Abstract
Background and objectives: Maintenance hemodialysis (MHD) patients with larger body or fat mass have greater survival than normal to low mass. We hypothesized that mid-arm muscle circumference (MAMC), a conveniently measured surrogate of lean body mass (LBM), has stronger association with clinical outcomes than triceps skinfold (TSF), a surrogate of fat mass.
Design, settings, participants, & measurements: The associations of TSF, MAMC, and serum creatinine, another LBM surrogate, with baseline short form 36 quality-of-life scores and 5-year survival were examined in 792 MHD patients. In a randomly selected subsample of 118 subjects, LBM was measured by dual-energy x-ray absorptiometry.
Results: Dual-energy x-ray absorptiometry–assessed LBM correlated most strongly with MAMC and serum creatinine. Higher MAMC was associated with better short form 36 mental health scale and lower death hazard ratios (HRs) after adjustment for case-mix, malnutrition-inflammation-cachexia syndrome, and inflammatory markers. Adjusted death HRs were 1.00, 0.86, 0.69, and 0.63 for the first to fourth MAMC quartiles, respectively. Higher serum creatinine and TSF were also associated with lower death HRs, but these associations were mitigated after multivariate adjustments. Using median values of TSF and MAMC to dichotomize, combined high MAMC with either high or low TSF (compared with low MAMC/TSF) exhibited the greatest survival, i.e., death HRs of 0.52 and 0.59, respectively.
Conclusions: Higher MAMC is a surrogate of larger LBM and an independent predictor of better mental health and greater survival in MHD patients. Sarcopenia-correcting interventions to improve clinical outcomes in this patient population warrant controlled trials.
Individuals with advanced chronic kidney disease (CKD), who undergo chronic dialysis treatment, have a high mortality rate (1,2). Although some studies have evaluated the association of nutrition with mortality (3,4), others have focused on body mass index (BMI) and suggested that dialysis patients with higher BMI enjoy a survival advantage in even such high BMI ranges that are ordinarily considered harmful for the general population (5–11). BMI, however, is an imperfect measure of adiposity (6,8,10) and does not differentiate lean from fat mass (12). Lean body mass (LBM) can serve as an index of muscle mass and somatic protein, whereas fat mass more directly reflects energy storage. Higher fat mass has been associated with inflammation and adverse outcomes in the general population (13), whereas higher muscle mass seems to be associated with better clinical outcomes (14). If CKD patients are similar, interventions to improve sarcopenia in CKD patients may improve survival.
In the general population, BMI is more strongly correlated with body fat mass and less with LBM when measured by such methods as dual-energy x-ray absorptiometry (DEXA) (15,16). However, in maintenance hemodialysis (MHD) patients, BMI may be equally correlated with both fat and lean mass (17). Other measures of body size or composition may better represent LBM or muscle mass in MHD patients. A large epidemiologic study used urinary creatinine before the start of dialysis treatment as a muscle mass surrogate and suggested that the survival advantage of obese MHD patients is mostly because of higher LBM (11). To our knowledge, only a few studies have compared outcome predictability of surrogates of body mass components in MHD patients.
In this study, we first examined the correlations of several anthropometric and biochemical measures with the DEXA-measured LBM as the reference standard in a small group of MHD patients to determine which serves as a better surrogate for LBM. In a larger cohort, we subsequently studied the outcome predictability of two surrogates of LBM, mid-arm muscle circumference (MAMC) and serum creatinine, and compared them with triceps skinfold (TSF) as a surrogate of fat mass after controlling for demographics, nutritional status, and inflammation. We also examined the association of MAMC with health-related quality of life (QoL) at the start of the cohort. We hypothesized that MAMC is a reliable correlate of LBM and a predictor of better QoL and greater survival in MHD patients.
Materials and Methods
Patient Population
We studied MHD patients who participated in the National Institutes of Health–sponsored Nutritional and Inflammatory Evaluation in Dialysis (NIED) Study (18–22). The original patient cohort was derived from a pool of approximately 1300 MHD outpatients in eight DaVita dialysis facilities in the South Bay Los Angeles area (see NIED Study website at www.NIEDStudy.org for more details). Inclusion criteria were outpatients who had been undergoing MHD for at least 8 weeks, who were 18 years of age or older, and who signed the Institutional Review Board–approved consent form. From October 1, 2001, through December 31, 2006, 893 MHD patients signed the informed consent form, and 792 of them underwent the periodic evaluations of the NIED Study. A modified version of the Charlson comorbidity index, i.e., without the age and kidney disease components, was used to assess the severity of comorbidities (23,24). The 792 MHD patients were followed for up to 63 months. A total of 118 of these patients (of approximately 200 randomly invited subjects) also agreed attend a substudy to undergo additional body compositions tests including DEXA at the Harbor-UCLA General Clinical Research Center. In each patient, body composition assessments were performed on the day after a routine hemodialysis treatment. All participants refrained from eating and drinking for at least 4 hours before the tests.
Anthropometric Measures
Body weight assessment and anthropometric measurements were performed while patients underwent hemodialysis treatment or within 5 to 20 minutes thereafter. Biceps and triceps skinfold thicknesses were measured with a conventional skinfold caliper using standard techniques (18,25). The mid-arm circumference was measured with a plastic tape. The average value of the triplicate measurements of the non–dialysis vascular access arm was obtained. MAMC was calculated as follows (26):
Dual Energy X-Ray Absorptiometry
As the reference test of body composition in the substudy of 118 subjects, DEXA was performed with a Hologic Series Delphi-A Fan Beam x-ray Bone Densitometer with software version 12.4 (Hologic, Bedford, MA), with participants wearing a hospital gown with no metal snaps and all artifacts removed (27–29). Participants were lying supine on the table, centered in the scan field with arms at their sides, palms down, and thighs separated. Scans were analyzed using the whole body fan beam method to determine LBM, fat mass, bone mineral content, and total body fluid as described elsewhere (28,29).
Near Infrared Interactance
To estimate the percentage of body fat and fat-free body mass at the start of the main cohort, near infrared (NIR) interactance technology was used at the time of anthropometric measurements. A commercial NIR interactance sensor with a coefficient of variation of 0.5% for total body fat measurements (portable 6100; Futrex, Gaithersburg, MD; www.futrex.com) was used. NIR measurements of body fat have been shown to be highly correlated with other body fat measures in MHD patients (20,29).
