Abstract
Background and objectives: Arteriovenous fistulas (AVFs) are widely accepted as the preferred hemodialysis vascular access type. However, supporting data have failed to consider morbidity and mortality incurred during failed creation attempts and may therefore overstate potential advantages. This study compares survival, quality-adjusted survival, and costs among incident hemodialysis patients after attempted placement of AVFs or arteiovenous grafts (AVGs).
Design, setting, participants, & measurements: Analogous Markov models were created, one each for AVF and AVG. Patients entered consideration at the time of first access creation, contemporaneous with dialysis initiation. Subsequent outcomes were determined probabilistically; transition probabilities, utilities, and costs were gathered from published sources. To ensure comparability between AVFs and AVGs, the timing and likelihood of access maturation were measured in a contemporary cohort of incident hemodialysis patients.
Results: Mean (SD) overall survival was 39.2 (0.8) and 36.7 (1.0) months for AVFs and AVGs, respectively: difference (95% confidence interval [CI]) 2.6 (1.8, 3.3) months. Quality-adjusted survival was 36.1 (0.8) and 32.5 (0.9) quality-adjusted life months (QALMs) for AVFs and AVGs, respectively: difference (95% CI) 3.6 (2.8, 4.3) QALMs. The incremental cost-effectiveness ratio (95% CI) for AVFs relative to AVGs was $446 (−6023, 6994) per quality-adjusted life year saved.
Conclusions: AVFs are associated with greater overall and quality-adjusted survival than AVGs. Observed differences were much less pronounced than might be expected from existing literature, suggesting that prospective identification of patients at high risk for AVF maturational failure might enable improvements in health outcomes via individualization of access planning.
Vascular access is an important determinant of morbidity and mortality for patients on hemodialysis (HD). Broad consensus exists that native arteriovenous fistulas (AVFs) are the preferred access type (1,2). Studies have repeatedly and consistently demonstrated that AVFs are associated with better survival, fewer episodes of mechanical and infectious complications, longer patency, reduced costs relative to arteriovenous grafts (AVGs), and that AVFs and AVGs are far superior to catheters in these respects (3–8). However, these studies have considered outcomes beginning at the time of first cannulation, and therefore do not reflect morbidity and mortality incurred upon failed attempts at access placement or during prolonged periods of maturation.
As many as 55% to 60% of AVFs never develop to the point of being usable for HD, and those that do often take up to 5 or more months to mature (9–12). Contemporary data for AVGs are scant, but prevailing sentiment suggests that they mature more reliably and rapidly than do AVFs (13).
Presently, 82% of U.S. patients initiate HD via a catheter, and most of these (63.2% of all incident patients) have no concomitant maturing arteriovenous access in place at the time of dialysis initiation (14). Among these patients in particular, “bridge” catheter exposure resulting from primary fistula failure or prolonged periods of maturation likely offset the benefits of AVFs relative to AVGs to some degree. To date, no study has formally incorporated perimaturational morbidity and mortality into the overall risk-benefit calculation regarding choice of arteriovenous access type.
We conducted these decision and cost-utility analyses to examine the effect of selecting AVF creation versus AVG placement among patients initiating HD without a functional or maturing access in place. By design, these analyses consider mortality, morbidity, and costs after successful maturation as well as those incurred in the course of attempted maturation.
Materials and Methods
Conceptual Model
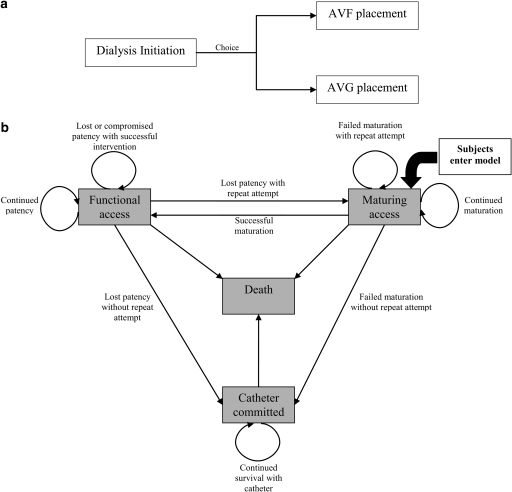
The base-case considered a decision between placement of an AVF or AVG occurring immediately after dialysis initiation (Figure 1a). Subsequently, outcomes and costs were determined by analogous Markov models: one each for the AVF and AVG arm (transition probabilities and utilities differed between arms). Patients entered the Markov model in the maturing access state (which implied concomitant catheter use) and transitioned among this and states of functional access, catheter commitment, and death in probabilistic fashion. Figure 1b demonstrates allowable transitions and associated clinical rationale. For example, patients could transition between maturing access and functional access upon successful access maturation or vice versa upon permanent loss of patency with subsequent placement of a new access. Catheter commitment connotes permanent abandonment of attempted arteriovenous access creation and does not include temporary catheter use while accesses matured; catheter morbidity and mortality during access maturation was instead accounted for in the transition probabilities and utilities assigned to maturing access states (see below). Death was modeled as an absorbing state and could be reached from any of the remaining states. In the base case, no crossover between AVFs and AVGs was allowed; this restriction was eased in a sensitivity analysis in which crossover from AVFs to AVGs was allowed after the first instance of AVF failure to mature or loss of patency (see below).
