Abstract
Background and objectives: The burden of HIV-associated chronic kidney disease (CKD) is growing in the United States, partially because of increased HIV-infection rates among African Americans. We determined the prevalence, incidence, and risk of rapid estimated GFR (eGFR) decline, ESRD, and death among HIV-infected (HIV+) African-American and non–African-American individuals cared for at the Comprehensive Care Center in Nashville, Tennessee, from January 1, 1998, through December 31, 2005.
Design, setting, participants, & measurements: Mixed effects, competing risks, and Poisson and Cox regression models were used to assess the risk of rapid eGFR decline (defined as ≥50% decrease in baseline eGFR), CKD5/ESRD, and death. The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate eGFR. Confounders were adjusted with a propensity score that related patient characteristics to the probability of being African American. Mixed effects models compared the rate of rapid eGFR decline for HIV-infected African Americans and non–African Americans.
Results: There were 2468 HIV-infected individuals in the study: 33% African American; 21% female. Among all patients, HIV-infected African Americans did not have a statistically significant increased risk for rapid eGFR decline compared with non–African Americans. However, African Americans had a significantly higher risk of ESRD and tended toward a higher risk of death.
Conclusions: HIV-infected African Americans did not have a statistically significant difference in the risk of eGFR decline when compared with HIV-infected non–African Americans. The findings in this study have potential public health significance.
African Americans make up only 13.5% of the United States population, but account for almost 50% of all new HIV infections (1). Furthermore, African-American men and women are 9 and 20 times more likely to die from the complications of HIV infection compared with Caucasian men and women, respectively (2,3). Despite the disparity in the risk of death from HIV infection among African Americans, the life span for HIV-infected persons has increased significantly since the 1980s (4). As a result, HIV-infected persons are living long enough to develop chronic diseases (4). For example, HIV-associated kidney disease is a leading cause of ESRD for African Americans, and HIV infection is increasingly recognized as an important risk factor for chronic kidney disease (CKD) within this population (5–10). The aim of the current study was to characterize the prevalence and incidence of rapid estimated GFR (eGFR) decline, CKD5/ESRD, and death among HIV-infected individuals receiving care in middle Tennessee (TN) during the highly active antiretroviral therapy (HAART) era. Death was evaluated as a competing risk for rapid decline in eGFR within this population. It was hypothesized that HIV-infected African Americans (AAs) would have more rapid decline in kidney function and higher risk of death compared with non-AAs. It was further hypothesized that the higher risk of death could potentially mask the risk of rapid eGFR decline in AAs because of competing risks.
Materials and Methods
Study Population and Study Follow-up
The Comprehensive Care Center (CCC) is a nonprofit clinic in Nashville, Tennessee, that has treated HIV-infected persons since 1994. A total of 3856 patients had at least one provider visit at the CCC January 1, 1998, through December 31, 2005. Date of entry into the cohort study was the first serum creatinine that was followed by a second serum creatinine measurement collected ≥3 months later, but <365 days from the first measurement within the study period. Requiring at least two serum creatinine measurements within the study period allowed for assessment of the chronicity of kidney disease. An individual was defined as lost to follow-up if they met one of the following criteria: (a) they had more than a 1-year gap between provider visits/serum laboratory measurements; (b) they had more than a 1-year gap between their last provider visit/serum laboratory measurement and the end of the study period; or (c) they had more than a 1-year gap between their last provider visit/serum laboratory measurement and the date of their death. Individuals were censored at death or loss to follow-up, whichever occurred first, during the study period.
Exclusion Criteria
Patients for whom race could not be identified, level of kidney function assessed by eGFR could not be calculated, or who did not have at least two serum creatinine measurements 90 to 365 days apart within the study period were excluded.
Data Sources
Data were obtained from the CCC clinical electronic medical record (EMR) from January 1, 1998, through December 31, 2005. Clinical data were used for all analyses and to calculate eGFR using the four-variable Modified Diet Renal Disease (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations (11,12). The CKD-EPI data are reported as the primary outcome in all analyses. All analyses used aggregate, de-identified data.
Clinical data were entered into the EMR at the time of the patient encounter, by automated data upload from reference laboratory results, or by clinic personnel. Antiretroviral therapy exposure was validated by systematic chart review. The Vanderbilt University Institutional Review Board approved this study, with waiver of informed consent.
