Abstract
Background and objectives: These analyses were designed to characterize renal disease progression in untreated adults with Fabry disease.
Design, setting, participants, & measurements: Data from the Fabry Registry for 462 untreated adults (121 men and 341 women) who had at least two estimated GFR (eGFR) values over a span of ≥12 months before starting enzyme replacement therapy were included.
Results: Most men (86 of 121, 71%) had more rapid loss of kidney function than the normal adult population (loss of eGFR > −1 ml/min per 1.73 m2 per year), whereas fewer women (133 of 341, 39%) had rapid loss of kidney function. Patients with rapid progression had significantly higher mean averaged urinary protein to urinary creatinine ratios (UP/Cr) than patients with slower progression (1.5 versus 0.2 for men; 1.4 versus 0.5 for women; P < 0.0001). Patients were grouped into quartiles based on averaged UP/Cr; renal function in men declined more rapidly with higher UP/Cr, with the steepest declines observed in men with UP/Cr > 1.5 (mean eGFR slope, −5.6 ml/min per 1.73 m2 per year; n = 30). eGFR slope declined more slowly in women, with the steepest declines observed in women with UP/Cr > 1.2 (mean eGFR slope, −1.3 ml/min per 1.73 m2 per year; n = 85). Regression models of eGFR slope indicated that UP/Cr is the most important indicator of renal disease progression in adult Fabry patients. In women, lower baseline eGFR and age were also associated with renal disease progression. Women who had clinical events had more rapid loss of kidney function.
Conclusions: Urinary protein excretion is strongly associated with renal disease progression in men and women with Fabry disease.
Fabry disease is an X-linked lysosomal storage disorder, characterized by decreased or absent activity of lysosomal α-galactosidase A (1), with progressive accumulation of globotriaosylceramide (GL-3) and other glycosphingolipids within many cells, including the vascular endothelium. In the kidney, this accumulation is observed in all glomerular cells, peritubular capillaries, vascular endothelial and smooth muscle cells, and distal tubular cells (2–4). Progressive GL-3 accumulation is associated with life-threatening complications, renal failure, cardiovascular dysfunction, and stroke (1,5).
Whereas the development of enzyme replacement therapy (ERT) represented a major advance in treating Fabry disease (6–8), patients with advanced renal disease have poorer clinical outcomes in response to ERT than do Fabry patients with milder disease (3,9). A better understanding of the natural history of Fabry disease may provide valuable information about the progressive loss of kidney function and risk of progressing to ESRD, as well as providing an appropriate context for evaluating response to therapy. The Fabry Registry is an observational database that compiles clinical and laboratory data on patients with Fabry disease. Longitudinal data from the Fabry Registry were analyzed to characterize changes in kidney function and cardiac and cerebrovascular events over time in adult Fabry patients before the initiation of ERT.
Materials and Methods
The Fabry Registry began enrolling patients in April 2001. As of July 3, 2009, the Fabry Registry included 2850 patients 18 years of age and older (1409 men and 1441 women). All patients with Fabry disease are eligible to enroll, regardless of age, gender, symptoms, or whether they are receiving ERT from any commercial source. Patient and physician participation is voluntary. Patients provide informed consent through local Institutional Review Boards/Ethics Committees and may decline to participate or withdraw consent at any time. Given the voluntary participation, patients' ages at clinical assessments and time intervals between assessments are variable.
Data from untreated adult Fabry Registry patients, including patients who subsequently started ERT, were included in these analyses. To be included, patients were required to have at least two serum creatinine values for estimated GFR (eGFR) reported over a span of 12 or more months during the natural history period (i.e., before ERT) and one or more urine protein/creatinine ratio (UP/Cr, g/g) values reported from within the time frame of 6 months before the first eGFR assessment to the date of the final eGFR assessment during the natural history period. All clinical data in these analyses were collected before any chronic dialysis or kidney transplantation. Data were analyzed from the first available eGFR assessment (baseline) until the final available eGFR assessment during the natural history period for each patient.
GFR was estimated from the serum creatinine using the chronic kidney disease epidemiology collaboration equation (10). Changes in eGFR over time (slopes) were calculated with mixed-effect models, using random effects to determine an intercept and eGFR slope over time for each patient. Patients were categorized by eGFR slopes (slope ≤ −1 and slope > −1), baseline eGFR categories (≥90, <90 to ≥60, and <60 ml/min per 1.73 m2), and UP/Cr quartiles.
Various parameters including age at baseline eGFR, averaged UP/Cr, baseline eGFR, and averaged systolic and diastolic BP were used as predictor variables; individual eGFR slopes for each patient were used as response variables in further regression analyses by gender.
The Kaplan-Meier method was used to calculate the median age at first serious clinical events, including cardiovascular, cerebrovascular or renal events, and death. Cardiovascular clinical events were defined as myocardial infarction, arrhythmia, cardiac syncope, congestive heart failure, angina pectoris, or significant cardiac procedures (e.g., pacemaker or other implantable cardiac device placement, bypass, stent placement, valve replacement, transplantation). Cerebrovascular events were defined as stroke. Renal events were defined as receiving chronic dialysis (40 days or longer), kidney transplantation, or eGFR <10 ml/min per 1.73 m2. Differences between the UP/Cr strata were examined with log rank tests.