Laboratory Tests
Predialysis blood samples and postdialysis serum urea nitrogen were obtained on a mid-week day, which coincided chronologically with the drawing of quarterly blood tests in the DaVita clinics. The single-pool Kt/V was used to represent the weekly dialysis dose. Except as indicated below, all laboratory measurements were performed by DaVita Laboratories (Deland, FL) using automated methods. In this study, 3-month averaged values of routine laboratory measures were used.
Additionally, serum high sensitivity C-reactive protein (CRP) was measured by a turbidimetric immunoassay (21,22). IL-6 and TNF-α were measured with immunoassay (30,31). CRP and the cytokines were measured in the General Clinical Research Center Laboratories. Plasma total homocysteine concentrations were determined by HPLC in the Harbor-UCLA Clinical Laboratories. Serum transthyretin (prealbumin) was measured using immunoprecipitin analysis (32).
Statistical Analyses
Pearson's correlation coefficient (r) was used for analyses of linear associations. Multivariate regression analyses were performed to obtain adjusted P values controlled for case-mix and other covariates. A restricted cubic splines graph was used as an exploratory data analysis strategy to show systematic relations between MAMC and mortality and to examine the linearity assumptions (33). Thereafter, to calculate the relative risks of death, hazard ratios (HRs) were obtained using Cox proportional hazard models after controlling for relevant covariates. Kaplan-Meier analyses were used to assess the differences in surviving proportions between quartiles of MAMC.
We performed incremental levels of multivariate adjustment: (1) case-mix variables included age, gender, race/ethnicity, diabetes, dialysis vintage, insurance (Medicare versus others), marital status, modified Charlson comorbidity score, dialysis dose (Kt/V), and residual renal function; (2) malnutrition inflammation complex syndrome (MICS) variables included serum phosphorus, albumin, creatinine, bicarbonate, calcium, ferritin, blood hemoglobin, white blood cell count, and lymphocyte percent; prescribed erythropoietin; normalized protein nitrogen appearance; and BMI; and (3) additional adjustment for three inflammatory markers (CRP, IL-6, and TNFα). Fiducial limits are given as means ± SD or median and interquartile range, when appropriate; hazard ratios include 95% confidence interval levels. Descriptive and multivariate statistics were carried out with Stata statistical software version 10.0 (Stata Corporation, College Station, TX).
Results
LBM Measurement in the Validation Substudy
In the substudy, 118 participating MHD patients were 56.0 ± 12.4 (SE) years old and included 60% men, 41% African Americans, 36% Hispanics, and 51% diabetics. Postdialysis average dry weight was 74.1 ± 18.1 kg (minimum: 36.9 kg; maximum: 131.0 kg), and averaged Kt/V (single pool) was 1.70 ± 0.27. Calculated LBM and total body fat mass by DEXA were 49.9 ± 10.7 (minimum: 30.2 kg, maximum: 90.8 kg) and 21.8 ± 11.1 kg (minimum: 4.8 kg, maximum: 53.0 kg), respectively. Table 1 shows the correlation coefficients of DEXA-measured LBM with MAMC, TSF, and serum levels of creatinine, albumin, and prealbumin. After multivariate adjustments, MAMC (r = 0.54, P < 0.001) and serum creatinine (r = 0.36, P < 0.01) had the strongest correlations with DEXA-measured LBM. We also validated TSF against DEXA-measured total body fat mass. The correlation coefficients before and after adjustment for case-mix were 0.74 (P < 0.001) and 0.76 (P < 0.001), respectively.
Table 1.
Pearson's correlation coefficients between LBM, measured by DEXA, and its possible surrogates in 118 randomly selected MHD patients
| Variable | Model 1a | Model 2b | Model 3c | Model 4d |
|---|---|---|---|---|
| Mid-arm muscle circumference | 0.72e | 0.58e | 0.65e | 0.54e |
| Serum creatinine | 0.40e | 0.19f | 0.25g | 0.36g |
| Serum albumin | 0.24g | −0.08 | −0.08 | −0.10 |
| Serum prealbumin | 0.02 | 0.05 | −0.14 | −0.12 |
| Triceps skinfold | 0.02 | −0.03 | −0.11 | −0.12 |
Bold = statistically significant correlation coefficients >0.10.
Model 1 includes each surrogate separately as dependent variable and LBM as independent variable in the model without adjustment.
Model 2 includes each surrogate separately plus case-mix variables include age, gender, race/ethnicity, diabetes, dialysis vintage, insurance (medicare), marital status, modified Charlson comorbidity score, dialysis dose (Kt/V), and kidney residual urine in the model.
Model 3 includes all five surrogates in the model.
Model 4 includes all five surrogates plus case mix variables in the model.
P < 0.001.
P = 0.05 to 0.01.
P = 0.01 to 0.001.
Characteristics of the Main Cohort Subjects
Baseline demographic, clinical, and laboratory values in 792 participating MHD patients of the main cohort are shown in Table 2. Patient mean age was 53 ± 15 (SE) years; 47% were women (n = 372), 51% were Hispanic (n = 404), and 31% were African American (n = 245). The median (and interquartile range) of dialysis vintage was 19 (7 to 42) months. We categorized participants into population-based quartiles of MAMC as a surrogate of LBM, which resulted in 196 to 199 subjects in each quartile. Table 2 also shows the relevant demographic, clinical, and laboratory measures within each MAMC quartile at baseline. In the higher MAMC quartiles, the proportions of women and Hispanics were lower and African Americans were higher. There were no significant differences in the prevalence of diabetes mellitus and other comorbidities according to the modified Charlson comorbidity score. The groups of patients with higher MAMC had higher BMI, biceps and triceps skinfold thicknesses, and NIR-measured total body fat.
Table 2.
