Abstract
Background and objectives: Arteriovenous fistulas (AVFs) are the preferred vascular access for hemodialysis but have a considerable failure rate. This study investigated whether routine preoperative vascular ultrasound results in better AVF outcome than physical examination.
Design, setting, participants, & measurements: Patients with end-stage kidney disease referred for permanent access formation were assessed by independent examiners using physical examination and ultrasound. After random allocation, the ultrasound report was disclosed to the surgeon for patients in the ultrasound group but not for the clinical group. End points were AVF failure and survival rates, analyzed by intention to treat and by use for hemodialysis.
Results: AVFs were made in 208 of 218 randomized patients. Clinical and ultrasound groups were similar in terms of patient characteristics, allocation to individual surgeons, and proportion of forearm AVFs. The ultrasound group had a significantly lower rate of immediate failure (4% versus 11%, P = 0.028) and, among failed AVFs, less thrombosis (38% versus 67%, P = 0.029). Primary AVF survival at 1 year was not statistically different (ultrasound = 65%, clinical = 56%, P = 0.081). Assisted primary AVF survival at 1 year was significantly better for the ultrasound group (80% versus 65%, P = 0.012). The number of patients requiring preoperative ultrasound to prevent one AVF failure was 12.
Conclusions: Routine preoperative vascular ultrasound in addition to clinical assessment improves AVF outcomes in terms of patency and use for dialysis. National Research Register, United Kingdom, trial number N0046131432.
The arteriovenous fistula (AVF) is the preferred vascular access for hemodialysis (1,2). However, AVF failure is a common problem, particularly for radiocephalic AVF, with a modest two-thirds survival by 1 year (3). AVF failure is often attributed to vascular comorbidity because an increasing number of elderly patients with diabetes and cardiovascular disease are accepted for hemodialysis treatment (4–9).
Physical examination has traditionally been used to identify a suitable artery and vein for AVF formation (10). Preoperative vascular mapping with ultrasound has recently been shown to predict AVF outcome (10–13). The Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend the use of routine ultrasound mapping for all patients but noted the lack of level one evidence to support this recommendation (1).
We conducted a randomized trial to evaluate the outcome of routine preoperative assessment with ultrasound in terms of patency and use of AVFs for hemodialysis.
Materials and Methods
Patients
The study was approved by the Local Ethics Committee of East Birmingham, United Kingdom; was registered with the U.K. National Research Register, trial number N0046131432; and adhered to the Declaration of Helsinki.
All patients with end-stage kidney disease at Heart of England Hospital (Birmingham, United Kingdom) who were referred for formation of AVF were invited to take part in the study. Included were all with either none or one previous AVF. Patients who had already participated in the study, who had more than one previous AVF, or had a previous upper-arm arteriovenous graft were excluded. Patients who gave informed written consent were recruited between August 31, 2004 and September 30, 2006 and were followed for up to 40 months. Clinical data were obtained from electronic patient and hemodialysis session records.
Preoperative Assessment
All patients were evaluated with physical and ultrasound examination by independent assessors blinded to each other. Physical examination was carried out by one of four vascular surgeons (one consultant, three trainees) with experience in AVF formation. Following a standardized protocol, the pulses at elbows and wrists and the superficial veins in the forearm and upper arm (with tourniquet) were assessed. Vessels were considered suitable if the artery had a good pulse and the vein was patent and of good caliber. The most distal possible site was chosen for AVF formation.
The ultrasound examination was carried out by a nephrology trainee (n = 216 scans) and a vascular access nurse specialist (n = 36 scans). Patients were assessed with a portable ultrasound scanner (SonoSite 180 plus, SonoSite, Inc., Bothell, WA) with a 5- to 10-MHz linear probe. A standardized scan protocol was used based on a technique described elsewhere, and the most distal site possible was recommended for AVF formation (14).