A two-sided t test was used to determine whether differences in continuous clinical parameters with normally distributed data were statistically significant between subgroups of patients with eGFR slopes ≤ −1 ml/min per 1.73 m2 per year versus patients with eGFR slopes > −1 ml/min per 1.73 m2 per year. These parameters included baseline age (both genders); baseline UP/Cr in men; and diastolic BP in both genders. A Wilcoxon test was used to determine whether differences in continuous clinical parameters with data that were not distributed normally were statistically different between subgroups of patients with eGFR slopes ≤ −1 versus > −1 ml/min per 1.73 m2 per year. These parameters included baseline UP/Cr in women and averaged UP/Cr in both genders. UP/Cr values data were transformed (9,11) before being used in the regression model.
The Kruskal-Wallis test was used to evaluate various clinical parameters across groups of patients categorized by baseline eGFR. Pearson correlation and Spearman rank correlation analyses were performed to determine the statistical dependence between various clinical parameters. P < 0.05 was considered statistically significant. Statistical analyses were performed using SAS statistical software version 9.1 (SAS Institute, Cary, NC).
Results
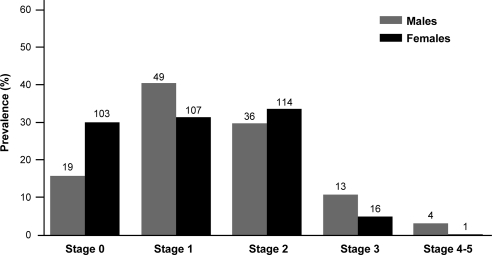
A total of 462 adult Fabry Registry patients (121 men and 341 women) had two or more eGFR values and one or more UP/Cr values over 12 or more months before initiation of ERT and before any chronic dialysis or transplantation. The mean age at first eGFR assessment was 34 years for men and 39 years for women. Eighty-one percent of men and 90% of women were white, which is consistent with the overall Fabry Registry population. Men were followed for 5 ± 4.6 (SD) years, and women were followed for 4 ± 3.8 years during the natural history period. At baseline, 17 of 121 men (14%) and 17 of 341 women (5%) exhibited stage 3 or worse chronic kidney disease (Figure 1).
Figure 1.
Summary of baseline chronic kidney disease (CKD) stage among Fabry Registry patients with longitudinal renal data. Percentages of patients in various stages of CKD at the time of their initial eGFR assessments are shown. The following definitions were used: stage 0, eGFR ≥ 90 ml/min per 1.73 m2, averaged UP/Cr < 0.3 (g/g), and urinary albumin to creatinine ratio (UA/Cr) < 0.03 (g/g), if UA/Cr data were available; stage 1, eGFR ≥90 ml/min per 1.73 m2, averaged UP/Cr ≥ 0.3, and UA/Cr ≥ 0.03, if UA/Cr data were available; stage 2, eGFR ≥60 to <90 ml/min per 1.73 m2; stage 3, eGFR 30 to <60 ml/min per 1.73 m2; and stages 4 to 5, eGFR < 30 ml/min per 1.73 m2. Data for men are shown in light gray bars and data for women are shown in dark gray bars, with the number of patients in each CKD stage shown above the bars. All data are from patients who had not been treated with ERT at the time of these assessments.
Patients were grouped into two categories: those with faster renal disease progression (loss of eGFR ≤ −1 ml/min per 1.73 m2 per year) and those with slower renal disease progression (eGFR slope > −1 ml/min per 1.73 m2 per year). Seventy-one percent of men (86 of 121) had faster progression versus 39% of women (133 of 341). Various clinical characteristics of these patients are shown in Table 1. At the time of the first eGFR assessment, men with faster renal disease progression were significantly older than men with slower renal disease progression (36 versus 30 years; P < 0.02 by t test). There was no significant age difference between the two groups of women (41 and 39 years). Mean baseline eGFR values and duration of follow-up were not significantly different between patients with faster or slower renal disease progression, respectively.
Table 1.
