Abstract
Background and objectives: Variation in kidney transplant access across the United States may motivate relocation of patients with ability to travel to better-supplied areas.
Design, setting, participants, & measurements: We examined national transplant registry and U.S. Census data for kidney transplant candidates listed in 1999 to 2009 with a reported residential zip code (n = 203,267). Cox's regression was used to assess associations of socioeconomic status (SES), distance from residence to transplant center, and relocation to a different donation service area (DSA) with transplant access and outcomes.
Results: Patients in the highest SES quartile had increased access to transplant compared with those with lowest SES, driven strongly by 76% higher likelihood of living donor transplantation (adjusted hazard ratio [aHR] 1.76, 95% confidence interval [CI] 1.70 to 1.83). Waitlist death was reduced in high compared with low SES candidates (aHR 0.86, 95% CI 0.84 to 0.89). High SES patients also experienced lower mortality after living and deceased donor transplant. Patients living farther from the transplant center had reduced access to deceased donor transplant and increased risk of post-transplant death. Inter-DSA travel was associated with a dramatic increase in deceased donor transplant access (HR 1.94, 95% CI 1.88 to 2.00) and was predicted by high SES, white race, and longer deceased-donor allograft waiting time in initial DSA.
Conclusions: Ongoing disparities exist in kidney transplantation access and outcomes on the basis of geography and SES despite near-universal insurance coverage under Medicare. Inter-DSA travel improves access and is more common among high SES candidates.
It has been nearly a decade since the Department of Health and Human Services issued the Final Rule regarding the operations of the Organ Procurement and Transplantation Network (OPTN), which directs the transplant community to reduce disparity in access to transplantation, to allocate organs over as wide of a geographic area possible, and to ensure that organs are allocated on the basis of medical necessity (1). Reflecting such directives, the kidney allocation algorithm has been adjusted to reduce the importance of HLA matching to improve access to transplantation for racial and ethnic minorities (2). However, with the exception of the recent revisions to the heart transplant allocation system (3), there have been no successful revisions to the current geographic boundaries of organ allocation.
Current deceased donor allocation policy is based on a system in which kidneys are initially offered to transplant centers in the local geographic area of recovery (donation service area [DSA]) before sharing within 1 of 11 geographic United Network for Organ Sharing (UNOS) regions, which each include ≥1 DSAs. As a result of substantial differences in the ratio of organs recovered to waiting candidates, there is dramatic variation in average waiting times across the UNOS regions, ranging from <2 years to nearly 7 years (4–7).
The role of socioeconomic status (SES) in determining access to transplantation services is complex because SES affects care throughout the transplant process (8,9). Patients with low SES often delay seeking medical care and lack access to specialty services, leading to delays in transplant referral, evaluation, and listing (10,11). Despite near-universal eligibility for Medicare coverage on the basis of ESRD provisions, insurance status continues to influence outcome and access to transplantation. For example, kidney transplant candidates with Medicare-only health insurance were recently shown to have a 78% lower likelihood of being pre-emptively listed for transplant compared with privately insured patients, thereby increasing waiting list morbidity and reducing post-transplant graft survival (12). Conversely, patients with college (odds ratio 1.20, P < 0.001) or postgraduate education (odd ratio 1.65, P < 0.001) were significantly more likely to be listed before dialysis.
The study presented here examined the associations of SES, distance from an individual's residence to the transplant center (quantified as travel time), and choosing to travel to a different DSA with kidney transplant access and outcomes in the United States. Specifically, we examined the differential effects of these sociodemographic factors among listed candidates and recipients of live and deceased donor organs. We sought to understand the potential contributions of SES, geographic differences in place of residence, and individual relocation behaviors to current disparities in transplant access and outcomes.
Materials and Methods
Data Source and Participant Selection
Data from OPTN/UNOS Standard Transplant Analytic Research files for patients listed for or transplanted with renal allografts in 1999 to 2009 were analyzed (13). Patients missing valid zip code of primary residence at listing were excluded from transplant access analyses, and patients without a valid residential zip code at transplant were excluded from post-transplant outcomes analyses. This study complied with all regulations regarding the Health Insurance Portability and Accountability Act and was approved by the Committee for the Projection of Human Subjects protections at Saint Louis University.
Exposure Measures: SES, Distance to Center, and Inter-DSA Travel
An index of neighborhood SES was computed based on census block-group data linked from the U.S. Census to reported zip code of patient residence. The SES index score was computed by the method of the Agency for Healthcare Research and Quality according to the formula 50 + (−0.07 × %crowded) + (0.08 × median property value) + (0.11 × median household income) + (−0.10 × %poverty) + (−0.11 × %education <12th grade) + (0.10 × %college) + (−0.08 × %unemployed), with possible values ranging from 0 to 100 (14). Higher SES index scores reflect higher SES levels. Patients were then categorized by quartiles of SES score.
Travel time from the candidate's residence to their transplant center at initial listing (and at transplantation if transplanted) was determined using distance from zip code to zip code according to an algorithm developed by Dr. David Goodman (15,16). Patients were categorized by quartile of travel time at initial listing, and at transplantation, respectively.
Inter-DSA “traveling” was defined as transfer of care to a center outside of a candidate's initial DSA after initial listing. Such transfer of care required initial listing at a center in one DSA, delisting, and then relisting at a center in a different DSA, as reported to the OPTN. This metric was chosen because waiting times by blood type are generally similar at centers within a given DSA because they share a local kidney allocation system. Travel to seek care outside of the initial DSA offers the candidate the opportunity to seek care at a center with different local wait time characteristics and to potentially improve access to deceased donor transplantation.
Covariates
Baseline patient demographic and clinical data were drawn from the OPTN Candidate and Transplant Registration forms. Demographic and clinical factors included age, race, ethnicity, blood type, peak panel reactive antibody level, education, insurance status, cause of end-stage renal failure, OPTN-reported comorbidities at listing, and year of listing and transplant events. Donor factors included age, race, ethnicity, cause of death, body mass index, type (living, standard criteria deceased, expanded criteria deceased, donation after cardiac death), number of HLA mismatches, cold ischemia time, and donor-recipient cytomegalovirus serostatus. Baseline information was considered at the time of listing or transplantation, respectively, as appropriate for each analysis. Median local waiting time was calculated by the Scientific Registry of Transplant Recipients from OPTN data for candidates waitlisted in each DSA for blood-type specific, non-multiorgan deceased donor transplants by listing year through 2006 (3). Geography was considered at the level of UNOS region or DSA.
Outcome Measures
Primary outcomes of interest included time from listing to (1) transplantation; (2) deceased-donor transplant, censored at living donor transplant; and (3) living donor transplant, censored at deceased donor transplant. Observation time for transplant access analyses was censored at death, removal from the list for illness or other cause, and end of study (November 2009). We also examined time from initial listing to death or waitlist removal for illness, censored at transplant or end of study. Associations of SES and distance to center with post-transplant patient survival and death-censored graft survival were examined among transplant recipients. Observation time for post-transplant outcomes was censored at end of study. Additional analyses considered cross-DSA travel as an outcome to examine clinical correlates of traveling for care. Observation time for traveling after initial listing was censored at death, waitlist removal because of illness, or end of study.