Figure 1.
Conceptual model. (a) In the base-case, the decision was modeled as a choice between AVF and AVG. (b) Subsequently, patients transitioned among states of maturing access, functional access, catheter commitment, and death in probabilistic fashion using analogous Markov models.
Transition Probabilities, Utilities, and Costs
The outcomes of interest were overall survival, quality-adjusted survival, and the incremental cost-effectiveness ratio (the difference in costs divided by the difference in quality-adjusted survival comparing two interventions). In most instances, transition probabilities were derived from the literature. To account for catheter-associated mortality during access maturation, unique probabilities of death from maturing AVF/AVG states (which were different from those from functioning AVF/AVG) were utilized (14). All identified probabilities were converted to a per-month scale to coincide with the cycle length of the Markov models; a 1-month cycle time was selected so as to provide a reasonable degree of granularity while still enabling sufficient time for identification and treatment of access-related events (e.g., mechanical complications, infections).
Available data regarding the likelihood and timing of access maturation and the likelihood of repeated access attempt upon failure to mature/loss of patency were not reasonably comparable from published reports. For this reason, we assembled a historical cohort of Fresenius Medical Care North America (FMCNA) patients who were incident to HD in the calendar year 2007, using a catheter at the time of initiation, and who subsequently had an arteriovenous access placed. After excluding subjects who changed modality (including transplantation), transferred care away from FMCNA, or were missing data on vascular access, we calculated the probability of access maturation in each month after placement (conditional upon no prior maturation) separately for AVF (n = 6459) and AVG (n = 2183). Similarly, we calculated the probability of repeated arteriovenous access attempt after maturation failure/permanent loss of patency.
Utilities for AVF, AVG, and catheter use were identified from published data (15). Each was divided by the highest identified utility (that for AVF), such that the resultant state utilities were 1.0, 0.95, and 0.91 for AVF, AVG, and catheter use, respectively. To reflect catheter-associated morbidity during access maturation, the utility for catheter was applied to maturing access states. Disutilities of −0.08 and −0.1 were applied to reflect morbidity associated with percutaneous (i.e., arteriogram, declot, catheter placement) and surgical procedures, respectively, when patients were probabilistically determined to require these (16). The monthly probability of blood stream infection related to each access state was identified from published data (17), and state rewards were reduced by the product of this probability times a disutility of −0.6 for sepsis (18).
Procedural costs related to access creation, angiography and intervention, and catheter placement and removal were identified from public Centers for Medicare and Medicaid Services (CMS) data (19). Erythropoietin usage for each access type was derived from the literature (20) and associated costs were identified from CMS data (19). Blood stream infection-related costs were calculated assuming 60% of events would require inpatient admission and 40% outpatient therapy alone (21). Outpatient treatment costs included expenditures related to antibiotic administration, catheter exchange (where applicable), and blood cultures (19,21,22). Inpatient costs were identified from the work of Reed et al. (23), which included costs incurred during follow-up care.
Statistical Analyses
The Markov model was constructed using the DATA 3.5 software package (TreeAge Software, Inc., Williamston, MA) on a Windows 98 operating system. Overall survival and quality-adjusted survival were expressed in terms of months and quality-adjusted life months (QALMs), respectively. Transitions were assumed to occur discretely within each cycle; state and transition awards were applied at end-cycle. A time horizon of 60 months was selected to encompass life expectancy for most HD patients. Second-order Monte Carlo simulation (with 5000 replicates) was used to incorporate parameter uncertainty related to transition probabilities. Cost-utility analysis was performed with (3% annual rate) and without discounting; because qualitative interpretation of results did not differ, nondiscounted rates are presented for simplicity.
One- and two-way sensitivity analyses were used to examine the robustness of findings with respect to individual and pair-wise combinations of transition probabilities, utilities, and costs. Finally, a sensitivity analysis was conducted in which a crossover strategy was introduced and compared with AVF-only and AVG-only strategies. The crossover arm assumed patients would undergo initial placement of an AVF, but that after maturational failure or patency loss, patients undergoing repeat access attempts would have AVGs placed.