Outcomes
The outcomes analyzed included incidence and hazards of rapid eGFR decline, CKD5/ESRD, and death. A rapid decline in eGFR was defined as ≥50% decrease in the baseline value. The occurrence of CKD5/ESRD was defined as having either a related International Classification of Diseases Ninth Revision (ICD-9) Code (see Supplemental data) or an eGFR <15 ml/min per 1.73 m2.
Covariates
The current study adjusted for sex, race, baseline age, level of kidney function, anemia, cardiovascular disease, absolute CD4+ lymphocyte count (CD4), HIV-1 RNA viral load, history of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, HAART, opportunistic infection, hypertension (HTN), chronic hepatitis C, diabetes mellitus, and HIV risk group: men-having-sex-with-men, heterosexual contact, intravenous drug use, other, or unknown. HTN, hepatitis C, anemia, diabetes mellitus, and cardiovascular disease diagnoses were based on ICD-9 codes (Supplemental Table 1). All analyses and covariates were chosen a priori based on clinical relevance.
Statistical Analyses
Descriptive statistics were expressed as frequencies and proportions for categorical variables and as means and SD, or median and interquartile ranges, for continuous variables depending on their distribution. Comparisons of the demographic, renal, and HIV parameters at baseline between AAs and non-AAs were performed by using the χ2 test for categorical variables and by using the Mann-Whitney U test for continuous variables. Incidence rates per 1000 patient-years were calculated and compared between AAs and non-AAs using Poisson regression (13). Rapid decrease in eGFR, incidence of ESRD, incidence of death, and a combination of rapid decline of eGFR or incidence of ESRD with death were analyzed using time-to-event analyses. The combined outcome, rapid decline in eGFR and incidence of death, was included in the analysis to account for individuals who experienced rapid decline in eGFR before death during the follow-up period. As a result, the occurrence of both rapid eGFR decline and incidence of death were accounted for as long as it occurred within the follow-up period.
Cox proportional hazard regression models were used to compute hazard ratios (HRs) of combined outcomes, rapid decrease in eGFR, CKD5/ESRD, and death from the time of enrollment. We further adjusted potential confounding covariates as listed above in the Cox proportional hazard regression models using a propensity score method (14). The propensity score of a subject is the probability of being African American given potential confounding covariates (Supplemental Table 1). Assumptions of proportional hazards for the final models were evaluated and met.
Competing risk analyses were performed to consider the different causes contributing to rapid eGFR decline, CKD5/ESRD, and death. To assess the predictive value of race for rapid eGFR decline, CKD5/ESRD, and death, we used the proportional subdistribution hazard model (15).
Mixed effects models were used to assess the change of eGFR over time for individuals. Restricted cubic splines with four knots were applied to best describe the nonlinear trend of eGFR over time for individuals (16). The model was adjusted for age, HTN, HAART, CD4, HIV-1 RNA viral load, opportunistic infection, and HIV risk (intravenous drug use) at baseline.
We analyzed the potential effect caused by subjects lost to follow-up. Baseline characteristics among individuals who were lost to follow-up were compared with individuals not lost to follow-up to assess the potential population differences between the two groups (Supplemental Table 2). All data analyses were performed with R-software version 2.7.2 (17). A significance level of 0.05 was used for statistical inferences.
Results
Baseline Individual Characteristics
A total of 2468 individuals qualified for the study. Table 1 depicts the baseline characteristics of the cohort by race category. Of the 2468 HIV-infected individuals, 820 (33%) were self-identified AAs and 1648 (67%) non-AAs. Racial categories were dichotomized in analyses: AAs versus other racial categories, designated non-AAs. Ninety-two percent of non-AAs were Caucasian. Median follow-up time for the study cohort was 2.1 years (range: 0.25, 7.9 years).
Table 1.