Briefly, the arterial scan followed the vasculature from the brachial artery in the mid-upper arm to the radial artery at the wrist in B-mode. Internal diameters were measured of the radial artery at the wrist and in the distal forearm and of the brachial artery at the elbow and in the mid-upper arm. The average of two diameter measurements at each site was the diameter reported. Minimum arterial diameters suitable for AVF formation were 2 mm in the forearm and 3 mm in the upper arm (10,15). Color power and spectral duplex ultrasound were recorded in the brachial artery just above the elbow and in the radial artery just above the wrist. The spectral Doppler waveform was considered adequate if it was triphasic or biphasic in the antegrade direction but inadequate if retrograde, damped, or absent (16). The ulnar artery was assessed in the distal forearm with B-mode and duplex ultrasound. Complete or near occlusion of the ulnar artery was considered a contraindication to access formation in the same arm because of the increased risk of hand ischemia.
After application of a tourniquet, the superficial veins were followed in cross section with B-mode from wrist to mid-upper arm with intermittent vein compression. The cephalic vein was scanned from wrist to mid-upper arm. The basilic vein was scanned from the elbow level to its drainage into the deep brachial veins and in the forearm if the cephalic vein was unsuitable (17). Internal diameters and vein depth were measured at the wrist, in the distal forearm, in the proximal forearm, at the level of the elbow, and in the upper arm. The average of two diameter measurements at each site was the diameter reported. Suitable veins had to be fully compressible and morphologically normal, with minimum diameters of 2 mm in the forearm and 3 mm in the upper arm (10).
Random Allocation
An independent trial coordinator, unaware of the results of the assessments, stratified patients by age (<65 years versus older) and dialysis status (prehemodialysis versus on hemodialysis) into four strata and randomized to clinical and ultrasound groups using four sets of consecutively numbered sealed envelopes containing the assessment allocation on the basis of a predefined computer-generated random sequence in blocks of eight. The allocation was revealed to the main researcher after consent, recruitment, and preoperative assessments were complete.
For the clinical group, the ultrasound findings were not disclosed to the surgeon. For the ultrasound group, the ultrasound findings were disclosed to the surgeon; in case of discrepancy between the clinical and ultrasound assessments, the surgeon could decide but usually followed the ultrasound recommendation. In the either group, the surgeon had the option to request further imaging (ultrasound, angiography) if deemed necessary. Patients were unaware of the random allocation.
Surgery
A team of 6 consultant vascular surgeons and 13 vascular surgical trainees shared the workload of AVF formation. Trainees generally operated with the consultant under direct supervision, but trainees with sufficient experience (n = 5) operated under indirect supervision.
AVF Use for Hemodialysis
After surgery, all AVFs were used by clinical need as defined by the attending nephrologist and by AVF maturity as assessed by an experienced hemodialysis nurse. When necessary, AVF salvage was undertaken by endovascular or surgical intervention for failed AVFs.
Study Objectives
We hypothesized that routine ultrasound use would result in (1) less AVF failure and (2) better AVF survival.
Outcome Definitions
Primary end point (early AVF outcomes):
Immediate AVF failure: AVF thrombosis on the day of surgery, defined as absence of a thrill or inadequate vein found at surgery (18,19)
Primary AVF failure: All AVF that were never adequate for hemodialysis after initial surgical formation, including immediate failure on the day of surgery, early thrombosis, and failure to mature (9,18).
Secondary end point (longer term AVF outcomes):
Primary AVF survival: Patency of all AVFs, defined by patency and usability for dialysis, from date of surgical formation to date of first access failure (20)
Assisted primary AVF survival: Patency of all AVFs, defined by patency and usability for dialysis, from date of surgical formation until the access was thrombosed, including time gained by successful salvage procedures (20).
AVF failure meant that AVFs were unusable for dialysis, requiring a salvage intervention, new access formation, or insertion of a hemodiaysis catheter (21). AVFs were considered usable for dialysis if they were used for at least six consecutive 4-hour dialysis sessions by two needle cannulation without assistance from a catheter, with a minimum blood pump rate of 200 ml/min after the third session.
Censoring occurred at the time of patient death, kidney transplantation, change to peritoneal dialysis, patient transfer to another unit, or the end of the follow-up period. Outcome assessment and data analysis were carried out without blinding to assessment groups using standard end points from the literature.