Baseline demographic, clinical, and laboratory values in 792 MHD patients at the start of the 5-year cohort study according to the quartiles of their mid-arm muscle circumference
| Variables | All Patients (n = 792) | Mid-Arm Muscle Circumference Quartiles |
||||
|---|---|---|---|---|---|---|
| Quartile 1 (n = 199) | Quartile 2 (n = 198) | Quartile 3 (n = 199) | Quartile 4 (n = 196) | P for Trenda | ||
| Demographic | ||||||
| Age, years | 53 ± 15 | 52 ± 15 | 55 ± 15 | 54 ± 15 | 52 ± 14 | 0.47 |
| Women, % | 47 | 66 | 46 | 33 | 42 | <0.01 |
| Race: % African-American | 31 | 22 | 27 | 28 | 46 | <0.01 |
| Ethnicity: % Hispanic | 51 | 61 | 56 | 55 | 34 | <0.01 |
| Primary insurance: Medicare% | 52 | 47 | 52 | 52 | 56 | 0.14 |
| Diabetes mellitus, % | 53 | 53 | 57 | 46 | 57 | 0.45 |
| Marital status: % married | 47 | 47 | 49 | 49 | 43 | 0.50 |
| Charlson comorbidity score | 1.87 ± 1.61 | 1.74 ± 1.65 | 1.91 ± 1.55 | 1.78 ± 1.60 | 2.02 ± 1.66 | 0.20 |
| Mortality, % | 28 | 33 | 28 | 25 | 28 | 0.06 |
| Body composition | ||||||
| Body mass index | 26.5 ± 6.1 | 22.4 ± 3.6 | 25.2 ± 4.5 | 26.3 ± 4.6 | 31.9 ± 7.0 | <0.01 |
| Triceps skinfold, mm | 17.5 ± 9.8 | 16.2 ± 8.0 | 17.5 ± 9.3 | 16.1 ± 10.2 | 20.1 ± 11.1 | 0.02 |
| Biceps skinfold, mm | 9.8 ± 7.7 | 7.9 ± 8.7 | 9.5 ± 6.8 | 9.0 ± 7.4 | 13.0 ± 7.1 | <0.01 |
| MAMC, cm | 25.8 ± 4.41 | 20.7 ± 2.1 | 24.2 ± 0.7 | 26.9 ± 0.9 | 31.6 ± 3.0 | <0.01 |
| NIR measured body fat, % | 26.5 ± 10.7 | 24.0 ± 9.0 | 26.0 ± 10.1 | 25.1 ± 10.8 | 31.1 ± 11.5 | <0.01 |
| Hemodialysis treatment | ||||||
| Dialysis vintage, monthsb | 19 (7 to 42) | 17 (6 to 44) | 18 (6 to 38) | 20 (9 to 44) | 22 (9 to 48) | 0.23 |
| Dialysis dose, Kt/V single pool | 1.61 ± 0.31 | 1.74 ± 0.33 | 1.66 ± 0.32 | 1.57 ± 0.25 | 1.49 ± 0.27 | <0.01 |
| nPNA, kg/day | 1.07 ± 0.24 | 1.11 ± 0.26 | 1.11 ± 0.24 | 1.04 ± 0.19 | 1.02 ± 0.23 | <0.01 |
| EPO dose, ×1000 U/wkb | 11.2 (6.4 to 18.2) | 11.4 (7.0 to 19.9) | 10.7 (6.7 to 16.7) | 10.3 (5.5 to 17.0) | 12.4 (6.2 to 20.5) | 0.91 |
| Biochemical measurements | ||||||
| Serum albumin, mg/dl | 3.9 ± 0.4 | 3.9 ± 0.4 | 3.9 ± 0.4 | 3.9 ± 0.3 | 3.9 ± 0.4 | 0.55 |
| Prealbumin, mg/dl | 28.4 ± 9.5 | 26.9 ± 9.4 | 28.4 ± 9.4 | 29.6 ± 9.6 | 29.0 ± 9.6 | 0.01 |
| creatinine, mg/dl | 10.2 ± 3.2 | 9.3 ± 2.8 | 10.0 ± 3.0 | 10.6 ± 3.4 | 10.9 ± 3.5 | <0.01 |
| Calcium, mg/dl | 9.3 ± 0.7 | 9.4 ± 0.7 | 9.3 ± 0.7 | 9.3 ± 0.6 | 9.4 ± 0.7 | 0.80 |
| iron, mg/dl | 67 ± 26 | 67 ± 28 | 69 ± 27 | 66 ± 24 | 64 ± 26 | 0.17 |
| Phosphorus, mg/dl | 5.8 ± 1.5 | 5.6 ± 1.4 | 5.7 ± 1.4 | 5.8 ± 1.5 | 6.0 ± 1.4 | <0.01 |
| Ferritin, ng/mlb | 481 (231 to 809) | 423 (115 to 618) | 453 (231 to 755) | 505 (254 to 834) | 548 (264 to 829) | <0.01 |
| Bicarbonate, mg/dl | 22.3 ± 2.8 | 22.3 ± 3.1 | 22.3 ± 2.9 | 22.3 ± 2.7 | 22.2 ± 2.7 | 0.93 |
| Homocysteine, μmol/L | 23 ± 10 | 22 ± 8 | 24 ± 11 | 23 ± 8 | 25 ± 12 | 0.02 |
| CRP, mg/lb | 3.7 (1.5 to 7.4) | 2.4 (1.2 to 5.4) | 3.6 (1.2 to 7.7) | 3.6 (1.6 to 7.6) | 4.9 (2.2 to 8.6) | <0.01 |
| IL-6, pg/mlb | 7.0 (4.0 to 13.3) | 6.8 (3.8 to 13.5) | 6.8 (3.8 to 15.8) | 6.2 (4.0 to 11.2) | 7.7 (4.3 to 13.2) | 0.91 |
| TNF-α,pg/mlb | 5.6 (3.8 to 8.8) | 5.8 (3.7 to 9.1) | 5.6 (3.7 to 9.0) | 5.7 (3.6 to 8.9) | 5.5 (4.1 to 8.6) | 0.88 |
| Blood hemoglobin, g/dl | 12.1 ± 0.9 | 12.1 ± 1.0 | 12.1 ± 1.0 | 12.1 ± 0.9 | 12.0 ± 0.9 | 0.52 |
| White blood count, ×1000 cells/μl | 7.2 ± 2.3 | 7.0 ± 2.1 | 7.2 ± 2.7 | 7.1 ± 2.1 | 7.5 ± 2.3 | 0.07 |
| Lymph., % of white blood count | 23 ± 8 | 23 ± 8 | 23 ± 7 | 24 ± 8 | 22 ± 8 | 0.41 |
All values are presented as mean ± SD or percentages except for variables that are not normally distributed (vintage, erythropoietin dose, ferritin, CRP, IL6, and TNF-α), in which we used interquartile range (IQR). Kt/V = dialysis dose.