Baseline characteristics of HIV+ individuals cared for at the CCC, 1998 through 2005
| N | non-AA n (%) | AA n (%) | P | ||
|---|---|---|---|---|---|
| 2468 | 1648 (66.7%) | 820 (33.2%) | |||
| Gender | |||||
| men | 1944 | 1385 (84%) | 559 (68%) | <0.001g | |
| Median age (years)a | 2468 | 38 | 39 | 0.45h | |
| Median creatinine (mg/dl) | 2468 | 0.9 | 1.0 | <0.001h | |
| Median weight (kg) | 2468 | 76 | 75 | 0.77h | |
| Median BMI (kg/m2) | 2112 | 25 | 25 | 0.15h | |
| Median calculated eGFRb | 2468 | 101 | 107 | <0.001h | |
| eGFR ≥60 ml/min per 1.73 m2 | 1601 (97%) | 774 (94%) | <0.001g | ||
| eGFR <60 ml/min per 1.73 m2 | 47 (3%) | 46 (6%) | <0.001g | ||
| ESRD (ICD-9 Code)c | 2468 | Yes | 2 (0%) | 7 (1%) | 0.004g |
| HIV risk group: IDU | 2468 | Yes | 199 (12%) | 162 (20%) | <0.001g |
| Median absolute CD4 count (cells per mm3)d | 2387 | 350 | 304 | <0.001h | |
| Median HIV-1 RNA VL (copies per milliliter)e | 2330 | 5718 | 16844 | <0.001h | |
| Median serum albumin (g/dl) | 2464 | 4.4 | 4.1 | <0.001h | |
| HAART at baseline | 2468 | Yes | 761 (46%) | 235 (29%) | <0.001g |
| HAART use before baseline | 1472 | Yes | 160 (18%) | 73 (12%) | <0.004g |
| ACEI/ARB at baseline | 2468 | Yes | 78 (5%) | 47 (6%) | 0.29g |
| Comorbid conditionsf | |||||
| OI before baseline | 2468 | Yes | 209 (13%) | 89 (11%) | 0.19g |
| cardiovascular disease | 2468 | Yes | 77 (5%) | 47 (6%) | 0.26g |
| diabetes mellitus | 2468 | Yes | 109 (7%) | 72 (9%) | 0.052g |
| hypertension | 2468 | Yes | 369 (22%) | 278 (34%) | <0.001g |
| hepatitis C | 2468 | Yes | 145 (9%) | 124 (15%) | <0.001g |
| anemia | 2468 | Yes | 219 (13%) | 155 (19%) | <0.001g |
N is the number of nonmissing values. Percentage (%) values follow the frequencies of the events n for HIV+ AAs and HIV+ non-AAs. P is for the differences between AA and non-AA groups. OI/ADE, opportunistic infection/AIDS-defining event; IDU, intravenous venous use; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
The median age at the first valid creatinine measurement.
eGFR calculated using the CKD-EPI equation (10).
ESRD diagnoses at baseline defined by ICD-9 coding.
Absolute CD4 count reported as cells per mm3.
HIV-1 RNA reported as copies per milliliter.
Comorbid conditions at baseline defined by ICD-9 coding.
Pearson test used.
Wilcoxon test used.
Among the 2468 individuals in the study, 1116 (45%) were defined as lost to follow-up. Baseline characteristics of individuals lost to follow-up were compared with those included in the study (Supplemental Table 2). There were no statistically significant differences in the likelihood of follow-up based on race.
Long-Term Outcomes among the Entire Cohort
A composite outcome was defined as rapid eGFR decline or death. The incidence rate and hazard ratios are reported for all subgroup analyses in Table 2. There were a total of 126 deaths and 63 rapid eGFR decline events during our follow-up period for the entire cohort. Among those who died, seven developed rapid eGFR decline before death. Therefore, among the 2468 individuals in the entire cohort, 182 subjects experienced the composite event. The risk for the composite event was 60% higher for AAs than for non-AAs (adjusted HR 1.6, 95% CIs 1.1, 2.3). Decline in eGFR and death were analyzed separately to assess whether there was a statistically significant difference in risk for each outcome (Table 2). AAs did not have a significantly increased risk for rapid eGFR decline (adjusted HR 1.1, 95% CIs 0.6, 2.1) or death after adjusting for covariates (adjusted HR 1.5, 95% CIs 0.9, 2.5), although there was a trend for higher risk of death. Twenty-one CKD5/ESRD events occurred during the study. AAs were significantly more likely than non-AAs to progress to CKD5/ESRD (adjusted HR 4.5, 95% CIs 1.8, 11.4).