Clinical characteristics of Fabry registry patients grouped by renal disease progression status
| Men |
Women |
|||
|---|---|---|---|---|
| Faster Progression (eGFR Slope ≤ −1) | Slower Progression (eGFR Slope > −1) | Faster Progression (eGFR Slope ≤ −1) | Slower Progression (eGFR Slope > −1) | |
| Patients with eGFR data, n | 86 | 35 | 133 | 208 |
| age at baseline, mean (SD), years | 36 (14) | 30 (10)b | 41 (12) | 39 (14) |
| eGFRa at baseline, mean (SD) | 89 (29) | 94 (22) | 96 (23) | 95 (21) |
| Follow up time, mean (SD), years | 5.1 (4.71) | 4.2 (4.11) | 4.3 (3.95) | 4.3 (3.6) |
| median (25th, 75th) | 3.2 (2.0, 6.7) | 2.4 (1.4, 5.3) | 2.8 (2.0, 5.4) | 3.4 (2.1, 5.1) |
| Patients with baseline UP/Cr data, n | 37 | 11 | 67 | 111 |
| UP/Cr, baseline mean (SD) | 1.4 (1.2) | 0.4 (0.85)b | 1.3 (1.5) | 0.5 (0.7)b |
| median (25th, 75th) | 1.1 (0.7, 1.7) | 0.1 (0.1, 0.2) | 0.6 (0.2, 2.0) | 0.1 (0.1, 0.8) |
| Patients with averaged UP/Cr data, n | 86 | 35 | 133 | 208 |
| UP/Cr, averaged mean (SD) | 1.5 (1.2) | 0.2 (0.3)b | 1.4 (1.5) | 0.5 (0.7)b |
| median (25th, 75th) | 1.1 (0.7, 1.9) | 0.1 (0.1, 0.2) | 0.5 (0.2, 2.1) | 0.2 (0.1, 1.8) |
| BP, n | 76 | 32 | 124 | 198 |
| systolic BP, averaged mean (SD), mmHg | 127 (12) | 127 (11) | 124 (14) | 122 (15) |
| median (25th, 75th) | 127 (118, 135) | 128 (121, 132) | 123 (114, 135) | 121 (111, 131) |
| diastolic BP, averaged mean (SD), mmHg | 78 (8) | 73 (8)b | 77 (9) | 75 (9) |
| median (25th, 75th) | 78 (72, 84) | 73 (70, 76) | 77 (69, 84) | 75 (69, 82) |
| Reported history of ACEi/ARB use, n (%) | 20 (23) | 6 (17) | 29 (22) | 35 (17) |
All data are from adult Fabry Registry patients (≥18 years) with two or more eGFR assessments (calculated by the chronic kidney disease epidemiology collaboration equation) over a period of ≥12 months during the natural history period (i.e., before any treatment with enzyme replacement therapy) and before any chronic dialysis or renal transplant events. “Averaged” data reflect the average of all values reported within 6 months of the date of the first eGFR assessment to the most recent assessment.
eGFR data are expressed as ml/min per 1.73 m2.
P < 0.05 for patients with faster versus slower progression within each gender (see Materials and Methods section for description of statistical analyses used for specific clinical parameters).
Patients with faster progression had significantly higher UP/Cr than those with slower progression, both at baseline and when each patient's UP/Cr values were averaged over the natural history period (Table 1). The median averaged UP/Cr was 1.1 versus 0.1 for men and 0.6 versus 0.1 for women with faster or slower disease progression, respectively (P < 0.0001 by Wilcoxon test). Faster progression was associated with higher averaged mean diastolic BP in men (P < 0.01 by t test) but not women.
Genotype data were available for 101 men and 311 women (Table 2). The distribution of mutation types was similar in both groups, with nonsense mutations appearing to be more common in the faster progression groups.
Table 2.
Categorization of genotype Fabry registry patients grouped by renal disease progression status
| Men |
Women |
|||
|---|---|---|---|---|
| Faster Progression (eGFR Slope ≤ −1) | Slower Progression (eGFR Slope > −1) | Faster Progression (eGFR Slope ≤ −1) | Slower Progression (eGFR Slope > −1) | |
| Mutation type, n (%) | ||||
| nonsense | 11 (12.8) | 1 (2.9) | 17 (12.8) | 16 (7.7) |
| missense | 39 (45.3) | 22 (62.9) | 73 (54.9) | 117 (56.3) |
| splice site | 4 (4.7) | 0 | 1 (0.8) | 6 (2.9) |
| frameshift | 8 (9.3) | 2 (5.7) | 8 (6.0) | 12 (5.8) |
| large deletion | — | — | — | 1 (0.5) |
| initiator codon | — | 1 (2.9) | — | 1 (0.5) |
| small deletion (no frameshift) | 2 (2.3) | 1 (2.9) | 4 (3.0) | 4 (1.9) |
| small insertion (no frameshift) | — | — | — | 4 (1.9) |
| other | 1 (1.2) | — | — | — |
| Unable to categorize genotype | 7 (8.1) | 2 (5.7) | 13 (9.8) | 34 (16.3) |
| Not reported | 14 (16.3) | 6 (17.1) | 17 (12.8) | 13 (6.3) |
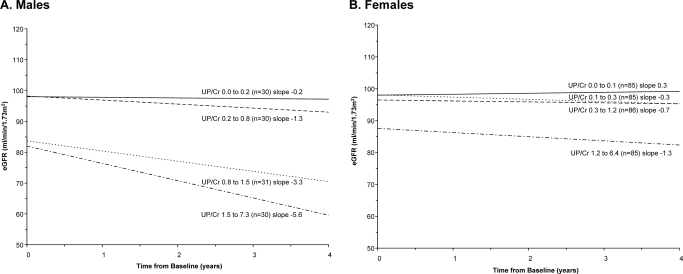
eGFR slopes were calculated based on UP/Cr quartile groups. Renal function in men declined more rapidly for those with increased urinary protein levels (Figure 2). The average eGFR slope was −0.2 ml/min per 1.73 m2 per year among the 30 men in the lowest averaged UP/Cr quartile and −5.6 ml/min per 1.73 m2 per year among the 30 men in the highest UP/Cr quartile. Renal function was more stable for women, but the highest levels of proteinuria were associated with more rapid declines in renal function (Figure 2). Average eGFR slope was 0.3 ml/min per 1.73 m2 per year among the 85 women with the lowest levels, and −1.3 ml/min per 1.73 m2 per year for the 85 women with the highest UP/Cr levels.
Figure 2.
Effect of proteinuria on eGFR over time in Fabry Registry patients with longitudinal renal data. Patients were grouped into quartiles with approximately equal numbers of patients, based on averaged UP/Cr levels. eGFR was calculated using the chronic kidney disease epidemiology collaboration equation, as described in the Materials and Methods section. Data for men are shown in A and data for women are shown in B.
