and SDNS were, except for minor variations, similar. After therapy with rituximab, patients with SRNS and SDNS were followed for 21.5 ± 11.5 and 16.8 ± 5.9 months, respectively. The extended follow-up of patients with SDNS emphasizes the impact of rituximab in causing sustained remission and marked corticosteroid sparing. These findings also confirm our previous experience with this medication, where we showed satisfactory short-term results in five patients with difficult SRNS.
This report has multiple limitations, the main limitation being the lack of a control group. However, all patients had prolonged illnesses that were refractory to standard therapy, and apart from tapering doses of steroids, most patients did not receive concomitant therapies. Although it is unlikely that the observed impact of therapy with rituximab was fortuitous, prospective controlled trials are necessary to confirm the efficacy of this agent. Second, because of the limitations in obtaining CD19 counts, serial monitoring was not done. The number of doses of rituximab used was thus based on experience rather than targeting specific CD19 levels. Considering the potential urinary losses of rituximab, we used more doses in patients with SRNS than in those with SDNS who were in remission when they received the medication. Data from previous studies suggested that two to four doses (each of 375 mg/m2) is associated with CD19 depletion to <1% (16); similar findings were seen our patients where sequential counts were done. Whereas the B-cell measurement is of interest, it is not essential for demonstration of efficacy, because in most studies, the B-cell counts can remain low for months. Even if they do not, there is limited evidence that the return of these cells is detrimental. Relapses of some diseases are known despite B-cell depletion (18). Additionally, there is emerging evidence that lack of response to rituximab is associated with persistence of B-lineage cells in specific body compartments; therefore, peripheral blood monitoring might not be adequate (19).
The risk of side effects attributed to rituximab is variable. The multicentric French report found transient adverse effects in 45%, including one with P. carinii pneumonia (16). Prytula et al. (11) reported acute reactions in 27% patients and a high incidence of severe side effects including anaphylaxis and serious infections. Another case series reported occasional cases of reversible cytokine shock and neutropenia, with no risk of severe infections (17). In this study, mild infusion-related reactions, none meriting discontinuation of therapy, were seen in 7% of patients; there were no serious adverse events. Because none of the patients had serious infections, routine prophylaxis with cotrimoxazole was discontinued. However, physicians must also be aware of potentially life-threatening side effects, including the occurrence of progressive multifocal leukoencephalopathy (20) and acute lung injury (21,22).
Although there might be a bias for reporting favorable outcomes, findings from this and previous studies suggest that therapy with rituximab benefits a proportion of patients with difficult SDNS or SRNS. Although there was a trend toward better response in patients with MCD in this report, we could not identify any feature that predicted the response to rituximab. Future studies are needed to analyze clinical, histologic, and other features associated with a satisfactory response to rituximab. Although therapy is expensive (500 mg rituximab costs approximately $800–1200 in India and $8000 in the United States), its efficacy and safety enable it to be considered a promising agent in these patients. Based on this experience, we propose the need for a randomized controlled trial comparing the efficacy and safety of rituximab to a calcineurin inhibitor (e.g., tacrolimus) in patients with SDNS refractory to treatment with MMF and cyclophosphamide. If therapy with rituximab effectively sustains remission, given its relative safety profile and lack of nephrotoxicity, its use might be preferred to long-term therapy with calcineurin inhibitors. Until then, treatment with rituximab should be considered in patients with SRNS and SDNS who are refractory to other medications or show evidence of calcineurin inhibitor nephrotoxicity.
Disclosures
None.