Statistical Analyses
Expecting primary AVF failure in 35%, we considered a reduction of failure with ultrasound by at least 15% as clinically important (9). At a significance level of 0.05 and a power level of 0.8, a minimum sample size of 280 patients (140 in each group) was required.
Univariate analysis of categorical variables was made with χ2 square test. After cube transformation, age was tested by independent t test (22). Number needed to treat was calculated as 1/absolute risk reduction. Absolute risk reduction was calculated as the difference and relative risk was calculated as the ratio of failure risk in the clinical versus that in the ultrasound group (23). Primary and assisted primary AVF survival was examined by life-table analysis. Multivariate analysis of AVF survival was examined by Cox regression. Predictor variables were excluded by a backward stepwise approach on the basis of the likelihood ratio (22). All analyses were by intention to treat and under the condition that AVF usability for hemodialysis was known. We used SPSS version 16 for statistical calculations. A P value of 0.05 or less was considered significant.
Results
Patients
Of 258 eligible patients, 218 consented to the study (Figure 1). All 208 patients undergoing surgery (clinical n = 101, ultrasound n = 107) received an AVF for which the immediate outcome was known. AVF usability for hemodialysis was known in 186 patients for primary survival and in 183 patients for assisted primary survival. Primary survival was unknown in 22 patients because of death or not having started dialysis by the end of follow-up. Assisted primary survival was unknown in a further three patients who had undergone AVF salvage but had not started dialysis (Figure 1). Thus, the number required by our power calculation was not reached.
Figure 1.
Consort diagram of patients in the study.
Baseline Characteristics of Randomized Groups
Clinical and ultrasound groups were similar in terms of baseline patient characteristics, difficulty of access, or surgeon's experience (Table 1). Upper-arm or right-arm AVF location and the rate of vein transpositions were not significantly different, although the latter was more common in the ultrasound group (Table 2).
Table 1.
Baseline characteristics
| Characteristic | Clinical Group (n = 106) | Ultrasound Group (n = 112) | P |
|---|---|---|---|
| Age | 0.760 | ||
| median [range] | 67 [20 to 89] | 69 [26 to 88] | |
| 25th percentile | 55 | 60 | |
| 75th percentile | 76 | 75 | |
| Gender | 0.496 | ||
| male | 70 (66.0%) | 69 (61.6%) | |
| female | 36 (34%) | 43 (38.4%) | |
| Ethnicity | 0.477 | ||
| Caucasian | 71 (67%) | 80 (71.4%) | |
| Indo-Asian | 21 (19.8%) | 25 (22.3%) | |
| African | 11 (10.4%) | 4 (3.6%) | |
| other | 3 (2.8%) | 3 (2.7%) | |
| Body mass indexa | 26.9 ± 6.2 | 27.1 ± 6.31 | 0.471 |
| Hypertension | 79 (75.2%) | 87 (78.4%) | 0.584 |
| Diabetes | 36 (34.3%) | 48 (43.2%) | 0.177 |
| Cardiac diseaseb | 38 (36.2%) | 33 (29.7%) | 0.312 |
| Vascular diseasec | 23 (21.9%) | 16 (14.4%) | 0.153 |
| Antiplatelet agentd | 55 (52.9%) | 47 (42.3%) | 0.122 |
| Hemodialysis | 0.955 | ||
| not on hemodialysise | 82 (77.4%) | 87 (77.7%) | |
| on hemodialysis | 24 (22.6%) | 25 (22.3%) | |
| Difficult accessf | 12 (11.3%) | 12 (10.7%) | 0.886 |
| Surgical experience | 0.549 | ||
| surgical consultant alone | 39 (39.8%) | 47 (46.5%) | |
| surgical consultant + trainee | 41 (41.8%) | 35 (34.7%) | |
| surgical trainees alone | 18 (18.4%) | 19 (18.8%) |
Body mass index data only available for approximately 62% of patients (clinical 57%, ultrasound 67%).
Cardiac disease was defined by a clinical history of previous angina, acute coronary syndrome, coronary artery bypass graft, or cardiac transplant.
Vascular disease was defined by a clinical history of claudication, critical leg ischemia, and endovascular or surgical intervention for peripheral arterial disease.