P values for dialysis dose (vintage), ferritin, erythropoietin dose, CRP, IL-6, and TNF-α are based on the logarithmic values of these measures.
These values are medians with the 25th to 75th percentiles in parentheses.
Health-Related QoL
Table 3 shows the baseline short form 36 scores among the 792 MHD patients who answered this QoL questionnaire (34). The scores are grouped according to the selected MAMC quartiles presented in Table 2. Some trends toward better scores, in particular better reported mental health scale, in the highest MAMC groups were observed (P < 0.01).
Table 3.
Baseline SF36a health-related quality of life scoresb across quartiles of mid-arm muscle circumference in 792 MHD patients
| Variables | Mid-arm Muscle Circumference Quartiles |
||||
|---|---|---|---|---|---|
| Quartile 1 (n = 199) | Quartile 2 (n = 198) | Quartile 3 (n = 199) | Quartile 4 (n = 196) | P for Trend | |
| SF-36 mental health dimension | 50 ± 20 | 55 ± 22 | 54 ± 20 | 53 ± 20 | 0.17 |
| SF-36 physical health dimension | 44 ± 21 | 47 ± 23 | 47 ± 21 | 46 ± 22 | 0.54 |
| SF-36 scales | |||||
| Body pain | 56 ± 29 | 61 ± 29 | 60 ± 29 | 56 ± 28 | 0.99 |
| General health | 44 ± 24 | 47 ± 21 | 44 ± 21 | 44 ± 21 | 0.97 |
| Mental health | 62 ± 21 | 67 ± 21 | 67 ± 19 | 69 ± 20 | <0.01 |
| Physical function | 44 ± 28 | 46 ± 31 | 48 ± 29 | 47 ± 30 | 0.36 |
| Role emotional | 42 ± 43 | 52 ± 44 | 48 ± 43 | 47 ± 42 | 0.42 |
| Role physical | 31 ± 39 | 37 ± 41 | 36 ± 42 | 36 ± 42 | 0.19 |
| Functionality | 57 ± 27 | 62 ± 29 | 63 ± 28 | 63 ± 27 | 0.08 |
| Vitality | 48 ± 21 | 50 ± 24 | 49 ± 21 | 47 ± 22 | 0.52 |
Short form quality of life score with 36 questions.
Of all the 792 patients under study, data of quality of life were available for 690 patients.
Linear Associations
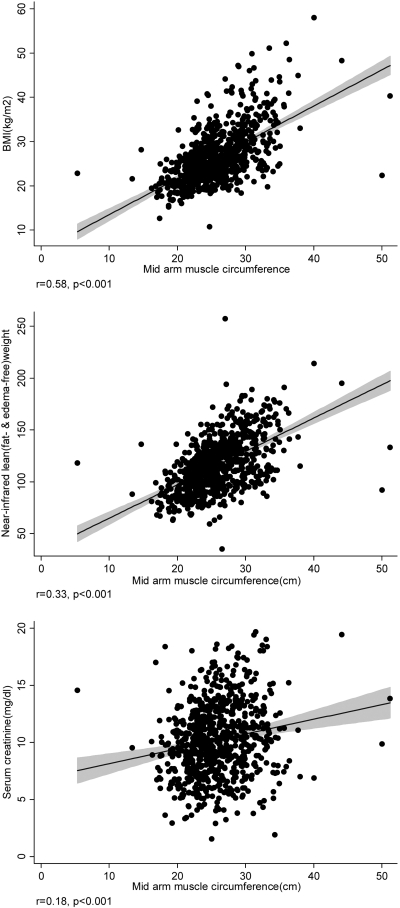
Examining the fully adjusted correlation coefficients of relevant clinical, nutritional, and inflammatory measures with MAMC at baseline in the 792 MHD patients, the MAMC was correlated with BMI (r = +0.58), triceps skinfold thicknesses (r = −0.27), NIR-measured lean body mass (r = +0.33), and serum creatinine concentration (r = +0.148; all P < 0.01). Figure 1 shows the unadjusted associations between MAMC and some relevant variables. The scatter plots exhibit widespread correlation especially for serum creatinine.
Figure 1.
Scatter plots, regression line, and 95% CI, reflecting the correlations between MAMC with BMI (top), NIR lean weight (middle), and serum creatinine concentration (bottom) in 792 MHD patients. Shaded areas reflect the 95% CIs.
Survival Predictability of Body Composition Surrogates
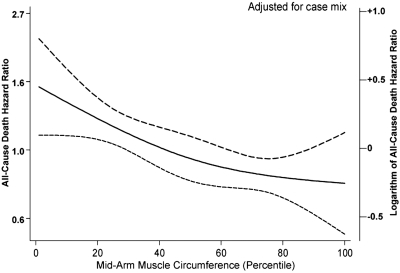
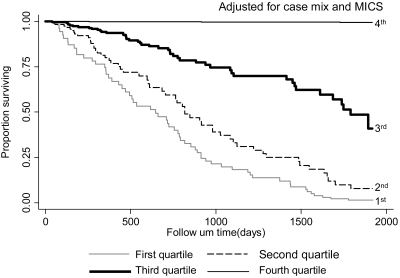
Over the 5 years of the cohort of 792 MHD patients, 222 (28%) patients died. Median and interquartile range for follow-up time were 730 and 927 days, respectively. Figure 2 shows the cubic splines graph illustrating the multivariate-adjusted association between baseline MAMC and mortality rate. Decreased risk of death was observed in MHD patients with higher MAMC (Cox model, P < 0.01). Figure 3 shows the Kaplan-Meier proportion of surviving patients across the four MAMC quartiles, indicating that the two lowest MAMC quartiles were incrementally associated with the highest mortality (P < 0.01). The death hazard ratios are shown in Table 4. The higher quartiles of MAMC tended to correlate with lower death hazard ratio in all adjusted models examined (P for trend < 0.05). A similar, albeit somewhat weaker, trend was also noticed for the survival predictability of serum creatinine, which is another surrogate of LBM (Table 4). As shown in Table 4, the highest quartile of TSF, a surrogate of fat mass, was associated with decreased death hazard ratio (95% confidence interval) in the case-mix–adjusted model (0.69; 0.45 to 1.07). This relationship was attenuated after additional multivariate adjustment for MICS and inflammatory markers.
Figure 2.