Acknowledgments
The Department of Biotechnology, Government of India, funded the mutational studies. Cooperation between All India Institute of Medical Sciences and Cedars Sinai Medical Center was through the Sister Center Program of the International Society of Nephrology. Mr. Hemant K. Mishra helped with the statistical analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Tarshish P, Tobin JN, Bernstein J, Edelman CM. Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8: 769–776, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Kyrieleis HA, Löwik MM, Pronk I, Cruysberg HR, Kremer JA, Oyen WJ, van den Heuvel BL, Wetzels JF, Levtchenko EN: Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol 4: 1593–1600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mekahli D, Liutkus A, Ranchin B, Yu A, Bessenav L, Girardin E, Van Damme-Lombaerts R, Palcoux JB, Cachat F, Lavocat MP, Bourdat-Michel G, Nobili F, Cochat P: Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: A multicenter study. Pediatr Nephrol 24: 1525–1532, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Abeyagunawardena AS, Sebire NJ, Risdon RA, Dillon MJ, Rees L, Van't Hoff W, Kumarasiri PV, Trompeter RS: Predictors of long-term outcome of children with idiopathic focal segmental glomerulosclerosis. Pediatr Nephrol 22: 215–221, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Li X, Li H, Ye H, Li Q, He X, Zhang X, Chen Y, Han F, He Q, Wang H, Chen J: Tacrolimus therapy in adults with steroid- and cyclophosphamide-resistant nephrotic syndrome and normal or mildly reduced GFR. Am J Kidney Dis 54: 51–58, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Nakayama M, Kamei K, Nozu K, Matsuoka K, Nakagawa A, Sako M, Iijima K: Rituximab for refractory focal segmental glomerulosclerosis. Pediatr Nephrol 23: 481–485, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Anderson DR, Grillo-López A, Varns C, Chambers KS, Hanna N: Targeted anti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin's B-cell lymphoma. Biochem Soc Trans 25: 705–708, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Dörner T, Kinnman N, Tak PP: Targeting B cells in immune-mediated inflammatory disease: A comprehensive review of mechanisms of action and identification of biomarkers. Pharmacol Ther 125: 464–475, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Mok CC: Therapeutic options for resistant lupus nephritis. Semin Arthrit Rheum 36: 71–81, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Haffner D, Fischer DC: Nephrotic syndrome and rituximab: Facts and perspectives. Pediatr Nephrol 24: 1433–1438, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Prytuła A, Iijima K, Kamei K, Geary D, Gottlich E, Majeed A, Taylor M, Marks SD, Tuchman S, Camilla R, Ognjanovic M, Filler G, Smith G, Tullus K: Rituximab in refractory nephrotic syndrome. Pediatr Nephrol 25: 461–468, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Fujinaga S, Hirano D, Nishizaki N, Kamei K, Ito S, Ohtomo Y, Shimizu T, Kaneko K: Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatr Nephrol 25: 539–544, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Bagga A, Sinha A, Moudgil A: Rituximab in patients with the steroid-resistant nephrotic syndrome. N Engl J Med 356: 2751–2752, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Liptak P, Ivanyi B: Primer. Histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol 2: 398–404, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Kushner RF, Weinsier RL: Evaluation of the obese patient. Practical considerations. Med Clin North Am 84: 387–399, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, Groothoff J, Llanas B, Niaudet P, Nivet H, Raynaud N, Taque S, Ronco P, Bouissou F: Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: A multicentric series of 22 cases. Pediatr Nephrol 23: 1269–1279, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Sellier-Leclerc AL, Macher MA, Loirat C, Guérin V, Watier H, Peuchmaur M, Baudouin V, Deschênes G: Rituximab efficiency in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 25: 1109–1115, 2010 [DOI] [PubMed] [Google Scholar]
- 18. McDonald V, Manns K, Mackie IJ, Machin SJ, Scully MA: Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost 8: 1201–1208, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Boumans MJ, Tak PP: Rituximab treatment in rheumatoid arthritis: How does it work? Arthritis Res Ther 11: 134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, Laubach J, Bawn SD, Gordon LI, Winter JN, Furman RR, Vose JM, Zelenetz AD, Mamtani R, Raisch DW, Dorshimer GW, Rosen ST, Muro K, Gottardi-Littell NR, Talley RL, Sartor O, Green D, Major EO, Bennett CL: Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: A report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113: 4834–4840, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaumais MC, Garnier A, Chalard F, Peuchmaur M, Dauger S, Jacqz-Agrain E, Deschênes G: Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol 24: 1753–1755, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Lands LC: New therapies, new concerns: Rituximab-associated lung injury. Pediatr Nephrol 25: 1001–1003, 2010 [DOI] [PubMed] [Google Scholar]