Table 2.
Univariate and multivariate analyses for decline in eGFR (eGFR based on CKD-EPI equation), ESRD, and death for HIV+ CCC individuals, 1998 through 2005
| Baseline Group (N) | Outcome | Events, n | Unadjusted Incidence Rate Ratio AA:non-AA (95% CIs)c | Adjusted HR AA:non-AA (95% CIs)c |
|---|---|---|---|---|
| Total populationa (2468) | eGFR decline and death | 182 | 1.9 (1.4, 2.5) | 1.6 (1.1, 2.3) |
| eGFR decline | 63 | 2.1 (1.3, 3.5) | 1.1 (0.6, 2.1) | |
| death | 126 | 1.7 (1.2, 2.4) | 1.5 (0.9, 2.5) | |
| CKD5/ESRDd | 21 | 4.9 (1.9, 12.2) | 4.5 (1.8, 11.4) | |
| Subgroup analysisa eGFR ≥60 ml/min per 1.73 m2 (2366) | eGFR decline and death | 155 | 1.6 (1.2, 2.2) | 1.2 (0.8, 1.9) |
| eGFR decline | 56 | 1.6 (0.9, 2.9) | 1.1 (0.5, 2.2) | |
| death | 115 | 1.6 (1.1, 2.3) | 1.4 (0.8, 2.3) | |
| Subgroup analysisb eGFR <60 ml/min per 1.73 m2 (102) | eGFR decline and death | 27 | 3.0 (1.4, 6.7) | 1.8 (0.5, 6.1)b |
| eGFR decline | 17 | 3.2 (1.2, 8.8) | 2.5 (0.9, 6.9)b | |
| death | 11 | 1.8 (0.5, 5.7) | 1.9 (0.6, 6.5)b |
Race categorized as HIV+ AA and HIV+ non-AA. HRs reported for AA referent to non-AA.
Total population and eGFR ≥60 ml/min per 1.73 m2 subgroups adjusted for the following baseline covariates: age, absolute CD4 count, HIV-1 RNA, baseline eGFR, race, gender, hypertension, anemia, HAART use, hepatitis C, cardiovascular disease, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, diabetes, intravenous drug use–HIV risk, and opportunistic infection/AIDS-defining event.
All other subgroup analyses based on univariate Cox model due to a small sample size and limited power.
95% CIs reported for the corresponding HR using Cox regression.
CKD5 defined as eGFR ≤15 to 29 ml/min per 1.73 m2; ESRD <15 ml/min per 1.73 m2 or by ICD-9 code.
Long-Term Outcomes among Persons with Baseline eGFR ≥60 ml/min per 1.73 m2
A total of 155 composite events occurred among the 2366 individuals with a baseline eGFR ≥60 ml/min per 1.73 m2. The adjusted risks for the composite event (adjusted HR 1.2, 95% CIs 0.8, 1.9) and rapid eGFR decline alone (adjusted HR 1.1, 95% CIs 0.5, 2.2) were not statistically different for AAs and non-AAs. AA race tended toward, but was not significantly associated with, an increased risk of death in the adjusted analyses (adjusted HR 1.4, 95% CIs 0.8, 2.3).
Long-Term Outcomes among Persons with Baseline eGFR <60 ml/min per 1.73 m2
Twenty-seven composite events occurred among the 102 individuals with an eGFR <60 ml/min per 1.73 m2. Seventeen individuals had a rapid decline in eGFR during the follow-up period and 11 deaths occurred within this subgroup analysis. Although AAs consistently had an overall higher risk of the composite event (adjusted HR 1.8, 95% CIs 0.5, 6.1; Table 2), rapid eGFR decline (adjusted HR 2.5, 95% CIs 0.9, 6.9; Table 2), and death (adjusted HR 1.9, 95% CIs 0.6, 6.5; Table 2), findings did not reach statistical significance, possibly because of the small sample size of the subgroup analysis.