Statistical Analyses
All analyses were conducted using SAS version 9.2. (Cary, NC). Differences in donor and recipient characteristics as a function of SES and travel time were assessed by χ2 test for categorical variables. The cumulative incidence of transplant access and of traveling after listing, stratified by key baseline factors of interest, was estimated by the Kaplan–Meier method. Multivariable Cox regression models were constructed to estimate associations of SES and distance to center with each study outcome (adjusted hazards ratio [aHR] and 95% confidence interval [CI]), including adjustment for recipient, donor, and transplant factors. The proportionality assumption was confirmed by graphical methods. Adjusted associations of cross-DSA travel with transplant access after initial listing were modeled in time-dependent multivariable Cox regression, whereas pretransplant traveling status was considered as a baseline variable in models of post-transplant outcomes.
Results
There were 230,303 individuals listed for kidney transplantation and 134,594 transplant recipients recorded in the OPTN during the study period. Among these, 203,267 candidates and 114,547 transplant recipients had reported information on residential zip codes and were selected for analysis. Distributions of candidate and transplant recipient characteristics according to SES and distance from transplant center are shown in Table 1A, and Table 1B, respectively. Patients living farther from their transplant centers were more likely to be male, white race, and non-Hispanic. There were minimal differences in age, employment, and education according to distance from center. Larger demographic differences were noted across SES strata. Candidates in the highest neighborhood SES strata were more likely male, white race, non-Hispanic, older, college-educated, and privately insured and less likely to be obese. Donor demographic factors differed significantly as a function of recipient SES and distance to transplant center. Among recipients of deceased donor transplant, organs with a higher risk of graft failure including expanded criteria grafts (16.3 versus 13.5%, P < 0.001) and organs donated after cardiac death (7.4% versus 5.6%, P < 0.001) were more commonly used in patients living closest to compared with farthest from the transplant center. Consequently, there was a higher incidence of delayed graft function observed in transplants performed for patients living closest to their transplant centers.
Table 1A.
Kidney transplant candidate characteristics according to SES and distance-to-center categories
| Characteristic | Full Cohort (n = 203,267) | SES at Listing (%)a |
Distance to Center at Listing (%)a |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 (n = 57,573) | Quartile 2 (n = 47,154) | Quartile 3 (n = 48,514) | Quartile 4 (n = 50,026)b | Quartile 1 (n = 56,274) | Quartile 2 (n = 48,253) | Quartile 3 (n = 49,975) | Quartile 4 (n = 48,765) | ||
| Age at listing (years) | b | b | |||||||
| 0 to 18 | 3.3 | 3.7 | 3.4 | 3.1 | 3.2 | 2.8 | 3.4 | 3.5 | 3.7 |
| 19 to 30 | 8.2 | 9.0 | 8.7 | 7.9 | 6.9 | 8.4 | 8.2 | 7.8 | 8.3 |
| 30 to 45 | 22.9 | 24.7 | 23.8 | 22.4 | 20.5 | 23.6 | 23.0 | 22.4 | 22.5 |
| ≥46 | 65.6 | 62.7 | 64.1 | 66.6 | 69.4 | 65.2 | 65.5 | 66.3 | 65.5 |
| Gender | b | b | |||||||
| male | 59.6 | 57.4 | 59.1 | 60.1 | 61.9 | 58.4 | 60.2 | 60.7 | 59.1 |
| female | 40.5 | 42.6 | 40.9 | 39.9 | 38.1 | 41.7 | 39.8 | 39.3 | 40.9 |
| Race or ethnic group | b | b | |||||||
| white | 46.7 | 17.1 | 47.0 | 62.1 | 65.6 | 29.9 | 44.8 | 59.4 | 55.1 |
| black | 29.6 | 44.9 | 33.4 | 21.3 | 16.5 | 44.6 | 28.1 | 21.7 | 22.0 |
| Hispanic | 15.7 | 30.4 | 12.8 | 9.5 | 7.6 | 17.2 | 17.2 | 12.1 | 16.3 |
| other | 7.9 | 7.5 | 6.8 | 7.1 | 10.3 | 8.3 | 9.9 | 6.8 | 6.6 |
| Blood type | b | b | |||||||
| A | 33.4 | 29.0 | 33.7 | 35.8 | 35.8 | 30.5 | 33.0 | 35.1 | 35.4 |
| B | 14.6 | 15.8 | 14.1 | 13.8 | 14.3 | 16.7 | 15.2 | 13.3 | 12.8 |
| AB | 4.0 | 3.7 | 3.8 | 4.1 | 4.5 | 4.0 | 4.2 | 4.2 | 3.7 |
| O | 48.1 | 51.6 | 48.4 | 46.3 | 45.5 | 48.9 | 47.6 | 47.5 | 48.2 |
| Panel reactive antibodies | b | b | |||||||
| ≤10% | 70.7 | 68.1 | 71.5 | 71.8 | 71.9 | 69.9 | 71.4 | 72.1 | 69.6 |
| 11% to 30% | 7.0 | 7.2 | 7.2 | 7.0 | 6.6 | 7.4 | 7.1 | 6.6 | 6.9 |
| >30% | 12.8 | 13.1 | 13.6 | 12.9 | 11.4 | 13.6 | 11.9 | 12.7 | 12.7 |