Spline model with 95% CIs reflecting adjusted mortality predictability of MAMC, expressed as a fraction of the average MAMC in the 792 MHD patients (from October 2001 to January 2007). Case-mix variables include age, gender, race/ethnicity, diabetes, dialysis vintage, insurance (Medicare), marital status, modified Charlson comorbidity score, dialysis dose (Kt/V), and kidney residual urine.
Figure 3.
Kaplan-Meier proportion of surviving MHD patients after 5 years of observation according to the quartiles of MAMC in 792 MHD patients (adjusted for case-mix and MICS). Case-mix variables: age, gender, race/ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, Charlson comorbidity score, dialysis dose (Kt/V), and kidney residual urine. MICS variables included serum phosphorus, albumin, creatinine, bicarbonate, calcium, ferritin, blood hemoglobin, white blood count, and lymphocyte percent; prescribed erythropoietin; normalized protein catabolic rate (nPCR), also known as normalized protein nitrogen appearance; and BMI.
Table 4.
Hazard ratios (HR) and 95% confidence interval (CI) of 5-year mortality according to quartiles of mid-arm muscle circumference, serum creatinine, and triceps skinfold in 792 MHD patients (October 2001 to January 2007)
| Variables | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for Trend |
|---|---|---|---|---|---|
| Mid-arm muscle circumference quartiles | |||||
| n | (n = 199) | (n = 198) | (n = 199) | (n = 196) | |
| Unadjusted | 1.00 | 0.86 (0.61, 1.23) [P = 0.42] | 0.68 (0.47, 0.99) [P = 0.04] | 0.79 (0.55, 1.14) [P = 0.21] | 0.10 |
| Case-mixa adjusted | 1.00 | 0.70 (0.49, 1.02) [P = 0.06] | 0.59 (0.40, 0.87) [P < 0.01] | 0.55 (0.37, 0.82) [P < 0.01] | <0.01 |
| Case-mix MICSb adjusted | 1.00 | 0.92 (0.62, 1.36) [P = 0.68] | 0.73 (0.47, 1.13) [P = 0.16] | 0.63 (0.37, 1.05) [P = 0.07] | 0.04 |
| Case-mix + MICS + inflammationc adjusted | 1.00 | 0.86 (0.58, 1.29) [P = 0.48] | 0.69 (0.45, 1.08) [P = 0.10] | 0.63 (0.38, 1.06) [P = 0.08] | 0.04 |
| Serum creatinine quartiles | |||||
| n | (n = 186) | (n = 182) | (n = 184) | (n = 184) | |
| Unadjusted | 1.00 | 1.13 (0.80, 1.59) [P = 0.46] | 0.71 (0.49, 1.02) [P = 0.07] | 0.36 (0.23, 0.56) [P < 0.01] | <0.01 |
| Case-mixa adjusted | 1.00 | 1.16 (0.82, 1.67) [P = 0.39] | 0.74 (0.49, 1.12) [P = 0.15] | 0.53 (0.31, 0.92) [P = 0.02] | <0.01 |
| Case-mix MICSb adjusted | 1.00 | 1.08 (0.73, 1.61) [P = 0.67] | 0.80 (0.51, 1.26) [P = 0.33] | 0.58 (0.32, 1.06) [P = 0.07] | 0.04 |
| Case-mix + MICS + inflammationc adjusted | 1.00 | 1.10 (0.74, 1.65) [P = 0.61] | 0.81 (0.51, 1.29) [P = 0.38] | 0.61 (0.33, 1.12) [P = 0.11] | 0.07 |
| Triceps skinfold quartiles | |||||
| n | (n = 211) | (n = 188) | (n = 195) | (n = 198) | |
| Unadjusted | 1.00 | 1.24 (0.86, 1.79) [P = 0.24] | 0.86 (0.59, 1.28) [P = 0.48] | 1.05 (0.73, 1.53) [P = 0.76] | 0.77 |
| Case-mixa adjusted | 1.00 | 0.91 (0.62, 1.35) [P = 0.66] | 0.64 (0.42, 0.97) [P = 0.03] | 0.69 (0.45, 1.07) [P = 0.10] | 0.03 |
| Case-mix + MICSb adjusted | 1.00 | 1.03 (0.66, 1.59) [P = 0.82] | 0.70 (0.43, 1.16) [P = 0.91] | 0.78 (0.44, 1.39) [P = 0.55] | 0.20 |
| Case-mix + MICS + inflammationc adjusted | 1.00 | 0.94 (0.60, 1.47) [P = 0.79] | 0.66 (0.39, 1.09) [P = 0.10] | 0.73 (0.41, 1.30) [P = 0.29] | 0.15 |
Case-mix variables include age, gender, race/ethnicity, diabetes, dialysis vintage, insurance (Medicare), marital status, modified Charlson comorbidity score, dialysis dose (Kt/V), and kidney residual urine.
MICS variables include albumin, log erythropoietin dose, creatinine, hemoglobin, serum phosphorus, normalized protein catabolic rate (nPCR), bicarbonate, calcium, log ferritin, white blood count, lymphocyte percent, and body mass index.
Inflammatory markers include C-reactive protein, IL-6, TNF-α.
The highest versus lowest MAMC quartile tended to correlate with the greatest survival across almost all demographic, clinical, and laboratory subgroups (data not shown). The BMI was the only variable with a relatively strong effect modification (P for interaction of BMI and MAMC was <0.001); the death hazard ratio (95% confidence interval) of the highest versus lowest MAMC in those with BMI <25 kg/m2 was 0.14 (0.04 to 0.71), whereas for BMI ≥25 kg/m2, it was not significant, i.e., 0.99 (0.70 to 1.26).
Survival Predictability of Combinations of MAMC and TSF
We dichotomized the MAMC and TSF using their median values (high versus low) and created four (2 × 2) combined groups. As shown in Table 5, using the concordant low MAMC/low TSF as the reference group, combined high MAMC with either high or low TSF exhibited the greatest survival, in that the death hazard ratios (and 95% confidence interval) for both high and combined high MAMC and low TSF were 0.52 (0.36 to 0.77) and 0.59 (0.39 to 0.88), respectively.
Table 5.