When patients were grouped by baseline eGFR categories, patients with lower baseline eGFR levels tended to be older and tended to have higher averaged UP/Cr values than patients with less severe renal disease, among both genders (Table 3). Lower baseline eGFR in women was also associated with higher BP values. In addition, a higher percentage of women with lower baseline eGFR reported a history of angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) use compared with those with better baseline renal function.
Table 3.
Clinical characteristics of Fabry registry patients grouped by baseline eGFR values
| eGFR |
P | |||
|---|---|---|---|---|
| ≥90 | <90 to ≥60 | <60 | ||
| Men | ||||
| number of male patients, N | 68 | 36 | 17 | |
| eGFRa at baseline, mean SD) | 109 (13.3) | 78 (8.6) | 42 (13.7) | |
| age at baseline eGFR, mean (SD), years | 30 (9.4) | 37 (12.5) | 47 (16.1) | <0.0001 |
| men with baseline UP/Cr data, n | 29 | 12 | 7 | |
| UP/Cr, baseline mean (SD) | 0.9 (1.0) | 1.5 (1.4) | 1.8 (1.3) | NS |
| median (25th, 75th) | 0.6 (0.2, 1.1) | 1.3 (0.6, 1.8) | 1.5 (0.8, 3.5) | |
| men with averaged UP/Cr data, n | 68 | 36 | 17 | |
| UP/Cr, averaged mean (SD) | 0.8 (0.7) | 1.3 (1.3) | 1.9 (1.6) | 0.0025 |
| median (25th, 75th) | 0.6 (0.2, 1.2) | 0.9 (0.1, 1.9) | 1.5 (1.1, 2.2) | |
| BP, n | 63 | 34 | 11 | |
| averaged systolic BP (median, 25th, 75th), mmHg | 128 (120, 137) | 126 (114, 132) | 122 (118, 134) | NS |
| averaged diastolic BP (median, 25th, 75th), mmHg | 77 (72, 84) | 77 (72, 82) | 72 (70, 78) | NS |
| reported history of ACEi/ARB use, n (%) | 12 (18) | 9 (25) | 5 (29) | NS |
| Women | ||||
| number of female patients, N | 210 | 114 | 17 | |
| eGFRa at baseline, mean (SD) | 109 (11.6) | 76 (8.0) | 47 (8.8) | |
| age at baseline eGFR, mean (SD), years | 35 (11.3) | 46 (10.7) | 59 (11.7) | <0.001 |
| women with baseline UP/Cr data, n | 109 | 59 | 10 | |
| UP/Cr, baseline mean (SD) | 0.7 (0.9) | 0.8 (1.1) | 2.5 (2.1) | 0.004 |
| median (25th, 75th) | 0.2 (0.1, 0.9) | 0.5 (0.1, 1.0) | 2.1 (0.5, 4.2) | |
| women with averaged UP/Cr data, n | 210 | 114 | 17 | |
| UP/Cr, averaged mean (SD) | 0.7 (0.9) | 1.0 (1.4) | 1.8 (1.9) | 0.002 |
| median (25th, 75th) | 0.2 (0.1, 0.9) | 0.4 (0.1, 1.4) | 1.0 (0.3, 2.9) | |
| BP, n | 196 | 110 | 16 | |
| averaged systolic BP (median, 25th, 75th), mmHg | 119 (111, 129) | 125 (113, 135) | 138 (131, 144) | <0.0001 |
| averaged diastolic BP (median, 25th, 75th), mmHg | 74 (68, 80) | 77 (70, 83) | 84 (74, 90) | 0.003 |
| reported history of ACEi/ARB use, n (%) | 34 (16) | 21 (18) | 9 (53) | 0.008 |
“Averaged” data reflect the average of all values reported within 6 months of the date of the first eGFR assessment to the most recent assessment. P values were calculated by the Kruskal-Wallis test; NS, not significant.
eGFR data are expressed as ml/min per 1.73 m2.
Associations between baseline eGFR, age, UP/Cr, and other clinical parameters were evaluated with univariate analyses (Pearson correlations and Spearman rank correlations; Table 3). Age, baseline eGFR, averaged UP/Cr, and BP were identified as candidate covariates and were used to develop regression models for eGFR slope (Table 4). Averaged transformed UP/Cr was the variable most strongly associated with renal disease progression (P < 0.0001). The magnitude of the regression coefficient was threefold greater for men than women (compare −6.402 ± 0.502 to −2.109 ± 0.239). In addition to averaged UP/Cr, the averaged value for diastolic BP was also a predictor variable in the multivariable regression model for men. Baseline eGFR and age were also significant factors in renal disease progression among Fabry women (Table 4). The regression model accounted for much more of the variance for men than for women (compare adjusted R2, 0.661 and 0.236, respectively).
Table 4.