Antiplatelet agent = aspirin or clopidogrel or both.
Not on hemodialysis included patients with advanced chronic kidney disease (majority), failing peritoneal dialysis or renal transplant with plan to transfer to hemodialysis, and patients having started hemodialysis for less than 90 days.
Difficult access was defined as patients in whom no suitable site for AVF formation could be identified by clinical examination alone.
Table 2.
Site of AVF (in all patients who underwent surgery n = 208)
| Site of AVF | Clinical (n = 101) | Ultrasound (n = 107) |
|---|---|---|
| All forearm | 64 (63) | 63 (59) |
| left radiocephalic | 47 (47) | 42 (39) |
| right radiocephalic | 16 (16) | 20 (19) |
| other forearma | 1 (1) | 1 (1) |
| All upper arm | 37 (37) | 44 (41) |
| left braciocephalic | 21 (21) | 26 (24) |
| right brachiocephalic | 12 (12) | 10 (9) |
| left brachiobasilic | 2 (2) | 2 (2) |
| right brachiobasilic | 1 (1) | 5 (5) |
| other upper armb | 1 (1) | 1 (1) |
| All AVF with superficialization/transposition | 5 (5) | 11 (10) |
Data presented as absolute number (%). Forearm versus upper-arm AVF: χ2 0.266; P = 0.606. Superficialization/transposition of vein versus none: χ2 2.079; P = 0.149.
Other forearm AVF: left radiobasilic (ultrasound); left ulnobasilic (clinical).
Other upper-arm AVF: right upper-arm ulnocephalic (clinical); left upper-arm ulnocephalic (ultrasound).
Early AVF Outcomes
Immediate failure was significantly worse in the clinical group (Table 3). Of all 16 immediate failures, 2 were due to an inadequate vein found at surgery (clinical n = 2, ultrasound n = 0).
Table 3.
Primary outcome—AVF failure rates
| Outcome | Clinical Group | Ultrasound Group | RR [95% CI]a | P | ARRb | NNT [95% CI]c |
|---|---|---|---|---|---|---|
| Immediate failure | ||||||
| all AVF: n failures | 12 | 4 | 8.1% | 12 [6, 132] | ||
| ITT: n = 218d | 11.3% | 3.6% | 0.315 [0.105, 0.944] | 0.028 | ||
| surgery: n = 208e | 11.9% | 3.7% | 0.315 [0.105, 0.944] | 0.028 | ||
| forearm AVF: n failures | 11 | 4 | 11.1% | 9 | ||
| ITT: n = 131 | 16.7% | 6.2% | 0.369 [0.124, 1.100] | 0.054 | ||
| surgery: n = 126 | 17.5% | 6.3% | 0.364 [0.122, 1.081] | 0.054 | ||
| Primary failure | ||||||
| all AVF: n failures | 33 | 24 | 11.0% | 9 | ||
| ITT: n = 218 | 31.1% | 21.4% | 0.688 [0.437, 1.084] | 0.103 | ||
| dialysis use: n = 186f | 36.3% | 25.3% | 0.697 [0.449, 1.082] | 0.104 | ||
| forearm AVF: n failures | 27 | 19 | 12.4% | 8 | ||
| ITT: n = 131 | 40.9% | 29.2% | 0.715 [0.444, 1.151] | 0.139 | ||
| dialysis use: n = 116 | 45.8% | 33.3% | 0.728 [0.460, 1.154] | 0.171 | ||
| Nonmaturationg | ||||||
| all AVF: n failures | 21 | 20 | 4.6% | 22 | ||
| ITT: n = 218 | 19.8% | 17.9% | 0.901 [0.519, 1.565] | 0.712 | ||
| dialysis use: n = 170 | 26.6% | 22.0% | 0.827 [0.485, 1.409] | 0.484 | ||
| forearm AVF: n failures | 16 | 15 | 5.0% | 20 | ||
| ITT: n = 126 | 25.4% | 23.8% | 0.938 [0.509, 1.729] | 0.836 | ||
| dialysis use: n = 101 | 33.3% | 28.3% | 0.849 [0.472, 1.526] | 0.584 |
AVF failures are shown for all patients, for the clinical and for the ultrasound group, respectively; n failures means absolute number of AVF failures; % failures means percentage of AVF failures to total number AVF.