Hazard ratios and 95% confidence interval of 5-year mortality across four groups (2 × 2) of high versus low MAMC or TSF in 792 MHD patients (October 2001 to January 2007)
| Low MAMC and Low TSF | Low MAMC and High TSF | High MAMC and Low TSF | High MAMC and High TSF | |
|---|---|---|---|---|
| Number of subjects | 194 | 202 | 205 | 191 |
| Number of deaths | 70 | 51 | 46 | 55 |
| Crude mortality | 36% | 25% | 22% | 28% |
| Unadjusted death HR | 1 | 0.66 (0.46 to 0.95) | 0.60 (0.41 to 0.87) | 0.71 (0.49 to 1.00) |
| P value | n/a | 0.02 | <0.01 | 0.05 |
| case-mix adj. death HR | 1 | 0.61 (0.41 to 0.89) | 0.59 (0.39 to 0.88) | 0.52 (0.36 to 0.77) |
| P value | n/a | 0.01 | <0.001 | <0.001 |
Discussion
We examined the associations of DEXA-measured LBM with several anthropometric and biochemical measures in 118 MHD patients and found that MAMC and, to a lesser extent serum creatinine, correlated with LBM. The TSF correlated with DEXA-measured fat mass. We examined the larger cohort of 792 MHD patients and found that higher MAMC tended to be associated with better short form 36 measured mental health score at baseline. It was also associated with greater 5-year survival even after adjustment for demographics, comorbid conditions, and measures of nutritional status and inflammation including BMI and serum levels of albumin, prealbumin, CRP, and inflammatory cytokines. The association was particularly strong among low BMI (<25 kg/m2) patients. Serum creatinine concentration showed a similar, although somewhat less consistent, survival association, especially after adjustment for nutritional and inflammatory markers. Higher TSF, a surrogate of body fat, also exhibited a trend toward greater survival, but it was a weaker survival predictor than MAMC or serum creatinine concentration. Combined high MAMC with either high or low TSF (compared with both low) exhibited the greatest survival. These findings suggest that quality and quantity of body compositions might have a bearing on MHD patient survival.
In MHD patients, lower BMI is consistently a predictor of higher mortality, whereas higher BMI even in the ranges of obesity and morbid obesity seems to confer survival benefits (5–6,35–39). Kopple et al. (7) found similar seemingly counter-intuitive associations using body weight adjusted for height, as did several other studies (10,36,40–42). The only exceptions to this so-called obesity paradox (43) or reverse epidemiology (44) seem to be Asian-American MHD patients (45), in whom obesity does not consistently seem protective. Although BMI is most frequently used in nutritional assessment surveys as a surrogate of body size and nutritional status, it does not precisely reflect body composition, nor does it differentiate between muscle and fat mass (12). MAMC, which was a better surrogate of DEXA-measured LBM in our validation substudy, may reflect both muscle mass and caloric and protein adequacy (46). Hence, it can serve as a general index of appropriate nutritional status, whereas its reduction, known as sarcopenia (47), may be a sign of malnutrition or wasting (46).
We also found an association between higher TSF, a surrogate of body fat, and greater survival in MHD patients. In a previous 30-month longitudinal prospective study of 535 MHD patients, low total body fat percentage was an independent risk factor for poor survival, although the more obese patients had a higher prevalence of diabetes mellitus (20). More importantly, a decline in body fat over a 6-month period was associated with twice the death risk of those patients who had an increase in body fat (20). Thus, in contrast to the general population, fat mass seems to be protective in dialysis patients. However, it is not clear whether fat confers better survival benefits compared with muscle mass. In this study, we found that the association of high MAMC with greater survival is seen with both low and high TSF, as shown in Table 5. Hence, muscle mass may possibly be more important than peripheral body fat mass in predicting survival, consistent with the hypothesis by Beddhu et al. (11) that “if fat is good, muscle is better.”
A previous study indicated a stronger and more significant correlation between BMI and fat mass than between BMI and LBM in CKD patients at stages 3 to 5 (48). Hence, many previous studies assumed that the protective effect of BMI on mortality in MHD patients is related to fat mass and not LBM or muscle (20,48,49). We found a trend between higher peripheral fat mass, reflected by higher TSF and lower mortality, whereas we found a relatively consistent and graded association between higher muscle mass, reflected by MAMC and greater survival, as shown in Figure 2. In agreement with these findings, Marckmann (50) scored MHD patients according to relative body weight, serum transferrin, MAMC, and TSF and found that dialysis patients with a poor nutritional status had increased mortality. Beddhu et al. (11) found that the protective effect of BMI in the MHD population is conferred to those patients with elevated muscle mass, reflected by higher urinary creatinine before the initiation of dialysis therapy. Araujo et al. (51) found that higher MAMC is predictive of a lower death hazard ratio in MHD patients. Honda et al. (52) showed that protein energy wasting, even in overweight dialysis patients, was associated with higher mortality. Finally, Huang et al. (53) recently examined the Hemodialysis Study data and found that lower MAMC and low peripheral fat mass were associated with higher all-cause mortality in MHD patients. However, inflammatory cytokines were not measured and hence were not adjusted for in the Hemodialysis Study analyses, nor was the MAMC validated against DEXA. To our knowledge, our study is the only large cohort study with such comprehensive measures of nutritional status and inflammation indicating survival benefits of muscle mass, which is also associated with greater QoL.
There are several potential reasons why muscle mass may be associated with survival (53). First, lower muscle mass may reflect poor nutritional status (54). In our study, MHD patients with lower MAMC exhibited lower values of nutritional markers such as BMI, biceps and triceps skinfolds, total fat mass, and serum concentrations of prealbumin and creatinine (Table 1). Second, low muscle mass may reflect higher levels of or unopposed inflammation, because muscle may confer anti-inflammatory effects in MHD patients (52). However, in our study, low muscle mass was not correlated with lower inflammatory markers (Table 1), and the association of MAMC and mortality was largely independent of MICS including its inflammatory components, whereas the survival advantage of higher peripheral fat or TSF was mitigated after adjustment for MICS. Third, uremic toxins may distribute in the muscle mass compartment (53). Higher muscle mass is correlated with higher non–edema-related body water (55), which can dilute the circulating toxins and cytokines, so that patients with lower muscle mass may have a higher concentration of uremic toxins. Fourth, an increased muscle mass is associated with physical activity and exercise training (56). Exercise can improve arterial stiffness in MHD patients (57), and arterial stiffness is an independent predictor of cardiovascular disease and death in MHD patients (58,59). Higher muscle mass associated with exercise may improve insulin resistance, which is another independent predictor of mortality in dialysis patients (60).