Regression Modeling of eGFR Slope
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Parameter Estimate | SE | P | Parameter Estimate | SE | P | |
| Predictor Variables | ||||||
| averaged UP/Cr (transformed)a | −6.402 | 0.502 | <0.0001 | −2.109 | 0.239 | <0.0001 |
| baseline eGFR | −0.012 | 0.006 | 0.065 | −0.026 | 0.004 | <0.0001 |
| age at baseline eGFR | −0.017 | 0.013 | 0.194 | −0.025 | 0.007 | 0.001 |
| averaged systolic blood pressure | 0.009 | 0.012 | 0.457 | 0.002 | 0.008 | 0.815 |
| averaged diastolic blood pressure | −0.041 | 0.017 | 0.015 | −0.004 | 0.011 | 0.687 |
| Intercept | 7.147 | 1.661 | <0.0001 | 4.774 | 0.901 | <0.0001 |
| Number of observations included | 108 | 322 | ||||
| R2 value for model | 0.677 | 0.248 | ||||
| Adjusted R2 value for model | 0.661 | 0.236 | ||||
eGFR was calculated using the chronic kidney disease epidemiology collaboration equation. Averaged values were calculated by taking the average of all values in the window −6 months from baseline eGFR to date of last eGFR.
Averaged UP/Cr was transformed before analysis by taking the 4th root (9,11).
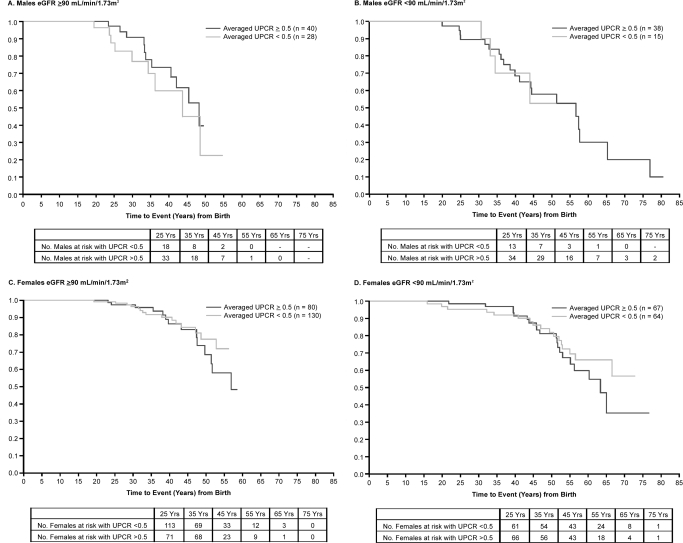
Patients with Fabry disease are susceptible to serious cerebrovascular and cardiovascular problems. Forty-eight of the 121 men (40%) and 70 of the 341 women (21%) experienced a major cardiovascular, cerebrovascular, or renal event during the natural history period, and one female patient died. Cardiac arrhythmia was reported as the initial event in 25 of 48 men (52%) and 35 of 70 women (50%). Kaplan-Meier estimates of time to first event, stratified by baseline eGFR and averaged UP/Cr, are shown in Figure 3. There was no effect of increased UP/Cr in men or women in either eGFR strata (log rank tests P > 0.300). Patients who experienced clinical events were older and had significantly lower baseline eGFR levels than patients without events (Table 5). Women who experienced clinical events had significantly faster renal disease progression (i.e., lower eGFR slope) than women who did not. Men with clinical events had significantly higher averaged UP/Cr levels and tended to have a lower eGFR slopes than men without events.
Figure 3.
Kaplan-Meier estimates of time to first clinical event. Clinical events included cardiac events (myocardial infarction, significant cardiac procedure, arrhythmia, angina, or congestive heart failure), cerebrovascular events (strokes), renal events (chronic dialysis, renal transplant, or eGFR < 10 ml/min per 1.73 m2), or death. Clinical events may have occurred either while patients were enrolled in the Fabry Registry or before enrollment, if such events were recorded on patients' medical history case report forms. Note that clinical data from patients in this cohort were restricted to data obtained before any chronic dialysis or renal transplant. Six men and one woman subsequently experienced renal events, and these are included in the Kaplan-Meier analysis. Patients were stratified by gender and baseline eGFR, as indicated in A–D. Data for patients with averaged UP/Cr ≥ 0.5 who experienced a clinical event during the natural history period are shown in dark gray, and data for patients with averaged UP/Cr < 0.5 are shown in light gray. The numbers of patients at risk for having events at the indicated ages are shown below the x-axis for each panel.
Table 5.