Relative risk of AVF failure with 95% CI [lower, upper limits].
Absolute risk reduction of AVF failure.
NNT [95% CI]: Number needed to treat (i.e., the number of ultrasound scans needed to avoid one AVF failure; the 95% CI is shown for immediate failure but not for primary failure or the forearm subgroups because these are not statistically significant.
ITT: analysis by intention to treat; i.e., for all patients randomized.
Analysis restricted to patients who had surgery.
Analysis restricted to AVF for which the outcome (dialysis use or failure) was known.
Nonmaturation: primary failure excluding immediate failure.
Primary failure occurred less in the ultrasound group, but this was not statistically significant (Table 3). Primary failure after excluding immediate failure was similar (clinical n = 21, ultrasound n = 20). On univariate comparison, primary failure was significantly worse in forearm compared with upper-arm AVFs (39.7% versus 15.7%; P = 0.001). Further analysis of primary failure by thrombosis versus other causes showed significantly more early thrombosis in the clinical group (clinical n = 22 of 33 (67%); ultrasound n = 9 of 24 (38%); P = 0.029).
The number of ultrasound scans needed to avoid one immediate failure event was low (Table 3).
Longer Term AVF Outcomes
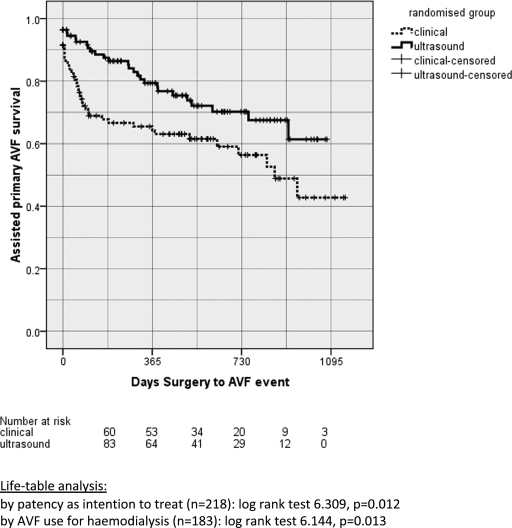
Primary AVF survival at 1 year was 56% for the clinical group and 65% for the ultrasound group, but this was not statistically significant (P = 0.081; Figure 2). The assisted primary survival at 1 year was significantly better for ultrasound (80% versus 65%, P = 0.012; Figure 3).
Figure 2.
Primary AVF survival of clinical and ultrasound groups.
Figure 3.
Assisted primary AVF survival for clinical and ultrasound groups.
With Cox regression, we tested which baseline clinical variables were independent predictors of AVF survival. First, we included all variables (i.e., means ultrasound, age, gender, ethnicity, diabetes, cardiac disease, vascular disease, antiplatelet medication, dialysis status, AVF site, or surgeon's experience) (Table 4). In the final analysis, after backward exclusion of nonsignificant variables, predialysis status, upper-arm AVF site, and male gender remained independent predictors of better primary survival; predialysis status, upper-arm AVF site, and ultrasound were independent predictors of better assisted primary survival (Table 4).
Table 4.