Among the potential limitations of our study is the selection bias during enrollment, but without this bias our associations may have actually been even greater given the relatively low mortality rate of our cohort. Detailed information about hemodialysis treatment and technique such as dialysis membrane did not exist. Skinfold measurements in our study quantified peripheral rather than central fat tissue. Central or visceral fat may be a risk factor for cardiovascular disease and death in both the general population (61) and in CKD patients (62,63), and both human and animal data suggest that there may be fat redistribution in kidney disease (64,65). The methods used for calculation of fat versus muscle mass proportions (66) have not been validated in MHD patients. Finally, as in any observational study, we cannot account for unmeasured or residual confounding. There are several strengths to this study including the long follow-up period (up to 63 months), comprehensive laboratory tests, including inflammatory cytokines, concomitant assessments of body composition, and use of DEXA as the reference method to find the best surrogate of LBM, especially because anthropometric measurements may be more closely correlated with DEXA than bioelectrical impedance in dialysis patients (29,67–69). Finally, participants were selected randomly without having prior knowledge of their inflammatory status.
Conclusions
In MHD patients, higher MAMC is a surrogate of larger LBM and a potential predictor of better mental health scale of QoL and greater survival. The survival advantage of MAMC seems more pronounced in lower BMI ranges and may be independent of TSF, which is a surrogate of peripheral fat mass and therefore a predictor of better survival. Larger prospective studies with detailed body composition analyses are needed to verify our findings, and sarcopenia-improving intervention in CKD patients should be evaluated.
Disclosures
None.
Acknowledgments
This study was supported by National Institutes of Health Grants DK61162 and R21DK078012 to K.K.-Z., research grants from DaVita Clinical Research, a philanthropic grant from Mr. Harold Simmons, and General Clinical Research Center (GCRC) Grant M01-RR00425 from the National Centers for Research Resources, National Institutes of Health. We thank DaVita Wild West and Gold Coast dietitians for supporting the study and the staff at Harbor-UCLA GCRC Core Laboratories for the management of blood samples and measuring inflammatory markers.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Association of Body Composition with Survival Among Patients on Hemodialysis,” on pages 2144–2145.
References
- 1. Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9: S16–S23, 1998 [PubMed] [Google Scholar]
- 2. Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Noori N, Kalantar-Zadeh K, Kovesdy CP, Murali SB, Bross R, Nissenson AR, Kopple JD: Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis 5: 683–692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD: Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol 5: 683–692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leavey SF, Strawderman RL, Jones CA, Port FK, Held PJ: Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis 31: 997–1006, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK: Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int 55: 1560–1567, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Kopple JD, Zhu X, Lew NL, Lowrie EG: Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int 56: 1136–1148, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Wolfe RA, Ashby VB, Daugirdas JT, Agodoa LY, Jones CA, Port FK: Body size, dose of hemodialysis, and mortality. Am J Kidney Dis 35: 80–88, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Lowrie EG, Li Z, Ofsthun N, Lazarus JM: Body size, dialysis dose and death risk relationships among hemodialysis patients. Kidney Int 62: 1891–1897, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA: Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol 13: 1061–1066, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Beddhu S, Pappas LM, Ramkumar N, Samore M: Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 14: 2366–2372, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Sarkar SR, Kuhlmann MK, Kotanko P, Zhu F, Heymsfield SB, Wang J, Meisels IS, Gotch FA, Kaysen GA, Levin NW: Metabolic consequences of body size and body composition in hemodialysis patients. Kidney Int 70: 1832–1839, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA: Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism 58: 1013–1022, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB: Body mass index as a measure of adiposity among children and adolescents: A validation study. J Pediatr 132: 204–210, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Strain GW, Zumoff B: The relationship of weight-height indices of obesity to body fat content. J Am Coll Nutr 11: 715–718, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Kakiya R, Shoji T, Tsujimoto Y, Tatsumi N, Hatsuda S, Shinohara K, Kimoto E, Tahara H, Koyama H, Emoto M, Ishimura E, Miki T, Tabata T, Nishizawa Y: Body fat mass and lean mass as predictors of survival in hemodialysis patients. Kidney Int 70: 549–556, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Williams AJ, McArley A: Body composition, treatment time, and outcome in hemodialysis patients. J Renal Nutr 9: 157–162, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Kalantar-Zadeh K, Dunne E, Nixon K, Kahn K, Lee GH, Kleiner M, Luft FC: Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol Dial Transplant 14: 169–175, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, Block G, Kopple JD: Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr 83: 202–210, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR: Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 347: 1557–1565, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Erbagci AB, Tarakcioglu M, Aksoy M, Kocabas R, Nacak M, Aynacioglu AS, Sivrikoz C: Diagnostic value of CRP and Lp(a) in coronary heart disease. Acta Cardiol 57: 197–204, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, Nissenson A: Chronic peritoneal dialysis in the United States: Declining utilization despite improving outcomes. J Am Soc Nephrol 18: 2781–2788, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML: A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med 108: 609–613, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Nelson EE, Hong CD, Pesce AL, Peterson DW, Singh S, Pollak VE: Anthropometric norms for the dialysis population. Am J Kidney Dis 16: 32–37, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Weber J, Kelley J: Assessing nutrition. In: Health Assessment in Nursing, 3rd Ed., edited by Nieginski E. Philadelphia, Lippincott Williams & Wilkins, 2003, p 165 [Google Scholar]
- 27. Donadio C, Halim AB, Caprio F, Grassi G, Khedr B, Mazzantini M: Single- and multi-frequency bioelectrical impedance analyses to analyse body composition in maintenance haemodialysis patients: Comparison with dual-energy x-ray absorptiometry. Physiol Meas 29: S517–S524, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Park JC, Kovesdy CP, Duong U, Streja E, Rambod M, Nissenson AR, Sprague SM, Kalantar-Zadeh K: Association of serum alkaline phosphatase and bone mineral density in maintenance hemodialysis patients. Hemodial Int 14: 182–192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bross R, Chandramohan G, Kovesdy CP, Oreopoulos A, Noori N, Golden S, Benner D, Kopple JD, Kalantar-Zadeh K: Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis 55: 885–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pecoits-Filho R, Barany P, Lindholm B, Heimburger O, Stenvinkel P: Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 17: 1684–1688, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Beutler B, Cerami A: The biology of cachectin/TNF: A primary mediator of the host response. Annu Rev Immunol 7: 625–655, 1989 [DOI] [PubMed] [Google Scholar]