Clinical characteristics of patients who experienced renal, cerebrovascular, or cardiovascular events
| Men |
Women |
|||
|---|---|---|---|---|
| No Clinical Events (n = 73) | Experienced Clinical Events (n = 48) | No Clinical Events (n = 271) | Experienced Clinical Events (n = 70) | |
| Patients with eGFR data, n | 73 | 48 | 271 | 70 |
| age at baseline eGFR, mean (SD), years | 31 (11) | 39 (15)b | 38 (13) | 44 (12)b |
| median (25th, 75th), years | 29 (23, 39) | 36 (27, 49) | 36 (27, 48) | 43 (37, 51) |
| eGFRa at baseline, mean (SD) | 97 (22) | 81 (31)b | 97 (22) | 88 (21)b |
| median (25th, 75th) | 97 (83, 113) | 89 (58, 100) | 100 (80, 112) | 86 (72, 104) |
| 57.7, 99.8 | ||||
| eGFRa slope, mean (SD), per year | −2.2 (2.33) | −3.1 (2.46)c | −0.4 (1.37) | −1.0 (1.41)b |
| median (25th, 75th) | −1.9 (−4.0, −0.2) | −2.8 (−4.2, −1.7) | −0.4 (−1.4, 0.4) | −1.0 (−1.7, 0.0) |
| Patients with baseline UP/Cr data, n | 37 | 11 | 160 | 18 |
| UP/Cr, baseline mean (SD) | 1.0 (1.1) | 1.7 (1.4) | 0.8 (1.2) | 0.7 (0.8) |
| median (25th, 75th) | 0.8 (0.2, 1.5) | 1.4 (0.8, 3.5) | 0.3 (0.1, 1.0) | 0.3 (0.1, 1.1) |
| Patients with averaged UP/Cr data, n | 73 | 48 | 271 | 70 |
| UP/Cr, averaged mean (SD) | 0.9 (0.9) | 1.4 (1.4)b | 0.8 (1.1) | 1.1 (1.5) |
| median (25th, 75th) | 0.7 (0.2, 1.3) | 1.0 (0.4, 2.0) | 0.2 (0.1, 1.1) | 0.5 (0.1, 1.5) |
| BP, n | 68 | 40 | 256 | 66 |
| systolic BP, averaged mean (SD), mmHg | 126 (10) | 129 (14) | 122 (14) | 125 (15) |
| median (25th, 75th) | 126 (119, 131) | 128 (119, 138) | 121 (112, 133) | 124 (113, 137) |
| diastolic BP, averaged mean (SD), mmHg | 76 (9) | 76 (8) | 75 (9) | 77 (10) |
| median (25th, 75th) | 77 (72, 83) | 76 (72, 82) | 75 (69, 82) | 77 (70, 84) |
| Reported history of ACEi/ARB use, n (%) | 14 (88) | 12 (71) | 49 (73) | 15 (54) |
“Averaged” data reflect the average of all values reported within 6 months of the date of the first eGFR assessment to the most recent assessment.
eGFR data are expressed as ml/min per 1.73 m2.
P < 0.05 by t test.
P = 0.054 by t test.
Discussion
Fabry nephropathy can begin at a very young age; glomerular GL-3 deposits have been detected in fetuses (12), and glomerular sclerosis and vascular lesions have been described in renal biopsies of children and adolescents who had not yet exhibited decreased eGFR or overt proteinuria (13,14). “Classically” involved adult men with advanced renal disease were fully described in 2002 (15). More recent analyses (5), including those reported herein, describe a much broader spectrum of kidney involvement and rates of loss of kidney function in men and women. A better understanding of the natural history of Fabry nephropathy may help identify patients at higher risk of progression, and appropriate therapy. Understanding the natural history of Fabry nephropathy is also needed to set treatment goals and interpret the responses to ERT.
Cross-sectional descriptions of Fabry nephropathy (15–17) are inherently limited, given the wide range of disease severity. Men progress to ESRD 10 years sooner than women (16,17). The majority of female patients have slowly progressive kidney disease, but a smaller subset seem to be more seriously affected with progression to ESRD at the same median age as men (16). Recent retrospective chart reviews evaluated longitudinal outcomes for a similar number of Fabry men (128 versus 121 in these analyses) but many fewer Fabry women (51 versus 341 in these analyses) (5).
In normal individuals, renal function declines with age at approximately −1 ml/min per 1.73 m2 per year during the sixth decade and more rapidly thereafter (18–20). Because 95% of the patients in this cohort were younger than 60 years of age, this rate was used to stratify patients into general categories of “faster” or “slower” progression. In men, renal function declined more rapidly among those with higher UP/Cr levels. Women generally had slower reductions in eGFR over time. However, the average decline in renal function for women with the highest UP/Cr levels (UP/Cr > 1.2 g/g) was greater than what would be expected for their age (18–20). Proteinuria was a predominant factor in predicting renal disease progression rate for both genders, with a greater impact in men than in women. In women, lower baseline eGFR and increased age at baseline were also associated with more rapid loss of kidney function (Table 4).
The rates of renal decline described herein for untreated Fabry men with substantial proteinuria are similar to that reported by Schiffmann et al. (−6.9 ml/min per 1.73 m2 per year) (5) in 22 men with baseline proteinuria ≥1 g/24 h, but slower than reported by Branton et al. (−12 ml/min per 1.73 m2 per year) for 14 “classically” affected adult men, all of whom eventually developed ESRD (15). Women in the Fabry Registry with the highest UP/Cr (n = 85) progressed more slowly than described by Schiffmann et al. (−4.6 ml/min per 1.73 m2 per year), but only five women were included in that analysis (5). In addition to the differences in patient populations and referral sources, different methods were used to estimate GFR; the current analyses used the chronic kidney disease epidemiology collaboration equation, whereas the previous reports used the modification of diet in renal disease equation or calculated changes in GFR based on two inulin clearance measurements (5,15).
Our findings confirm that proteinuria is a risk factor for renal progression in Fabry patients (3,9,11); patients with proteinuria ≥1 g/24 h have been shown to be less responsive to ERT with agalsidase-β than patients with less proteinuria (3,9). ACEi/ARBs, in conjunction with ERT can reduce proteinuria and stabilize renal function in Fabry patients (11). This is an important issue, as ERT alone does not decrease overt proteinuria in Fabry disease (3,9,21). Institution of ACEi/ARB therapy during ERT will not necessarily confer renal protection (22), unless doses are titrated to achieve sustained reduction in proteinuria to <0.5 g/24 h (11).