Secondary outcome—Cox regression for primary and assisted primary AVF survival
| Predictor Variables | Primary AVF Survival |
Assisted Primary AVF Survival |
||
|---|---|---|---|---|
| HR | 95% CI: Lower, Upper | HR | 95% CI: Lower, Upper | |
| Randomization to ultrasound | 0.669 | 0.436, 1.027 | 0.573 | 0.348, 0.945 |
| Agea | 1.001 | 0.986, 1.015 | 0.999 | 0.983, 1.016 |
| Genderb | 0.672 | 0.426, 1.060 | 0.857 | 0.504, 1.458 |
| Ethnicityc | 0.951 | 0.579, 1.564 | 1.364 | 0.782, 2.380 |
| Diabetes | 1.659 | 1.002, 2.747 | 1.648 | 0.922, 2.945 |
| Cardiac disease | 1.033 | 0.597, 1.788 | 1.037 | 0.555, 1.936 |
| Vascular disease | 1.105 | 0.605, 2.018 | 1.434 | 0.735, 2.794 |
| Antiplatelet drug | 0.733 | 0.422, 1.273 | 0.609 | 0.324, 1.145 |
| Dialysis statusd | 2.060 | 1.217, 3.487 | 2.668 | 1.451, 4.907 |
| Surgical experiencee | ||||
| trainee alone | 0.912 | 0.487, 1.708 | 1.419 | 0.731, 2.756 |
| consultant + trainee | 0.945 | 0.580, 1.540 | 0.751 | 0.418, 1.349 |
| AVF sitef | 0.449 | 0.273, 0.741 | 0.460 | 0.257, 0.823 |
Cox regression for AVF survival by intention to treat (for primary and assisted primary patency, respectively) with AVF survival as the dependent variable; the initial regression model with all predictor variables is shown in the table above with HR for AVF failure and 95% CIs.
Age was transformed to [(age^3)/10,000] to correct for skew.
Female gender as baseline hazard.
Caucasian as baseline hazard versus non-Caucasian.
“Not yet on hemodialysis” as baseline hazard versus “on hemodialysis.”
Consultant operating alone as baseline hazard, compared to trainee alone and to consultant with trainee operating.
Forearm AVF as baseline hazard versus upper-arm AVF.
Primary AVF Survival: For the initial model with all predictor variables shown in the table above, χ2 was 25.312 with 12 degrees of freedom and P = 0.013. The final model after backward stepwise exclusion of nonsignificant variables included only dialysis status (hazard ratio [HR] 2.196; 95% confidence interval [CI] [1.323, 3.644]), AVF site (HR 0.448; 95% CI [0.277, 0.724]), gender (HR 0.637; 95% CI [0.412, 0.985]), randomization to ultrasound (HR 0.691; 95% CI [0.453, 1.054]), and diabetes (HR 1.504; 95% CI [0.965, 2.344]). The final model χ2 was 23.263 with 5 degrees of freedom and P = 0.000.
Assisted Primary AVF Survival: For the initial model with all predictor variables shown in the table above, χ2 was 26.759 with 12 degrees of freedom and P = 0.008. The final model after backward stepwise exclusion of nonsignificant variables included only dialysis status (HR 2.601; 95% CI [1.473, 4.594]), AVF site (HR 0.491; 95% CI [0.284, 0.849]), randomization to ultrasound (HR 0.594 95% CI [0.364, 0.969]), and diabetes (HR 1.657; 95% CI [0.989, 2.777]). The final model χ2 was 19.613 with 4 degrees of freedom and P = 0.001.
Discussion
Our randomized trial shows that routine preoperative ultrasound in addition to physical examination improves AVF patency and dialysis use in a patient population without complex access problems. Specifically, ultrasound results in less immediate failure, less early AVF thrombosis, and better assisted primary AVF survival. Ultrasound is effective with a small number of ultrasound scans needed to prevent failure.
Our finding that immediate failure is significantly reduced by preoperative ultrasound is consistent with another randomized trial, which showed significantly less failure (6% versus 25%) on the day of surgery in the ultrasound compared with the clinical group (19). Inadequate vessels can be identified by ultrasound, which reduces the rate of immediate failure (11,12,24).
Our data show no statistically significant difference between the clinical and ultrasound groups for primary failure or for primary survival of AVF. Particularly, we saw no difference when primary failure is counted without immediate failure. This could indicate that ultrasound improves AVF patency but not AVF maturation, in agreement with published experience that maturation failure remains an important problem, even when preoperative ultrasound is used (25–27). However, our study is underpowered and cannot give a definitive answer.
Our study shows a significantly better assisted primary AVF survival (i.e., survival time gained by AVF salvage) in the ultrasound group. The clinical group had a significantly greater early AVF loss due to a higher rate of early thrombosis and thus had poorer potential for salvage.
We found that factors other than preoperative assessment affect AVF outcome, including gender, diabetes, and AVF site. This is in keeping with the literature (5,6,27,28). As noted by others, prior hemodialysis strongly predicted poorer outcome (28). Our stratified randomization produced equal allocation of patients with prior hemodialysis.