| unreported | 9.6 | 11.6 | 7.7 | 8.3 | 10.1 | 9.1 | 9.7 | 8.7 | 10.8 |
| College degree | 35.6 | 25.8 | 32.2 | 37.1 | 48.6b | 33.8 | 39.5 | 36.3 | 33.1b |
| Employed | 8.9 | 5.3 | 7.7 | 10.1 | 12.8b | 7.8 | 10.0 | 9.9 | 7.8b |
| Body mass index (BMI) | b | b | |||||||
| nonobese (BMI < 25) | 72.4 | 76.6 | 71.4 | 69.1 | 71.7 | 73.8 | 73.3 | 71.2 | 71.0 |
| overweight (25 ≤ BMI < 30) | 14.9 | 12.6 | 15.0 | 16.3 | 16.0 | 14.1 | 14.8 | 15.4 | 15.2 |
| obese (BMI > 30) | 12.8 | 10.9 | 13.7 | 14.7 | 12.3 | 12.1 | 11.9 | 13.4 | 13.8 |
| Primary cause of ESRD | |||||||||
| diabetes | 11.5 | 10.9 | 11.9 | 12.3 | 11.1b | 11.1 | 11.0 | 11.8 | 12.1b |
| hypertension | 11.7 | 13.8 | 12.2 | 10.8 | 9.8b | 14.3 | 11.6 | 10.5 | 10.1b |
| GN | 11.2 | 8.4 | 11.1 | 12.5 | 13.1b | 9.9 | 11.4 | 11.8 | 11.8b |
| other | 65.6 | 66.9 | 64.8 | 64.4 | 66.0b | 64.7 | 66.0 | 65.9 | 66.0b |
| Comorbidities | |||||||||
| diabetes | 37.8 | 42.1 | 38.2 | 36.5 | 33.8b | 38.5 | 36.6 | 37.0 | 39.1b |
| hypertension | 52.9 | 53.9 | 54.1 | 53.0 | 50.5b | 54.6 | 51.1 | 51.6 | 54.0b |
| cerebrovascular disease | 2.0 | 2.0 | 2.1 | 2.1 | 1.9b | 2.1 | 1.9 | 2.0 | 2.2b |
| peripheral vascular disease | 4.7 | 4.8 | 4.7 | 5.0 | 4.3b | 4.6 | 4.4 | 4.6 | 5.2b |
| Region at listing | b | b | |||||||
| 1 | 3.9 | 3.5 | 2.8 | 3.6 | 5.6 | 5.4 | 4.0 | 4.9 | 1.1 |
| 2 | 13.1 | 10.9 | 11.5 | 13.5 | 16.9 | 17.9 | 13.8 | 13.6 | 6.3 |
| 3 | 12.2 | 11.1 | 17.5 | 12.3 | 8.6 | 7.6 | 9.4 | 12.1 | 20.5 |
| 4 | 9.2 | 13.4 | 9.5 | 6.7 | 6.4 | 7.8 | 10.7 | 6.6 | 11.9 |
| 5 | 20.7 | 26.4 | 17.7 | 16.0 | 21.4 | 15.4 | 25.1 | 21.5 | 21.5 |
| 6 | 2.4 | 0.8 | 2.7 | 3.5 | 2.9 | 2.1 | 2.3 | 2.2 | 3.0 |
| 7 | 9.3 | 7.6 | 6.0 | 12.2 | 11.6 | 9.9 | 8.3 | 9.6 | 9.3 |
| 8 | 4.1 | 2.0 | 4.4 | 6.4 | 4.2 | 3.9 | 3.7 | 3.6 | 5.6 |
| 9 | 7.8 | 12.0 | 5.2 | 5.7 | 7.7 | 13.4 | 9.7 | 5.8 | 1.6 |
| 10 | 8.3 | 5.8 | 8.0 | 11.0 | 8.8 | 9.6 | 6.8 | 9.2 | 7.2 |
| 11 | 9.0 | 6.5 | 14.9 | 9.3 | 6.0 | 7.0 | 6.2 | 11.0 | 12.0 |
| Year of listing | b | b | |||||||
| 1999 to 2001 | 23.0 | 23.7 | 23.7 | 22.8 | 21.7 | 24.1 | 21.9 | 22.3 | 23.6 |
| 2002 to 2003 | 16.9 | 17.3 | 17.1 | 16.7 | 16.3 | 17.0 | 16.4 | 16.2 | 17.8 |
| 2004 to 2005 | 18.9 | 18.8 | 18.8 | 18.8 | 18.8 | 18.8 | 18.8 | 18.8 | 18.8 |
| 2006 to 2007 | 21.3 | 20.7 | 20.7 | 20.7 | 20.7 | 20.5 | 20.5 | 20.5 | 20.5 |
| 2008 to 2009 | 19.9 | 39.5 | 39.5 | 39.5 | 39.5 | 39.3 | 39.3 | 39.3 | 39.3 |
| Insurance status | b | b | |||||||
| public | 32.5 | 42.9 | 33.8 | 29.2 | 22.5 | 37.4 | 30.6 | 28.3 | 33.0 |
| private | 46.6 | 32.9 | 42.7 | 51.1 | 61.7 | 40.6 | 51.8 | 52.3 | 42.5 |
| other | 20.9 | 24.2 | 23.5 | 19.8 | 15.8 | 22.1 | 17.6 | 19.4 | 24.5 |
Percentages reflect proportions within the given SES or distance-to-center category with the indicated demographic or clinical traits (“column percentages”). SES quartiles at listing were distributed as ≤41, 42 to 45, 46 to 49, and ≥50. Distance-to-center quartiles in terms of travel time to listing center (minutes) were distributed as ≤15, 16 to 33, 34 to 88, and ≥89.
P values for χ2 test of variation in the distribution of clinical traits according to SES or according to distance-to-center category: bP < 0.001.
Table 1B.
Characteristics of transplant recipients according to SES and distance-to-center category
| Characteristic | Full Cohort (n = 114,547) | SES at Listing (%)a |
Distance to Center at Listing (%)a |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 (n = 28,645) | Quartile 2 (n = 35,173) | Quartile 3 (n = 26,417) | Quartile 4 (n = 24,312) | Quartile 1 (n = 30,131) | Quartile 2 (n = 28,938) | Quartile 3 (n = 28,090) | Quartile 4 (n = 27,388) | ||
| Age at transplant (years) | b | b | |||||||
| 0 to 18 | 4.5 | 5.6 | 4.5 | 4.0 | 4.0 | 3.9 | 4.7 | 4.7 | 4.9 |
| 19 to 30 | 9.2 | 10.5 | 9.8 | 8.7 | 7.6 | 9.5 | 9.1 | 8.9 | 9.3 |
| 30 to 45 | 25.1 | 26.8 | 25.6 | 24.5 | 23.0 | 25.4 | 25.4 | 24.8 | 24.8 |
| ≥46 | 61.2 | 57.2 | 60.2 | 62.8 | 65.4 | 61.3 | 60.8 | 61.7 | 60.9 |
| Gender | b | b | |||||||
| male | 60.4 | 58.6 | 60.3 | 60.9 | 61.9 | 59.0 | 60.9 | 61.4 | 60.2 |
| female | 39.6 | 41.4 | 39.7 | 39.1 | 38.1 | 41.0 | 39.2 | 38.6 | 39.8 |
| Race or ethnic group | b | b | |||||||
| white | 53.3 | 20.1 | 56.3 | 68.9 | 71.3 | 33.9 | 51.5 | 65.8 | 63.8 |
| black | 26.2 | 44.6 | 27.3 | 16.7 | 13.4 | 41.9 | 24.1 | 19.1 | 18.6 |