- 32. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Durrleman S, Simon R: Flexible regression models with cubic splines. Stat Med 8: 551–561, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol 12: 2797–2806, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Degoulet P, Legrain M, Reach I, Aime F, Devries C, Rojas P, Jacobs C: Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron 31: 103–110, 1982 [DOI] [PubMed] [Google Scholar]
- 36. Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW: Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 16: 2386–2394, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Wong JS, Port FK, Hulbert-Shearon TE, Carroll CE, Wolfe RA, Agodoa LY, Daugirdas JT: Survival advantage in Asian American end-stage renal disease patients. Kidney Int 55: 2515–2523, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, Cruess DF, Kimmel PL: Body mass index, dialysis modality, and survival: Analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int 65: 597–605, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Glanton CW, Hypolite IO, Hshieh PB, Agodoa LY, Yuan CM, Abbott KC: Factors associated with improved short term survival in obese end stage renal disease patients. Ann Epidemiol 13: 136–143, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Aoyagi T, Naka H, Miyaji K, Hayakawa K, Ishikawa H, Hata M: Body mass index for chronic hemodialysis patients: Stable hemodialysis and mortality. Int J Urol 8: S71–S75, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Kutner NG, Zhang R: Body mass index as a predictor of continued survival in older chronic dialysis patients. Int Urol Nephrol 32: 441–448, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Johnson DW, Herzig KA, Purdie DM, Chang W, Brown AM, Rigby RJ, Campbell SB, Nicol DL, Hawley CM: Is obesity a favorable prognostic factor in peritoneal dialysis patients? Perit Dial Int 20: 715–721, 2000 [PubMed] [Google Scholar]
- 43. Kalantar-Zadeh K, Kopple JD: Obesity paradox in patients on maintenance dialysis. Contrib Nephrol 151: 57–69, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Kalantar-Zadeh K: Causes and consequences of the reverse epidemiology of body mass index in dialysis patients. J Renal Nutr 15: 142–147, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S: Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 46: 489–500, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Gibney MJ, EM, Ljungqvist J: Clinical Nutrition: Anthropometric Assessment of Body Composition, 3rd Ed., Oxford, UK, Blackwell, 2005, p 20 [Google Scholar]
- 47. Cano NJ, Miolane-Debouit M, Leger J, Heng AE: Assessment of body protein: energy status in chronic kidney disease. Semin Nephrol 29: 59–66, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Leinig C, Pecoits-Filho R, Nascimento MM, Goncalves S, Riella MC, Martins C: Association between body mass index and body fat in chronic kidney disease stages 3 to 5, hemodialysis, and peritoneal dialysis patients. J Renal Nutr 18: 424–429, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Johansen KL, Young B, Kaysen GA, Chertow GM: Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr 80: 324–332, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Marckmann P: Nutritional status and mortality of patients in regular dialysis therapy. J Intern Med 226: 429–432, 1989 [DOI] [PubMed] [Google Scholar]
- 51. Araujo IC, Kamimura MA, Draibe SA, Canziani ME, Manfredi SR, Avesani CM, Sesso R, Cuppari L: Nutritional parameters and mortality in incident hemodialysis patients. J Renal Nutr 16: 27–35, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Honda H, Qureshi AR, Axelsson J, Heimburger O, Suliman ME, Barany P, Stenvinkel P, Lindholm B: Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr 86: 633–638, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Huang CX, Tighiouart H, Beddhu S, Cheung AK, Dwyer JT, Eknoyan G, Beck GJ, Levey AS, Sarnak MJ: Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int 77: 624–629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM: Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: A prospective study. Kidney Int 55: 1945–1951, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Gotch FA: Kt/V is the best dialysis dose parameter. Blood Purif 18: 276–285, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Horber FF, Kohler SA, Lippuner K, Jaeger P: Effect of regular physical training on age-associated alteration of body composition in men. Eur J Clin Invest 26: 279–285, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Mustata S, Chan C, Lai V, Miller JA: Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol 15: 2713–2718, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, Ishimura E, Tabata T, Nishizawa Y: Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 12: 2117–2124, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness on survival in end-stage renal disease. Circulation 99: 2434–2439, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Shinohara K, Shoji T, Emoto M, Tahara H, Koyama H, Ishimura E, Miki T, Tabata T, Nishizawa Y: Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol 13: 1894–1900, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Bays H, Blonde L, Rosenson R: Adiposopathy: How do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients? Expert Rev Cardiovasc Ther 4: 871–895, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Elsayed EF, Tighiouart H, Weiner DE, Griffith J, Salem D, Levey AS, Sarnak MJ: Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am J Kidney Dis 52: 49–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Postorino M, Marino C, Tripepi G, Zoccali C: Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol 53: 1265–1272, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Chen H, Liu Z, Li S, Chen Y, Yang B, Cai J, Wang Q, Li L: The relationship between body fat distribution and renal damage in Chinese with obesity. Exp Clin Endocrinol Diabetes 116: 99–103, 2008 [DOI] [PubMed] [Google Scholar]
- 65. Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE: A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41: 733–741, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Siri W: Body composition from fluid spaces and density: Analysis of methods. In: Techniques for Measuring Body Composition, edited by Brozek J, Henschel A. Washington, DC, National Research Council, 1961, pp 223–244 [Google Scholar]
- 67. Avesani CM, Draibe SA, Kamimura MA, Cendoroglo M, Pedrosa A, Castro ML, Cuppari L: Assessment of body composition by dual energy X-ray absorptiometry, skinfold thickness and creatinine kinetics in chronic kidney disease patients. Nephrol Dial Transplant 19: 2289–2295, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Kamimura MA, Avesani CM, Cendoroglo M, Canziani ME, Draibe SA, Cuppari L: Comparison of skinfold thicknesses and bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body fat in patients on long-term haemodialysis therapy. Nephrol Dial Transplant 18: 101–105, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Kamimura MA, Jose Dos Santos NS, Avesani CM, Fernandes Canziani ME, Draibe SA, Cuppari L: Comparison of three methods for the determination of body fat in patients on long-term hemodialysis therapy. J Am Diet Assoc 103: 195–199, 2003 [DOI] [PubMed] [Google Scholar]