Our study supports the recommendation by KDOQI and European guidelines for routine preoperative ultrasound (1,2). However, a recent trial, which preselected only patients with normal preoperative physical findings and then randomized some to additional ultrasound mapping, found no advantage for ultrasound in terms of immediate patency or early AVF survival (29). In comparison, our patients were considerably older and not preselected, and our data show that such patients generally benefit from ultrasound. In our experience, ultrasound is unlikely to add information in the small subgroup of young nondiabetic men with normal physical findings.
Potential strengths of our study are its randomized design, the strict end point definition for functional AVF patency by use on dialysis, a real-life setting including the option of selective imaging for the clinical group, and a low dropout rate in terms of surgery not being done (n = 10). A potential weakness is that the operating surgeons were not blinded to the randomization group because it was necessary to justify the decision for AVF formation on the basis of the details of the preoperative assessment.
In conclusion, our randomized clinical trial demonstrates a benefit of routine preoperative ultrasound in AVF outcomes with a small number needed to treat to save one fistula. Although nonmaturation may remain an important problem, preoperative ultrasound combined with appropriate AVF salvage leads to better long-term AVF use in hemodialysis.
Disclosures
None.
Acknowledgments
We thank the British Renal Society for supporting this research project with a generous grant. We are indebted to others who made important contributions to the study, including Dr. John Henderson, consultant radiologist at Heart of England Hospital Birmingham, for his invaluable training in ultrasound; Dr. Jocelyn Bell, trial coordinator at the Research and Development Department of Heart of England Hospital, for carrying out the randomization; Mr. Tim Marshall, statistician at the University of Birmingham (now retired), for his invaluable contribution and advice on design of the study (he produced the power calculation and the computer-generated random sequence); Dr. Allan White, statistician at the University of Birmingham, for review of the statistical analysis; Professor Andrew Bradbury, professor of vascular surgery and consultant at Heart of England Hospital, for his invaluable suggestion to design this study as a randomized trial; Mr. Mark Grannell and Mr. Yh Tan, vascular surgical trainees at Heart of England Hospital, for their faithful readiness to carry out clinical patient assessments; Mr. Carl Richardson, renal access specialist nurse at Heart of England Hospital, for his initial contribution of ultrasound scanning; and Mr. Roger Atkins, Mrs. Sarah Powers, and many other renal nurses at Heart of England Hospital for their willingness to practically support the study. Finally we thank the participating patients who gave their time and cooperation to make this study possible. This study was presented in abstract form at the British Renal Society meetings in Birmingham, United Kingdom, June 11 through 13, 2007, and in Glasgow, United Kingdom, May 13 through 16, 2008, as well as at the Vascular Access Society meeting in Rome, Italy, April 20 through 22, 2009.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. National Kidney Foundation Kidney Disease Outcomes Quality Initiative: 2006 update vascular access. Guideline 2: Selection and placement of hemodialysis access. Am J Kidney Dis 48[Suppl 1]: S192–S200, 2006 [Google Scholar]
- 2. Tordoir J, Cannaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R. EBPG on vascular access. Nephrol Dialysis Transplant 22[Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Rooijens PPGM, Tordoir JHM, Stijnen T, Burgmans JPJ, de Smet AAEA, Yo TI. Radiocephalic wrist arteriovenous fistula for hemodialysis: Meta-analysis indicates a high primary failure rate. Eur J Vasc Endovasc Surg 28: 583–589, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV: Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int 67: 2462–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, Allon M: Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 56: 275–280, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Golledge J, Smith CJ, Emery J, Farrington K, Thompson HH: Outcome of primary radiocephalic fistula for haemodialysis. Br J Surg 86: 211–216, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K: Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis 42: 1000–1012, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Tomson C, Udayaraj U, Gilg J, Ansell D. Comorbidities in U.K. patients at the start of renal replacement therapy. In: U.K. Renal Registry: The Ninth Annual Report, edited by Ansell D, Feest T, Tomson C, Williams A, Warwick G. Bristol, United Kingdom, Renal Registry, 2006: 87–101 [Google Scholar]