| Hispanic | 13.4 | 28.0 | 10.6 | 7.7 | 6.5 | 16.3 | 15.4 | 9.9 | 11.7 |
| other | 7.0 | 7.3 | 5.8 | 6.8 | 8.8 | 7.9 | 8.9 | 5.3 | 5.8 |
| Blood type | b | b | |||||||
| A | 36.8 | 32.4 | 37.6 | 39.3 | 38.3 | 33.8 | 36.2 | 38.6 | 39.0 |
| B | 13.2 | 14.8 | 12.6 | 12.3 | 13.2 | 15.1 | 13.9 | 12.1 | 11.5 |
| AB | 4.8 | 4.6 | 4.6 | 4.7 | 5.2 | 4.9 | 5.0 | 4.6 | 4.5 |
| O | 45.2 | 48.2 | 45.2 | 43.7 | 43.3 | 46.2 | 44.8 | 44.7 | 45.0 |
| Panel reactive antibodies | b | b | |||||||
| ≤10% | 74.1 | 71.3 | 74.4 | 75.3 | 75.5 | 72.3 | 74.1 | 74.8 | 75.2 |
| 11% to 30% | 9.3 | 10.5 | 9.2 | 8.7 | 8.6 | 10.2 | 9.6 | 8.7 | 8.6 |
| >30% | 13.8 | 15.1 | 14.3 | 13.3 | 12.2 | 14.8 | 13.2 | 13.7 | 13.5 |
| unreported | 2.8 | 3.1 | 2.1 | 2.7 | 3.7 | 2.7 | 3.1 | 2.8 | 2.6 |
| College degree | 35.8 | 25.3 | 32.5 | 38.8 | 49.9 | 33.6 | 39.4 | 35.9 | 34.5b |
| Employed | 14.3 | 9.6 | 12.6 | 16.5 | 20.0b | 12.9 | 16.2 | 15.3 | 12.8b |
| BMI | b | b | |||||||
| nonobese (BMI < 25) | 47.8 | 48.8 | 46.8 | 46.2 | 49.7 | 47.9 | 49.4 | 46.8 | 47.0 |
| overweight (25 ≤ BMI < 30) | 28.2 | 27.5 | 27.9 | 28.6 | 29.1 | 28.2 | 28.3 | 28.4 | 27.9 |
| obese (BMI > 30) | 24.0 | 23.7 | 25.4 | 25.2 | 21.2 | 23.9 | 22.3 | 24.9 | 25.0 |
| Primary cause of ESRD | |||||||||
| diabetes | 23.0 | 23.7 | 23.8 | 23.2 | 20.9b | 22.7 | 22.3 | 22.9 | 24.2b |
| hypertension | 23.0 | 31.9 | 23.2 | 18.4 | 17.1b | 30.1 | 22.3 | 20.1 | 18.9b |
| GN | 22.1 | 19.7 | 22.1 | 22.9 | 23.9b | 20.6 | 22.5 | 22.5 | 22.7b |
| other | 32.0 | 24.7 | 30.9 | 35.5 | 38.1b | 26.6 | 32.9 | 34.5 | 34.2b |
| Duration of pretransplant dialysis (years) | b | b | |||||||
| 0 | 18.5 | 12.7 | 16.2 | 20.5 | 26.0 | 15.7 | 19.3 | 20.4 | 18.7 |
| 1 | 16.7 | 11.5 | 16.3 | 19.0 | 20.8 | 14.6 | 17.3 | 17.8 | 17.4 |
| 2 | 19.2 | 17.4 | 20.4 | 19.9 | 18.7 | 17.7 | 18.9 | 19.7 | 20.5 |
| 3 | 15.7 | 16.4 | 16.9 | 15.5 | 13.5 | 15.8 | 15.4 | 15.4 | 16.3 |
| 4 | 12.1 | 13.9 | 12.9 | 11.6 | 9.6 | 13.4 | 11.8 | 11.6 | 11.7 |
| 5 | 17.8 | 28.1 | 17.3 | 13.5 | 11.5 | 22.8 | 17.3 | 15.2 | 15.5 |
| Peritoneal dialysis modality | 12.3 | 10.9 | 13.4 | 12.7 | 11.6b | 10.4 | 11.6 | 12.8 | 14.4b |
| Recipient comorbidities | |||||||||
| diabetes | 28.9 | 30.3 | 29.8 | 28.8 | 26.2b | 29.6 | 27.8 | 28.6 | 29.7b |
| hypertension | 69.6 | 71.9 | 70.6 | 68.7 | 66.3b | 72.4 | 68.5 | 67.9 | 69.2b |
| cerebrovascular disease | 2.1 | 2.0 | 2.2 | 2.2 | 2.0 | 2.1 | 2.0 | 2.1 | 2.2 |
| peripheral vascular disease | 3.4 | 3.2 | 3.7 | 3.5 | 3.08b | 3.3 | 3.2 | 3.5 | 3.61b |
| Year of transplantation | b | b | |||||||
| 1999 to 2001 | 25.3 | 25.8 | 25.8 | 25.8 | 25.8 | 26.1 | 26.1 | 26.1 | 26.1 |
| 2002 to 2003 | 19.0 | 19.5 | 19.5 | 19.5 | 19.5 | 19.0 | 19.0 | 19.0 | 19.0 |
| 2004 to 2005 | 21.4 | 21.2 | 21.2 | 21.2 | 21.2 | 20.9 | 20.9 | 20.9 | 20.9 |
| 2006 to 2007 | 22.3 | 21.8 | 21.8 | 21.8 | 21.8 | 22.1 | 22.1 | 22.1 | 22.1 |
| 2008 to 2009 | 12.1 | 11.8 | 11.8 | 11.8 | 11.8 | 12.0 | 12.0 | 12.0 | 12.0 |
| Donor type | b | b | |||||||
| living | 31.1 | 22.9 | 28.7 | 33.4 | 41.5 | 25.8 | 33.2 | 33.4 | 32.1 |
| standard criteria deceased | 54.0 | 60.7 | 56.6 | 51.9 | 44.5 | 56.6 | 52.0 | 52.2 | 54.9 |
| expanded criteria deceased | 10.5 | 11.8 | 10.3 | 10.1 | 9.9 | 12.1 | 10.7 | 10.1 | 9.2 |
| donated after cardiac death | 4.5 | 4.6 | 4.4 | 4.5 | 4.2 | 5.5 | 4.1 | 4.3 | 3.8 |
| Donor age (years) | b | b | |||||||
| 0 to 18 | 11.4 | 12.7 | 12.3 | 10.9 | 9.1 | 11.6 | 10.8 | 11.0 | 12.1 |
| 19 to 30 | 21.2 | 23.1 | 21.9 | 20.4 | 19.0 | 21.1 | 21.5 | 20.7 | 21.7 |
| 30 to 45 | 31.2 | 29.8 | 31.2 | 31.7 | 32.3 | 30.5 | 31.5 | 31.3 | 31.5 |
| Donor race or ethnic group | b | b | |||||||
| white | 36.2 | 56.4 | 72.1 | 76.4 | 75.0 | 63.3 | 66.3 | 74.6 | 75.5 |
| black | 69.8 | 17.6 | 13.3 | 10.2 | 10.4 | 17.2 | 13.2 | 11.0 | 10.4 |
| Hispanic | 13.0 | 21.8 | 11.2 | 9.6 | 9.9 | 15.0 | 15.5 | 11.1 | 10.9 |
| other | 13.2 | 4.2 | 3.4 | 3.8 | 4.7 | 4.4 | 5.0 | 3.3 | 3.2 |
| Donor gender | b | b | |||||||
| male | 4.0 | 56.1 | 53.7 | 53.0 | 52.0 | 54.9 | 53.8 | 52.9 | 53.4 |
| female | 53.8 | 43.9 | 46.3 | 47.0 | 48.0 | 45.1 | 46.2 | 47.1 | 46.7 |
| HLA mismatches | b | b | |||||||
| 0 | 46.2 | 10.0 | 12.1 | 13.2 | 12.9 | 9.8 | 11.9 | 13.2 | 13.3 |
| 1 | 12.0 | 2.5 | 3.0 | 3.2 | 3.0 | 2.5 | 2.9 | 3.1 | 3.2 |
| 2 | 2.9 | 7.8 | 9.4 | 9.6 | 10.1 | 8.1 | 9.2 | 9.7 | 9.8 |