Mixed Effects Model Analysis
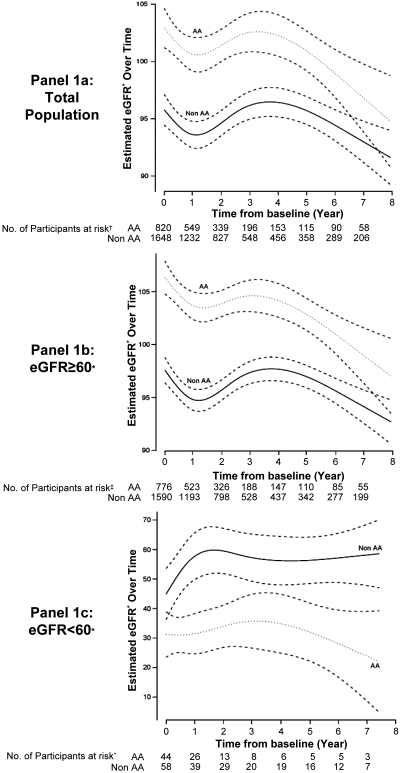
Mixed effects model analyses were performed to compare change in eGFR for AAs and non-AAs during the entire study period. Point estimates of eGFR were compared in the entire cohort, for individuals with a baseline eGFR ≥60 ml/min per 1.73 m2 and for individuals with a baseline eGFR <60 ml/min per 1.73 m2 (Figure 1, a through c). As shown in Figure 1a, AAs started with higher eGFR among the entire cohort. There was a slight but statistically significant convergence (P < 0.01) between the groups as time progressed. An almost identical trend was observed among individuals who started the study with eGFR ≥60 ml/min per 1.73 m2 (Figure 1b). Although the results suggest a more rapid decrease in eGFR for AAs with a baseline eGFR <60 ml/min per 1.73 m2 as shown in Figure 1c, this observation is limited by a significantly smaller sample size.
Figure 1.
Mixed effects model analysis. (a) Total population. (b) eGFR ≥60 subgroup. (c) eGFR <60. *All eGFR measurements expressed in ml/min per 1.73 m2. Models adjusted for the following baseline covariates: age, absolute CD4 count, HIV-1 RNA, baseline eGFR, race, gender, hypertension, anemia, HAART use, hepatitis C, opportunistic infection/AIDS-defining event, intravenous drug use–HIV risk, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, HAART use, and anemia.
Competing Risk Analysis
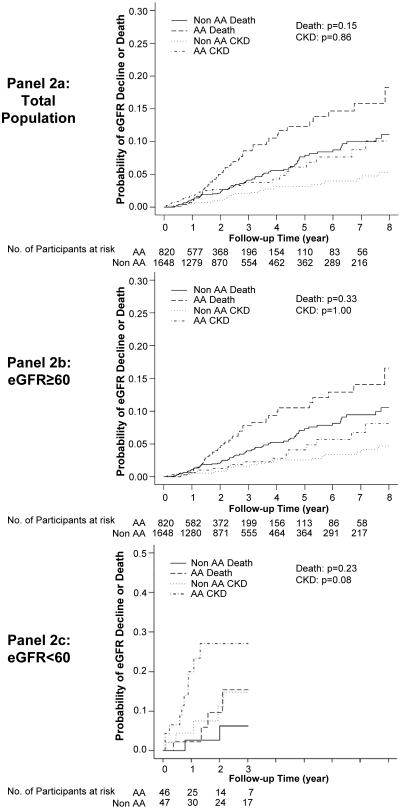
Competing risk models were performed to determine whether there was a differential risk of eGFR decline or death between AAs and non-AA individuals assuming that death prevents the observation of eGFR decline (Figure 2). Death was not a significant competing risk for rapid eGFR decline when comparing AAs and non-AAs in the entire cohort or in the subgroup analysis of individuals with a baseline eGFR ≥60 ml/min per 1.73 m2. This observation did not support the original hypothesis that an increased risk of death among HIV-infected AAs may potentially mask higher rates of rapid decline in eGFR.
Figure 2.
Competing risk analysis. (a) Total population. (b) eGFR ≥60 subgroup. (c) eGFR <60. *All eGFR measurements expressed in ml/min per 1.73 m2. Models adjusted for the following baseline covariates: age, absolute CD4 count, HIV-1 RNA, baseline eGFR, race, gender, hypertension, anemia, HAART use, hepatitis C, opportunistic infection/AIDS-defining event, intravenous drug use–HIV risk, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, HAART use, and anemia.