- 9. Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Malovrh M: The role of sonography in the planning of arteriovenous fistulas for hemodialysis. Semin Dial 16: 299–303, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Malovrh M: Native arteriovenous fistula: Preoperative evaluation. Am J Kidney Dis 39: 1218–1225, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Parmar J, Aslam M, Standfield N: Pre-operative radial arterial diameter predicts early failure of arteriovenous fistula (AVF) for haemodialysis. Eur J Vasc Endovasc Surg 33: 113–115, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Ku YM, Kim YO, Kim JI, Choi YJ, Yoon SA, Kim YS, Song SW, Yang CW, Kim YS, Chang YS, Bang BK. Ultrasonographic measurement of intima-media thickness of radial artery in pre-dialysis uraemic patients: Comparison with histological examination. Nephrol Dial Transplant 21: 715–720, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Ferring M, Henderson J, Wilmink A, Smith S: Vascular ultrasound for the pre-operative evaluation prior to arteriovenous fistula formation for haemodialysis: Review of the evidence. Nephrol Dial Transplant 23: 1809–1815, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Brown PW: Preoperative radiological assessment for vascular access. Eur J Vasc Endovasc Surg 31: 64–69, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Oates C. Pulsatile flow. In: Cardiovascular Haemodynamics and Doppler Waveforms Explained, edited by Oates C. London, Greenwich Medical Media, 2001: 61–116 [Google Scholar]
- 17. Robbin ML, Lockhart ME. Ultrasound assessment before and after hemodialysis access. In: Introduction to Vascular Ultrasonography, edited by Zwiebel W, Pellerito J. Philadelphia, Elsevier Saunders, 2005: 325–340 [Google Scholar]
- 18. Miller C, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Mihmanli I, Besirli K, Kurugoglu S, Atakir K, Haider S, Ogut G, Numan F, Canturk E, Sayin AG: Cephalic vein and hemodialysis fistula: Surgeon's observation versus color Doppler ultrasonographic findings. J Ultrasound Med 20: 217–222, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Sidawy A, Gray R, Besarab A, Henry M, Ascher E, Silva MJ, Miller A, Scher L, Trerotola S, Gregory R, Rutherford R, Kent K: Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 35: 603–610, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Konner K, Hulbert-Shearon T, Roys E, Port F: Tailoring the initial vascular access for dialysis patients. Kidney Int 62: 329–338, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Altman D. Practical Statistics for Medical Research, London, Chapman and Hall, 1991 [Google Scholar]
- 23. Greenhalgh T. How to Read a Paper: The Basics of Evidence-Based Medicine, London, BMJ Books, 2001: 215 [Google Scholar]
- 24. Robbin ML, Gallichio MH, Deierhoi MH, Young CJ, Weber TM, Allon M: US vascular mapping before hemodialysis access placement. Radiology 217: 83–88, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Sivanesan S, How TV, Bakran A: Sites of stenosis in AV fistulae for haemodialysis access. Nephrol Dial Transplant 14: 118–120, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Grogan J, Castilla M, Lozanski L, Griffin A, Loth F, Bassiouny H: Frequency of critical stenosis in primary arteriovenous fistulae before hemodialysis access: Should duplex ultrasound surveillance be the standard of care? J Vasc Surg 41: 1000–1006, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Peterson W, Barker J, Allon M. Disparities in fistula maturation persist despite preoperative vascular mapping. Clin J Am Soc Nephrol 3: 437–441, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weale AR, Bevis P, Neary WD, Boyes S, Morgan JD, Lear PA, Mitchell DC: Radiocephalic and brachiocephalic arteriovenous fistula outcomes in the elderly. J Vasc Surg 47: 144–150, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Nursal TZ, Oguzkurt L, Tercan F, Torer N, Noyan T, Karakayali H, Haberal M: Is routine preoperative ultrasonographic mapping for arteriovenous fistula creation necessary in patients with favorable physical examination findings? Results of a randomized controlled trial. World J Surg 30: 1100–1107, 2006 [DOI] [PubMed] [Google Scholar]