| 3 | 9.2 | 17.2 | 19.1 | 19.3 | 19.5 | 17.6 | 19.1 | 19.1 | 19.3 |
| 4 | 18.8 | 22.9 | 21.5 | 20.7 | 20.2 | 22.5 | 21.2 | 20.8 | 21.0 |
| 5 | 21.4 | 26.1 | 23.3 | 22.7 | 22.6 | 25.9 | 23.5 | 22.7 | 22.6 |
| 6 | 23.7 | 13.4 | 11.7 | 11.3 | 11.9 | 13.7 | 12.3 | 11.3 | 10.8 |
| Duration of cold ischemia (hours) | b | b | |||||||
| 0 to 12 | 12.1 | 33.3 | 39.2 | 42.7 | 49.0 | 37.6 | 43.2 | 42.1 | 39.3 |
| 13 to 24 | 40.5 | 43.5 | 41.4 | 39.8 | 34.3 | 42.4 | 38.0 | 39.4 | 40.7 |
| 25 to 36 | 40.1 | 19.4 | 16.4 | 14.7 | 13.8 | 16.6 | 15.5 | 15.7 | 17.3 |
| >36 | 16.2 | 3.8 | 3.0 | 2.8 | 2.9 | 3.5 | 3.3 | 2.9 | 2.8 |
| Donor-recipient cytomegalovirus seropairing | b | b | |||||||
| D−/R− | 3.1 | 8.3 | 13.3 | 16.6 | 18.5 | 11.2 | 13.9 | 16.7 | 14.1 |
| D+/R− | 13.9 | 13.2 | 16.2 | 18.3 | 18.3 | 14.5 | 16.7 | 17.8 | 16.6 |
| D−/R+ | 16.4 | 21.0 | 21.0 | 19.8 | 17.9 | 21.2 | 18.8 | 19.2 | 20.9 |
| D+/R+ | 20.1 | 44.3 | 36.4 | 32.6 | 30.6 | 40.1 | 36.3 | 33.0 | 35.4 |
| unreported | 36.3 | 13.2 | 13.1 | 12.8 | 14.7 | 13.0 | 14.3 | 13.4 | 13.0 |
| Delayed graft function | 17.9 | 22.1 | 18.1 | 16.2 | 14.4 | 20.7 | 17.9 | 16.0 | 16.6 |
| Region at transplant | b | b | |||||||
| 1 | 4.2 | 3.8 | 3.2 | 4.2 | 6.3 | 5.7 | 4.5 | 5.1 | 1.5 |
| 2 | 14.7 | 12.3 | 13.2 | 14.6 | 19.6 | 18.1 | 15.5 | 15.4 | 9.2 |
| 3 | 12.4 | 12.1 | 15.8 | 12.1 | 8.0 | 8.0 | 10.3 | 12.6 | 19.0 |
| 4 | 8.8 | 12.8 | 9.0 | 6.7 | 6.1 | 7.3 | 10.4 | 7.0 | 10.5 |
| 5 | 16.5 | 22.6 | 13.3 | 13.5 | 17.0 | 13.9 | 21.3 | 15.2 | 15.5 |
| 6 | 2.8 | 1.1 | 3.0 | 4.5 | 2.8 | 2.5 | 2.5 | 2.8 | 3.5 |
| 7 | 10.6 | 8.3 | 8.2 | 13.9 | 13.3 | 10.5 | 8.5 | 10.4 | 13.2 |
| 8 | 4.9 | 2.4 | 6.1 | 6.4 | 4.5 | 4.6 | 4.2 | 4.2 | 6.7 |
| 9 | 7.0 | 10.7 | 4.8 | 5.0 | 8.0 | 11.6 | 8.5 | 5.9 | 1.6 |
| 10 | 8.9 | 6.6 | 8.9 | 11.5 | 9.0 | 10.3 | 7.4 | 10.5 | 7.4 |
| 11 | 9.3 | 7.4 | 14.6 | 7.7 | 5.5 | 7.4 | 7.0 | 11.0 | 11.9 |
| Insurance status | b | b | |||||||
| public | 34.0 | 43.4 | 35.0 | 30.4 | 25.5 | 38.7 | 32.5 | 31.3 | 33.2 |
| private | 40.2 | 25.9 | 37.0 | 45.9 | 55.6 | 33.9 | 44.8 | 44.4 | 38.1 |
| other | 25.8 | 30.7 | 28.0 | 23.7 | 19.0 | 27.4 | 22.7 | 24.3 | 28.7 |
| Traveled before transplant | 5.5 | 3.9 | 5.2 | 5.9 | 7.2 | 2.5 | 3.3 | 5.8 | 10.6 |
Percentages reflect proportions within the given SES or distance-to-center category with the indicated pretransplant demographic or clinical traits (“column percentages”). SES quartiles at transplant were distributed as ≤41, 42 to 46, 47 to 50, and ≥51. Distance to center quartiles in terms of travel time to transplant center (minutes) were distributed as ≤15, 16 to 36, 37 to 94, and ≥95.
P values for χ2 test of variation in the distribution of baseline clinical traits according to SES or according to distance-to-center category: bP = 0.0002 to 0.001.
Effect of SES on Access to Transplantation
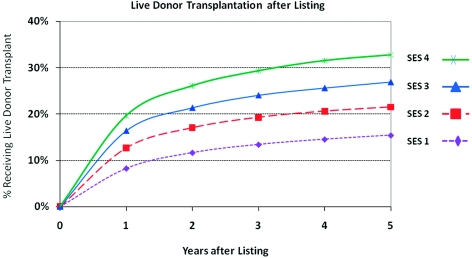
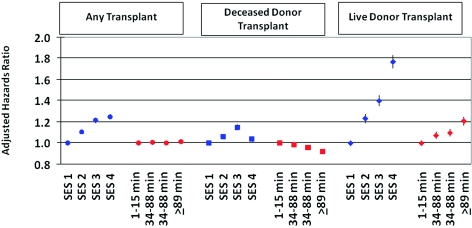
The influence of candidate SES on access to renal transplantation was profound and was driven in particular by live donor transplantation. By 3 years after listing, the cumulative incidence of live donor transplant was 29.4% among the highest SES patients versus 13.5% among those in the lowest SES strata (Figure 1). This association persisted with covariate adjustment, including recipient race, such that high SES candidates had 75% higher likelihood of live donor transplant than those with lowest SES (aHR 1.76, 95% CI 1.70 to 1.83) (Figure 2). In contrast, SES bore a modest, nonlinear association with deceased donor transplantation, being highest among patients in the third quartile of SES (aHR compared with lowest SES strata: 1.15, 95% CI 1.12 to 1.17). SES was also strongly associated with lower transplant-censored waitlist mortality. Compared with the lowest SES group, waitlisted candidates with the highest SES experienced a 14% reduction in the risk of death or removal for illness (aHR 0.86, 95% CI 0.84 to 0.89). Associations were similar in analyses stratified by initial listing DSA to adjust for the effect of the local organ supply.
Figure 1.
Cumulative incidence of live donor transplant access according to SES quartile at initial listing censored at deceased donor transplantation. SES quartiles at transplant were distributed as ≤41, 42 to 45, 46 to 49, and ≥50.
Figure 2.