Discussion
The current study was undertaken to explore the effect of race on the prevalence, incidence, and risk of rapid eGFR decline and mortality in the setting of HIV infection. HIV-infected AAs and non-AAs with eGFR ≥60 ml/min per 1.73 m2 at baseline had a similar risk for rapid decline in eGFR. In contrast, HIV-infected AAs with baseline eGFR <60 ml/min per 1.73 m2 suggests a higher incidence of rapid eGFR decline and death compared with HIV-infected non-AAs, but these risks did not reach statistical significance. The lack of statistical significance may be partially explained by the small size within this subgroup analysis.
An important observation in this study, however, is the difference in baseline eGFR for HIV-infected AAs compared with HIV-infected non-AAs (Figure 1). HIV-infected AAs with baseline eGFR ≥60 ml/min per 1.73 m2 began with higher eGFR when compared with HIV-infected non-AAs. The opposite trend was observed among individuals with a baseline eGFR <60 ml/min per 1.73 m2. An accelerated decline in eGFR was observed for both ethnic groups with eGFR ≥60 ml/min per 1.73 m2 during the beginning of the study period. The reasons for the accelerated kidney disease progression are likely due to a combination of predisposing social, environmental, and genetic risk factors. In an earlier study of HIV-infected individuals cared for in an urban clinic, AAs were at a slightly but statistically significant increased risk for incident CKD (9). Once CKD had commenced, AAs developed ESRD markedly faster than did Caucasian individuals. The observation of an increased risk for ESRD among HIV-infected AAs is supported by our results, although our interpretation is limited by the small sample size of individuals and a high lost to follow-up rate.
Similar to prior studies, our data suggest that the racial differences in the rates of ESRD may be partially explained by a more aggressive natural disease history in AAs and the observation that AAs were less likely to receive HAART at baseline (9). It is possible that differential adherence to HAART may have also confounded our findings. As with most large retrospective cohort studies, we were unable to account for patient-level adherence, but this will be an important aspect of future analyses. Although this particular study did not aim to explore mechanisms, it is possible that certain genetic and environmental factors, such as the myosin heavy-chain 9 gene and socio-economic status, could also be contributing (18).
The risk of death among HIV-infected AAs in the post-HAART era warrants further exploration and has significant public health implications. For example, recent studies indicate that HIV/AIDS–associated mortality rates have decreased by >50% in some populations during the HAART era (19,20). A recent study of Third National Health and Nutrition Examination Survey (NHANES) participants reported racial differences in mortality among individuals stratified by the presence of CKD (21). In the subgroup of the 2892 patients who had CKD in this study, AAs had a significantly higher risk of death, which was modified by age and male sex. AAs younger than 65 years were 78% more likely to die than Caucasian individuals, whereas no significant differences in mortality were observed among individuals who were older than 65 years. Our findings, although not statistically significant, suggest a trend toward higher risk of death in AAs in a relatively young cohort of HIV-infected individuals. It remains unclear, however, what role race plays in the increased risk of death among AAs with CKD who have concurrent HIV infection.
Our study has several limitations. First, we cannot rule out the possibility of residual confounding by unmeasured factors in this observational study. The definitive cause of kidney disease and proteinuria were not available. Increased levels of proteinuria have been linked to cardiovascular disease, death, and progression of kidney disease (22). Controlling for proteinuria may explain some of the observed risk of eGFR decline and death. Second, a small subgroup sample size may have contributed to the lack of significance observed in our competing risk analysis. Finally, among the 2468 individuals, 1116 (45%) were defined as lost to follow-up based on available renal function data. Thus, we analyzed baseline characteristics of individuals based on lost to follow-up status and there was no significant difference in baseline characteristics for lost to follow-up between AAs and non-AAs.
In conclusion, our results demonstrated that there was an increased rate of ESRD and a marked decline in eGFR once HIV-infected AAs progressed to an eGFR <60 ml/min per 1.73 m2. To our knowledge, this is the first study that analyzes rapid eGFR decline as a competing risk for death in an HIV-infected population and further validates eGFR values using the CKD-EPI equation (11). The specific reasons for the differences observed in this study, which have been observed in the general non–HIV-infected CKD population as well, are not explained by adjustment of obvious HIV-associated risk factors. It is likely that the current observations are due to the interaction of multiple factors, including, but not limited to, possible genetic, social, and other clinical risk factors, such as lower baseline absolute CD4 count and HAART use among HIV-infected AAs, which require further study.