Recent recommendations for patients with Fabry disease receiving ERT include ACEi/ARB treatment to reduce urinary protein excretion to <0.5 g/24 h (23–25), with the goal of reducing the rate of kidney function loss to less than −1 ml/min per 1.73 m2 per year. Sixty-four percent of men and 43% of women in this cohort had averaged UP/Cr values ≥0.5 (data not shown), and only 22% of men and 19% of women reported receiving ACEi/ARBs at any time during the observation period. Because the Fabry Registry has limited information about ACEi/ARB dosing, we focused more attention on averaged proteinuria levels. Assuming that the reno-protective effects of ACEi/ARBs are associated with reductions in proteinuria, we reasoned that the averaged UP/Cr values reflected the reno-protective effects of any ACEi/ARB therapy.
Proteinuria seems to be the most important predictor for renal progression, but proteinuria had a much greater predictive value for men than women (Table 4). Women tolerate low levels of proteinuria with less progression than men (Figure 2). Similar differences between men and women have been described for the Prevention of Renal Valscular Endstage Disease cohort (26), albeit at lower levels of proteinuria. Whether the gender difference represents differential susceptibility to progressive damage or pathophysiologic differences manifested as proteinuria is worthy of further study.
Proteinuria seems to be more important than other identified risk factors in women, but the majority of the progression risk was not identified for women. Mutation subtypes and α-galactosidase enzyme activity may impact disease progression (15). We did not include α-galactosidase activity in the regression model because enzyme assays were not standardized, and there are challenges to extrapolating residual enzyme activity measured in peripheral blood to organ-specific activity, especially in women, where lyonization can play an important role in X chromosome–linked diseases (1).
A substantial portion of patients in this cohort (40% of men and 21% of women) experienced a major renal, cerebrovascular, or cardiovascular event during the follow-up period. Although no effect of averaged UP/Cr was observed on time to clinical events, the most common type of clinical event was cardiac arrhythmia; this is consistent with previous analyses of Fabry patients (5,17). Whereas UP/Cr is an important indicator of renal disease progression, it may not be directly associated with the cardiovascular aspects of Fabry disease progression. However, renal disease can be both a risk factor for and a consequence of cardiac disease (27,28), and the cardiovascular and renal manifestations of Fabry disease may be interrelated. Indeed, men who experienced clinical events had significantly higher averaged UP/Cr levels than men who did not, confirming the need for regular cardiac, cerebrovascular, and renal assessments for Fabry disease (24,29).
There are important limitations to analyzing registry data, especially in rare diseases where the number of patients is small. Missing data limit the use of appropriate covariates in building regression models of associations between prognostic factors. This cohort may not be representative of the overall Fabry patient population because of phenotypic variability. Including only patients with at least two serum creatinine measurements imposes a bias against very mildly affected patients who may have had only one baseline assessment, whereas more severely affected patients may have only had one baseline evaluation before initiating ERT and would also be excluded from these analyses. We expect that these two sources of confounding may have partially offset each other, with consequent regression dilution bias (30).
Taken together, these findings indicate that adult patients with Fabry disease with overt proteinuria lose renal function more rapidly than those with little or no proteinuria. Among women, baseline age and eGFR levels were also prognostic indicators for more rapid loss of kidney function. Proteinuria was significantly higher for men with major clinical events compared with those who did not. Other risk factors have been associated with stroke in Fabry patients (31), and additional studies are needed to define risk factors associated with cardiovascular events. In view of the progression of Fabry nephropathy (5), urinary protein excretion and eGFR levels should be closely monitored in all Fabry patients, regardless of other signs or symptoms. Based on the gender-specific risk factors identified herein, reasonable predictions of renal outcomes can be defined at the baseline evaluation. This has important prognostic implications and provides a basis for interpreting the effects of interventions, such as ERT and/or ACEi/ARB therapy on renal outcome measures.
Disclosures
Genzyme Corporation sponsors the Fabry Registry. Members of the Fabry Registry Board of Advisors include C.W., J.P.O., A.O., M.M., D.P.G., G.E.L., B.V., S.W., and D.G.W. Authors who have received research funds or travel support from Genzyme include C.W., J.P.O., A.O., M.M., D.P.G., G.E.L., A.S., L.M., R.M., B.V., S.W., and D.G.W. Authors who have received speaking fees from Genzyme include C.W., J.P.O., A.O., M.M., D.P.G., G.E.L., R.M., B.C., B.V., and S.W. R.L. and D.B.J. are Genzyme employees. All honoraria received by G.E.L. are donated to the Gaucher Stichting, a national foundation that supports research in the field of lysosomal storage disorders. D.G.W. has served as a paid consultant to Genzyme and also has consultancies and has received travel funds from Abbot, Amgen, Amicus, Gilead, Parion, Relypsa, and Shire.