Adjusted associations of prelisting SES and distance to center with the likelihood of transplant access by multivariable Cox regression. Time to deceased donor transplant was censored at receipt of a live donor allograft. Time to live donor transplant was censored at receipt of a deceased donor allograft. Regression models were adjusted for baseline demographic, clinical, and geographic factors at initial listing, as displayed in Table 1A. Results were similar after stratification by DSA of initial listing.
Effect of Distance to Center on Transplant Access
Overall kidney transplant access was generally similar across distance strata (Figure 2). However, after adjustment for baseline factors including differences in SES, waitlisted candidates living farthest from transplant centers were less likely to receive a deceased donor transplant (aHR 0.92, 95% CI 0.90 to 0.94) compared with those in the closest distance category. Conversely, access to living donor transplantation showed a graded increase with farther distance from transplant center. Candidates living in the farthest distance quartile were 20% more likely to receive a living donor transplant than patients in the closest quartile (aHR 1.20, 95% CI 1.16 to 1.24).
Improving Access via Inter-DSA Travel
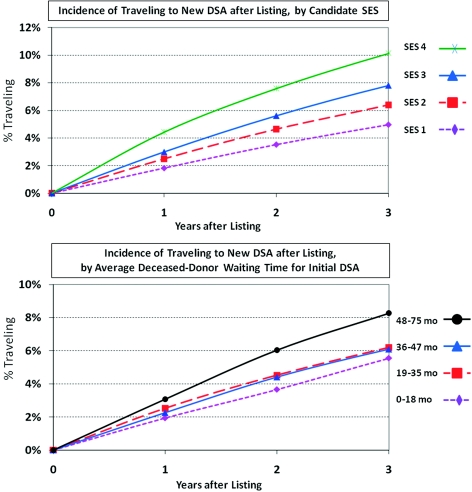
Analyses of correlates of inter-DSA traveling were performed among those listed in 1999 to 2006, for whom average DSA-specific deceased donor waiting times were available (n = 140,918). Inter-DSA traveling after listing increased in a graded manner with SES such that 10.2% of the highest SES candidates traveled by 3 years after initial listing compared with 5.0% of those in the lowest SES group (Figure 3). Traveling was also substantially more common in those with the longest versus shortest expected deceased donor waiting times (8.3% versus 5.5% by 3 years, Figure 3). Independent predictors of traveling included far distance to the initial center, high SES, white race, sensitization, long local expected deceased donor waiting time, and region of listing (Table 2). Traveling was more common in recent years of the study, rising approximately 44% among patients listed in 2006 as compared to those listed in 1999 (aHR 1.44, 95% CI 1.31 to 1.58).
Figure 3.
Incidence of inter-DSA travel after initial listing according SES quartile and according to median DSA-specific waiting time. Inter-DSA traveling was defined as transfer of care to a center outside of a candidate's initial DSA after initial listing, censored at death, waitlist removal because of illness, and end of study. Median waiting blood-type-specific waiting time for deceased donor transplant was defined for initial DSA at year of listing.
Table 2.
Clinical correlates of inter-DSA traveling by multivariable Cox regression
| Baseline Trait at Listing | Traveler % (n = 7278) | Nontraveler % (n = 133,640) | aHR for Traveling (95% CI) |
|---|---|---|---|
| Distance to center (travel time in minutes) | b | ||
| ≤15 | 19.9 | 28.3 | Reference |
| 16 to 33 | 21.1 | 23.4 | 1.10 (1.02 to 1.19)a |
| 34 to 88 | 25.2 | 24.3 | 1.37 (1.27 to 1.47)b |
| >89 | 33.7 | 24.1 | 2.36 (2.19 to 2.53)b |
| SES quartile | b | ||
| 1 (≤41) | 22.7 | 28.9 | Reference |
| 2 (42 to 45) | 21.3 | 23.5 | 1.19 (1.11 to 1.28)b |
| 3 (46 to 49) | 23.9 | 23.8 | 1.43 (1.33 to 1.54)b |
| 4 (≥50) | 32.1 | 23.7 | 1.97 (1.83 to 2.11)b |
| Age at listing (years) | b | ||
| 0 to 18 | 1.4 | 3.6 | 0.49 (0.40 to 0.61)b |
| 19 to 30 | 8.7 | 8.5 | 1.00 (0.91 to 1.10) |
| 30 to 45 | 25.8 | 23.6 | Reference |
| ≥46 | 64.1 | 64.3 | 0.92 (0.87 to 0.97)a |
| Gender | |||
| male | 60.3 | 59.3 | Reference |
| female | 39.7 | 40.7 | 0.89 (0.85 to 0.94)b |
| Race or ethnic group (%) | b | ||
| white | 50.2 | 47.6 | Reference |
| black | 28.2 | 29.4 | 0.82 (0.77 to 0.87)b |
| Hispanic | 12.0 | 15.3 | 0.65 (0.60 to 0.71)b |
| other | 9.6 | 7.7 | 0.97 (0.89 to 1.06) |
| Panel reactive antibodies | b | ||
| ≤10% | 63.7 | 68.1 | Reference |
| 11% to 30% | 8.8 | 7.7 | 1.04 (0.96 to 1.13) |
| >30% | 17.7 | 13.7 | 1.14 (1.07 to 1.21)b |
| unreported | 9.8 | 10.5 | 0.69 (0.63 to 0.75)b |
| Comorbidities | |||
| hypertension | 55.8 | 77.3b | 0.41 (0.39 to 0.43)b |
| cerebrovascular disease | 1.6 | 3.02b | 0.63 (0.52 to 0.75)b |
| peripheral vascular disease | 3.6 | 4.83b | 0.83 (0.73 to 0.94)a |
| Region at listing | b | ||
| 1 | 1.9 | 3.9 | 0.9 (0.72 to 1.12) |
| 2 | 16.5 | 13.5 | 1.72 (1.47 to 2.01)b |
| 3 | 12.7 | 12.1 | 1.37 (1.17 to 1.61)b |
| 4 | 10.1 | 8.6 | 2.13 (1.81 to 2.51)b |
| 5 | 20.6 | 20.9 | 1.14 (0.98 to 1.34) |
| 6 | 1.8 | 2.5 | 0.84 (0.67 to 1.06) |
| 7 | 9.5 | 9.6 | 1.62 (1.37 to 1.91)b |
| 8 | 2.6 | 4.2 | Reference |
| 9 | 11.2 | 7.1 | 2.08 (1.76 to 2.47)b |
| 10 | 6.2 | 8.5 | 1.09 (0.92 to 1.29) |
| 11 | 7.1 | 9.0 | 1.08 (0.91 to 1.28) |
| Year of listing | b | ||
| 1999 | 9.7 | 10.6 | Reference |
| 2000 | 9.5 | 11.4 | 0.91 (0.82 to 1.01) |
| 2001 | 10.6 | 11.3 | 0.97 (0.87 to 1.07) |
| 2002 | 11.4 | 11.9 | 1.00 (0.90 to 1.10) |
| 2003 | 12.5 | 12.4 | 1.05 (0.95 to 1.16) |
| 2004 | 13.4 | 13.2 | 1.08 (0.98 to 1.19) |
| 2005 | 16.0 | 14.0 | 1.32 (1.20 to 1.45)b |
| 2006 | 16.9 | 15.3 | 1.44 (1.31 to 1.58)b |
| Median wait time at initial DSA (months)c | b | ||
| <19 | 11.4 | 20.1 | Reference |
| 19 to 35 | 31.6 | 35.4 | 1.16 (1.07 to 1.26)b |
| 36 to 47 | 23.3 | 23.6 | 1.12 (1.03 to 1.23)a |
| >47 | 33.7 | 21.0 | 1.76 (1.61 to 1.93)b |
Sample limited to patients listed in 1999 to 2006 for availability of baseline DSA-specific waiting times (n = 140,918). Inter-DSA traveling was defined as transfer of care to a center outside of a candidate's initial DSA after initial listing, censored at death, waitlist removal because of illness, and end of study. Percentages reflect proportions of travelers and nontravelers, respectively, with the indicated demographic or clinical traits at listing (column percentages).