Disclosures
None.
Acknowledgments
This work was supported by the following research grants: 5 T32 DK007569-17 and K24 DK62849 from the National Institute of Diabetes, Digestive and Kidney Diseases (T.A.I.), National Kidney Foundation Research Fellowship Award (T.P.A.), National Center on Minority Health and Health Disparities/National Institutes of Health Loan Repayment Award (T.P.A.), Clinical Translational Science Award 1UL-1RR024975 (T.P.A.), Vanderbilt-Meharry Center for AIDS Research (National Institutes of Health P30 AI054999–T.R.S., S.E.S., and P.F.R.), K23 AT002508 from the National Center for Complementary and Alternative Medicine (T.H.), The Tennessee Valley VA Clinical Research Center of Excellence (T.H.), and the National Center for Research Resources and National Institutes of Allergy and Infectious Diseases K24 A1065298 (T.R.S. and S.E.S.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at http://www.cjasn.org/.
References
- 1. CDC: HIV/AIDS and African-Americans. Available at: http://www.cdc.gov/HIV/topics/AA/index.htm Accessed December 30, 2009
- 2. Satcher D, Rubens P: Multicultural Medicine and Health Disparities, New York, McGraw-Hill Medical Publishing, 2006 [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC): HIV/AIDS Among Women. Available at: www.cdc.gov/HIV/topics/women/resources/factsheets/women.htm Accessed July 20, 2010
- 4. Centers for Disease Control and Prevention (CDC): Trends in HIV/AIDS diagnoses- 33 states, 2001–2004. MMWR Morb Mortal Wkly Rep 54:1149–1153, 2005 [PubMed] [Google Scholar]
- 5. Winston JA, Burns GC, Klotman PE: The human immunodeficiency virus (HIV) epidemic and HIV-associated nephropathy. Semin Nephrol 18: 373–377, 1998 [PubMed] [Google Scholar]
- 6. Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, Grimm R, Liu J, Louis T, Manning W, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li Q, Li S, Qiu Y, Li S, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zhang R, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L: Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis 49[1 Suppl 1]: A6–A7, S1–S296, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Eggers PW, Kimmel PL: Is there an epidemic of HIV Infection in the US ESRD program? J Am Soc Nephrol 15: 2477–2485, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O'Hare AM: Racial differences in end-stage renal disease rates in HIV infection versus diabetes. J Am Soc Nephrol 18: 2968–2974, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD: Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: A tale of two races. J Infect Dis 197: 1548–1557, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 11. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. for the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI): A new equation to estimate the glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koch GG, Aktinson SS, Stokes ME: Poisson regression. In: Encyclopedia of Statisitcal Sciences 7th edition, New York, John Wiley and Sons, Inc., 1986, pp 32–41 [Google Scholar]
- 14. Agostino R: Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17: 2265–2281, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Fine JP, Gray JR: A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 16. Harrell FE: Regression Modeling Strategies, New York, Springer, 2001 [Google Scholar]
- 17. R Development Core Team: R: A language and environment for statistical computing, Vienna, Austria, R Foundation for Statistical Computing, 2009. Available at: http://www.R-project.org Accessed July 21, 2010 [Google Scholar]
- 18. Freedman BI, Hicks PJ, Bostrom MA, Cunningham ME, Liu Y, Divers J, Kopp JB, Winkler CA, Nelson GW, Langefeld CD, Bowden DW: Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int 75: 736–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz EJ, Szczech LA, Ross MJ, Klotman ME, Winston JA, Klotman PE: Highly active antiretroviral therapy and the epidemic of HIV+ end-stage renal disease. J Am Soc Nephrol 16: 2412–2420, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, Porter K: Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 300: 51–59, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Mehrotra R, Kermah D, Fried L, Adler S, Norris K: Racial differences in mortality among those with CKD. J Am Soc Nephrol 19: 1403–1410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taal MW, Brenner BM: Predicting initiation and progression of chronic kidney disease: Developing renal risk scores. Kidney Int 70: 1694–1705, 2006 [DOI] [PubMed] [Google Scholar]