Acknowledgments
We thank the many patients who agreed to participate in the Fabry Registry and the physicians and research coordinators that have entered clinical data on these patients. We also acknowledge our colleagues at Genzyme Corporation (Cambridge, MA), Fanny O'Brien, PhD, and J. Alexander Cole, DSc, MPH, for assistance with statistical analyses and Badari Gudivada for statistical programming support.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Desnick RJ, Ioannou YA, Eng CM: Alpha-galactosidase A deficiency: Fabry disease. In: The Metabolic Bases of Inherited Disease, 8th ed., edited by Scriver C, Beaudet A, Sly W, Valle D. New York, McGraw-Hill, 2001, pp 3733–3774 [Google Scholar]
- 2. Fogo A, Bostad L, Svarstad E, Cook WJ, Moll S, Barbey F, Geldenhuys L, West M, Ferluga D, Vujkovac B, Howie A, Burns A, Reeve R, Waldek S, Noel LH, Grunfeld JP, Valbuena C, Oliveira JP, Muller J, Breunig F, Zhang X, Warnock DG: Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant 25: 2168–2177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Germain D, Waldek S, Banikazemi M, Bushinsky D, Charrow J, Lee P, Loew T, Vedder AC, Abichandani R, Wilcox WR, Guffon N: Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 18: 1547–1557, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Thurberg BL, Rennke H, Colvin RB, Dikman S, Gordon RE, Collins AB, Desnick RJ, O'Callaghan M: Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int 62: 1933–1946, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Schiffmann R, Warnock DG, Banikazemi M, Bultas J, Linthorst GE, Packman S, Sorensen SA, Wilcox WR, Desnick RJ: Fabry disease: Progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant 24: 2102–2111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ: Safety and efficacy of recombinant human alpha-galactosidase A–replacement therapy in Fabry's disease. N Engl J Med 345: 9–16, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Schiffmann R, Kopp JB, Austin HA, III, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO: Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 285: 2743–2749, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, Linthorst GE, Desnick RJ, Germain DP: Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet 75: 65–74, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ: Agalsidase-beta therapy for advanced Fabry disease: A randomized trial. Ann Intern Med 146: 77–86, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tahir H, Jackson LL, Warnock DG: Antiproteinuric therapy and Fabry nephropathy: Sustained reduction in proteinuria in patients receiving enzyme replacement therapy with agalsidase-beta. J Am Soc Nephrol 18: 2609–2617, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Elleder M, Poupetova H, Kozich V: Fetal pathology in Fabry's disease and mucopolysaccharidosis type I. Cesk Patol 34: 7–12, 1998 [PubMed] [Google Scholar]
- 13. Gubler MC, Lenoir G, Grunfeld JP, Ulmann A, Droz D, Habib R: Early renal changes in hemizygous and heterozygous patients with Fabry's disease. Kidney Int 13: 223–235, 1978 [DOI] [PubMed] [Google Scholar]
- 14. Tøndel C, Bostad L, Hirth A, Svarstad E: Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis 51: 767–776, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, Goldfarb L, Brady RO, Balow JE, Austin HA, III, Kopp JB: Natural history of Fabry renal disease: Influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 81: 122–138, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Ortiz A, Oliveira JP, Waldek S, Warnock DG, Cianciaruso B, Wanner C: Nephropathy in males and females with Fabry disease: Cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplant 23: 1600–1607, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt-Rasmussen U, Sims K, Waldek S, Pastores GM, Lee P, Eng CM, Marodi L, Stanford KE, Breunig F, Wanner C, Warnock DG, Lemay RM, Germain DP: Females with Fabry disease frequently have major organ involvement: Lessons from the Fabry Registry. Mol Genet Metab 93: 112–128, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 19. Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, Larson TS: Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis 43: 112–119, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Breunig F, Weidemann F, Strotmann J, Knoll A, Wanner C: Clinical benefit of enzyme replacement therapy in Fabry disease. Kidney Int 69: 1216–1221, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Schiffmann R, Askari H, Timmons M, Robinson C, Benko W, Brady R, Ries M: Weekly enzyme replacement therapy may slow decline of renal function in Fabry patients who are on long-term biweekly dosing. J Am Soc Nephrol 18: 1576–1583, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oqvist B, Brenner BM, Oliveira JP, Ortiz A, Schaefer R, Svarstad E, Wanner C, Zhang K, Warnock DG: Nephropathy in Fabry disease: The importance of early diagnosis and testing in high-risk populations. Nephrol Dial Transplant 24: 1736–1743, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Ortiz A, Oliveira JP, Wanner C, Brenner BM, Waldek S, Warnock DG: Recommendations and guidelines for the diagnosis and treatment of Fabry nephropathy in adults. Nat Clin Pract Nephrol 4: 327–336, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Warnock DG, Daina E, Remuzzi G, West M: Enzyme replacement therapy and Fabry nephropathy. Clin J Am Soc Nephrol 5: 371–378, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Halbesma N, Brantsma AH, Bakker SJ, Jansen DF, Stolk RP, De Zeeuw D, De Jong PE, Gansevoort RT: Gender differences in predictors of the decline of renal function in the general population. Kidney Int 74: 505–512, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Eng CM, Germain DP, Banikazemi M, Warnock DG, Wanner C, Hopkin RJ, Bultas J, Lee P, Sims K, Brodie SE, Pastores GM, Strotmann JM, Wilcox WR: Fabry disease: Guidelines for the evaluation and management of multi-organ system involvement. Genet Med 8: 539–548, 2006 [DOI] [PubMed] [Google Scholar]
- 30. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J: Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet 335: 765–774, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Sims K, Politei J, Banikazemi M, Lee P: Stroke in Fabry disease frequently occurs before diagnosis and in the absence of other clinical events: Natural history data from the Fabry Registry. Stroke 40: 788–794, 2009 [DOI] [PubMed] [Google Scholar]