P values for χ2 test of variation in the distribution of baseline clinical traits according to traveler status: aP = 0.002 to 0.01, bP < 0.001.
P value for the relative association of a baseline clinical trait with traveling after initial listing by multivariable Cox regression: a>P = 0.002 to 0.01, bP < 0.001.
Median blood-type-specific waiting time for deceased donor transplant according to initial DSA at year of listing.
Becoming a traveler dramatically increased overall access to any transplant (aHR 1.74, 95% CI 1.69 to 1.79). Among travelers, the likelihood of deceased donor transplant was nearly doubled (aHR 1.94, 95% CI 1.88 to 2.00) whereas the relative likelihood of living donor transplant increased approximately 33% (aHR 1.33, 95% CI 1.25 to 1.42). However, traveling itself was not associated with death or removal for illness when censored at transplantation (aHR 0.98, 95% CI 0.93 to 1.03).
Post-Transplant Outcomes
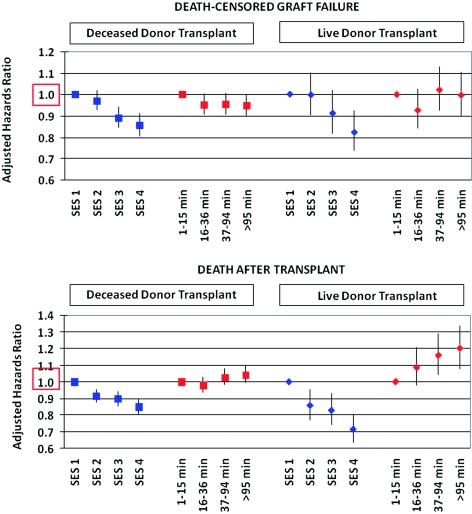
Outcomes after kidney transplant improved with increasing SES. Compared with patients in the lowest SES quartiles, the relative risk of death-censored graft failure decreased in a graded manner with SES quartile, from an approximately 5% relative risk reduction for those in the second quartile to 18% for those in the highest quartile (Figure 4). By 10 years, live donor and deceased donor allograft survival were 77% and 69% for high SES recipients, respectively, compared with 63% and 53% for recipients with lowest SES (Log-rank P values for both comparisons <0.0001). Higher SES was also strongly associated with reduced risk of subsequent patient death. Deceased donor (aHR 1.09, 95% CI 1.00 to 1.04) and live donor (aHR 1.20, 95% CI 1.07 to 1.34) graft recipients living farthest from the transplant center also had increased risk of post-transplant death (Figure 4). There was a borderline, nonsignificant association of longer distance from center with somewhat lower risk of deceased donor graft failure (aHR 0.95, 95% CI 0.90 to 1.00); however, this pattern was not present after stratification by broad categorization of organ quality (standard criteria versus expanded criteria or donation after cardiac death). Traveling to a different DSA before transplant was not significantly associated with post-transplant patient or allograft survival among transplant recipients.
Figure 4.
Adjusted associations of pretransplant SES and distance to center with post-transplant outcomes by multivariable Cox regression. Regression models were adjusted for baseline demographic, clinical, and geographic factors at the time of transplant as displayed in Table 1B.
Discussion
Disparity in access to kidney transplantation is evident as a function of patient SES and place of residence. In this analysis of national registry data, we found that greater affluence is associated with improved access to living donor allografts, a greater likelihood of traveling to a new DSA with an associated increase in deceased-donor transplant access, and superior post-transplant survival. We found that living farther from the transplant center is associated with modestly decreased access to deceased-donor kidney transplants and inferior post-transplant survival after controlling for SES and other donor and recipient characteristics.
The effect of sociodemographic factors such as race and ethnicity on kidney transplantation has been the subject of significant evaluation over the past 2 decades (17,18). African Americans face barriers in referral to transplant (12,19), completion of the evaluation and listing (17,20), and obtaining a deceased donor allograft in part because of HLA matching. Recent publications have also reported lower access to living donor kidneys among African Americans (21–23). It is encouraging that changes in the organ allocation system, which reduced emphasis on HLA matching, have attenuated the disparities faced by African Americans (2). Despite some progress in improving racial disparity through modification in the organ allocation system, there have been no substantial changes in current allocation policies to address the ongoing geographic disparity or the effect of SES on access to renal transplantation.
Geographic differences in renal transplant access as a function of state, DSA, and UNOS region are well documented (4,6). Because of current imbalances in donor supply and the size of waiting lists, the median waiting time for deceased-donor kidney transplant exceeds 5 to 6 years in the most competitive UNOS regions compared with <2 years in some southern and western states (24). This analysis demonstrates that differences in transplant access and outcomes on the basis of geography exist within individual regions. We found that patients living farthest from transplant centers had somewhat reduced access to deceased donor kidney transplantation but improved access to living donors. Increased distance from initial center was also a predictor of inter-DSA traveling, along with important factors such as prolonged DSA-specific waiting times for deceased donor organs and high SES. Those living farthest from their centers experienced inferior post-transplant patient survival after adjustment for baseline demographic and clinical factors.
Contrary to our expectations, kidney graft survival appears relatively unaffected by longer distance to center, which may reflect effect of other factors beyond access to care such as donor quality. Specifically, recipients living closest to transplant centers received a higher percentage of expanded criteria donor kidneys and experienced more delayed graft function. This may reflect transplant professionals' willingness to accept higher risk organs to improve transplant access in areas with long waiting times, principally larger urban areas close to major transplant centers. Furthermore, unmeasured differences in organ quality may persist and likely correlate with the aggressiveness of donor acceptance practices.
Socioeconomic factors clearly drive outcomes in many areas of healthcare, including access to primary and specialty healthcare, compliance with therapy, ability to afford medications, and outcome after surgical procedures. Higher SES increases access to transplant and improves outcome for patients with end-stage kidney disease (25,26). Higher SES results in early nephrology referral, higher rates of pre-emptive transplantation, reduced dialysis time, and improved outcomes on dialysis (27). For kidney transplant, we found that improved access appears to be a function of higher rates of living donor transplantation and an increased ability to travel to DSAs with shorter waiting times. After transplant, improved outcomes for high SES patients may be mediated by increased ability to afford and adhere to medication regimens, access to a broader spectrum of services, and differences in organ quality not adequately accounted for in the multivariate analysis. SES differences may be exacerbated by current Medicare policies that require a 20% copayment for immunosuppression and limit coverage to only 36 months post-transplantation, significantly impairing patients' ability to care for their transplant in the absence of private health insurance.
This analysis is limited by the retrospective design and degree of precision available for some variables of interest. The neighborhood SES measure used is a surrogate for that of the individual. However, the zip-code-based SES index used here was strongly correlated with individual parameters reflective of SES in the OPTN database such as insurance status, educational achievement, and race/ethnicity. Furthermore, it is likely that the effect of SES and distance are underestimated because this analysis only includes patients who successfully completed the process of evaluation and listing. Other potential limitations include the presence of unaccounted for variables not adjusted for in the model that affect organ quality. Our definition of travelers only includes patients who were initially listed at one center and then transferred care to a center in a new DSA. It does not capture those patients who chose to travel to a distant center for their initial care, which may, in fact, underestimate the effect of this behavior.
In conclusion, ongoing disparities persist in access to kidney transplantation and post-transplant outcomes on the basis of sociodemographic factors. Recent policy decisions have attenuated racial/ethnicity-based disparities in deceased donor transplants but have not effectively tackled the ongoing barriers faced by patients living in regions with long waiting times. Improved access to deceased donor transplantation can be obtained through inter-DSA travel, although this option is more available to candidates with greater income and private insurance. This differential opportunity appears to contradict the goals of the Final Rule, which state that the organ allocation system should ‘assure that allocation of scarce organs will be based on common medical criteria, not accidents of geography‘ (1). Broader sharing of organs across regions has the potential to decrease regional disparities in waiting times and may attenuate the motivation for high SES patients to travel to nonlocal DSAs to disproportionately improve access to deceased donor organs. These results suggest the need to consider addressing geographic variation within the proposed revisions to the kidney allocation system being considered by the OPTN.
Disclosures
None.
Acknowledgments
This work was supported by an American Recovery and Reinvestment Act grant from the National Institute of Diabetes Digestive and Kidney Diseases (NIDDK; RC1 1RC1DK086450-01). Dr. Axelrod was supported by a grant from the Hitchcock Foundation. Dr. Lentine also received career development support from NIDDK grant (K08DK073036). An abstract describing portions of this work was presented at the American Transplant Congress in Boston, MA, May 30 through June 3, 2009. Data reported here were supplied by UNOS as the contractor for OPTN. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by OPTN, the U.S. Government, NIDDK, or the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Department of Health and Human Services: The “Final Rule”. Available at: http://www.gaonet.gov/special.pubs/organ/appendd.pdf Accessed August 29, 2009.
- 2. Roberts JP, Wolfe RA, Bragg-Gresham JL, Rush SH, Wynn JJ, Distant DA, Ashby VB, Held PJ, Port FK: Effect of changing the priority for HLA matching on the rates and outcomes of kidney transplantation in minority groups. N Engl J Med 350: 545–551, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell BD, Stern MP, Haffner SM, Hazuda HP, Patterson JK: Risk factors for cardiovascular mortality in Mexican Americans and non-Hispanic whites. San Antonio Heart Study. Am J Epidemiol 131: 423–433, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Ellison MD, Edwards LB, Edwards EB, Barker CF: Geographic differences in access to transplantation in the United States. Transplantation 76: 1389–1394, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Roberts JP, Dykstra DM, Goodrich NP, Rush SH, Merion RM, Port FK: Geographic differences in event rates by model for end-stage liver disease score. Am J Transplant 6: 2470–2475, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB: Geographic variability in access to primary kidney transplantation in the United States, 1996–2005. Am J Transplant 7: 1412–1423, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Kemmer N, Safdar K, Kaiser T, Zacharias V, Neff GW: Impact of geographic location on access to liver transplantation among ethnic minorities. Transplantation 85: 166–170, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Kasiske BL, London W, Ellison MD: Race and socioeconomic factors influencing early placement on the kidney transplant waiting list. J Am Soc Nephrol 9: 2142–2147, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Goldfarb-Rumyantzev AS, Koford JK, Baird BC, Chelamcharla M, Habib AN, Wang BJ, Lin SJ, Shihab F, Isaacs RB: Role of socioeconomic status in kidney transplant outcome. Clin J Am Soc Nephrol 1: 313–322, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Cass A, Cunningham J, Arnold PC, Snelling P, Wang Z, Hoy W: Delayed referral to a nephrologist: outcomes among patients who survive at least one year on dialysis. Med J Aust 177: 135–138, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM: Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol 20: 1333–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keith D, Ashby VB, Port FK, Leichtman AB: Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clin J Am Soc Nephrol 3: 463–470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Organ Procurement and Transplant Network: About OPTN data. Available at http://optn.transplant.hrsa.gov/data Accessed January 4, 2010
- 14. Ramirez EA: Cardiovascular health in Puerto Ricans compared to other population groups in the United States. Ethn Dis 1: 188–199, 1991 [PubMed] [Google Scholar]
- 15. Birkmeyer JD, Siewers AE, Marth NJ, Goodman DC: Regionalization of high-risk surgery and implications for patient travel times. JAMA 290: 2703–2708, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Chan L, Hart LG, Goodman DC: Geographic access to health care for rural Medicare beneficiaries. J Rural Health 22: 140–146, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Epstein AM, Ayanian JZ, Keogh JH, Noonan SJ, Armistead N, Cleary PD, Weissman JS, David-Kasdan JA, Carlson D, Fuller J, Marsh D, Conti RM: Racial disparities in access to renal transplantation—Clinically appropriate or due to underuse or overuse? N Engl J Med 343: 1537–1544, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hicks LS, Cleary PD, Epstein AM, Ayanian JZ: Differences in health-related quality of life and treatment preferences among black and white patients with end-stage renal disease. Qual Life Res 13: 1129–1137, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Weng FL, Joffe MM, Feldman HI, Mange KC: Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis 46: 734–745, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Minniefield WJ, Yang J, Muti P: Differences in attitudes toward organ donation among African Americans and whites in the United States. J Natl Med Assoc 93: 372–379, 2001 [PMC free article] [PubMed] [Google Scholar]
- 22. Gore JL, Danovitch GM, Litwin MS, Pham PT, Singer JS: Disparities in the utilization of live donor renal transplantation. Am J Transplant 9: 1124–1133, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Segev DL, Montgomery RA: Regional and racial disparities in the use of live non-directed kidney donors. Am J Transplant 8: 1051–1055, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Axelrod DMK, Brewer E., Becker B., Segev DL., Rao PS: Kidney and pancreas transplantation in the United States 1999–2008: The changing face of living donation. Am J Transplantation 2010, in press [DOI] [PubMed] [Google Scholar]
- 25. Kemmer N, Zacharias V, Kaiser TE, Neff GW: Access to liver transplantation in the MELD era: Role of ethnicity and insurance. Dig Dis Sci 54: 1794–1797, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Bryce CL, Angus DC, Arnold RM, Chang CC, Farrell MH, Manzarbeitia C, Marino IR, Roberts MS: Sociodemographic differences in early access to liver transplantation services. Am J Transplant 9: 2092–2101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Navaneethan SD, Aloudat S, Singh S: A systematic review of patient and health system characteristics associated with late referral in chronic kidney disease. BMC Nephrol 9: 3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]