Results
Transition Probabilities
Transition probabilities and costs identified from the literature are given in Tables 1a and 1b, respectively. Risk of death with functioning AVF was converted to a per-month scale. (When data were modeled such that the underlying risk of death varied with time on dialysis, [17] results were nearly identical [not shown]; results that follow assume a constant underlying rate of death for simplicity.) Risks of death with functioning AVG, catheter, and maturing AVF and AVG were calculated by multiplying the baseline risk of death with a functioning AVF times the state-specific relative risk (Table 1a).
Table 1a.
Transition probabilities and costs based on published literature
| Transition | Probability or Relative Risk (95% CI) |
|---|---|
| Probability of established AVF continuing to function without intervention (25) | 0.95/month |
| Probability of failed attempt to reestablish patency of previously functioning AVF (28) | (33/303) = 0.11/event |
| Probability of established AVG continuing to function without intervention (25) | 0.91/month |
| Probability of failed attempt to re-establish patency of previously functioning AVG (28) | (14/207) = 0.068/event |
| Probability of death with functioning AVF (14) | 0.012/month |
| Relative risk (95% CI) of death with maturing AVF (reference: functioning AVF) (14) | 1.49 (1.44 to 1.55)a |
| Relative risk (95% CI) of death with functioning AVG (reference: functioning AVF) (14) | 1.39 (1.32 to 1.47)a |
| Relative risk (95% CI) of death with maturing AVG (reference: functioning AVF) (14) | 1.74 (1.65 to 1.84)a |
| Relative risk (95% CI) of death with catheter (reference: functioning AVF) (14) | 2.18 (2.11 to 2.26)a |
Estimates adjusted for demographic case mix, body mass index, insurance status, duration of predialysis nephrology care, residual estimated GFR, ESRD network, and 13 comorbid conditions (14).
Table 1b.
Costs based on published literature
| Event | Cost |
|---|---|
| Cost AVF creation (19) | $792.74/event |
| Cost AVG placement (19) | $573.82/event |
| Cost of percutaneous intervention (19) | $453.35/event |
| Cost of catheter insertion (19) | $284.57/event |
| Cost of catheter removal (19) | $137.77/event |
| Cost of outpatient treatment for bacteremia | $158.19/eventa |
| Cost of hospitalization for bacteremia | $20,647/eventb |
| Cost of erythropoietinc | |
| AVF | $606.81/month |
| AVG | $646.73/month |
| catheter (including maturing AVF and maturing AVG states) | $741.83/month |
Estimated based on costs for a 3-week course of vancomycin administered 1 g intravenously 3 times weekly during dialysis at $6.46/g plus the cost of two sets of blood cultures at $50/set (19). Costs were converted to a monthly scale on the basis of the monthly probability of blood stream infection (treated in the ambulatory setting) by access type (17,21).
Price reflects a weighted average of total costs (in-hospital and follow-up) for methicillin-sensitive and methicillin-resistant Staphylococcus aureus (23). Costs were converted to a monthly scale on the basis of the monthly probability of blood stream infection requiring hospitalization by access type (17,21).
Monthly probabilities of access maturation were calculated based on data from the FMCNA cohort (Table 2); for AVF and AVG, maturation rates declined markedly after month 7 such that subsequent transitions were no longer modeled. Overall, 57.2% and 70.6% of attempted AVFs and AVGs, respectively, matured to the point of use for HD. Upon failed maturation attempt or permanent loss of established patency, 50.6% (316/624) of patients with AVFs and 51.0% (75/147) of patients with AVGs had a new arteriovenous access placed.
Table 2.
Maturation rate of AVFs and AVGs by month postplacement among incident FMCNA patients
| Maturation | Number AVFs Matured/Number AVFs Eligible (%) | Number AVGs Matured/Number AVGs Eligible (%) |
|---|---|---|
| After | ||
| month 1 | 400/6459 (6.2) | 421/2183 (19.3) |
| month 2 | 376/6059 (6.2) | 587/1762 (33.3) |
| month 3 | 668/5683 (11.8) | 302/1175 (25.7) |
| month 4 | 694/5015 (13.8) | 116/873 (13.3) |
| month 5 | 566/4321 (13.1) | 48/757 (6.3) |
| month 6 | 400/3755 (10.7) | 28/709 (4.0) |
| month 7 | 588/3355 (17.5) | 38/681 (5.6) |
| Cumulative maturationa | 57.2% | 70.6% |
This was not modeled but is presented for clinical interest.
Base-Case Analyses
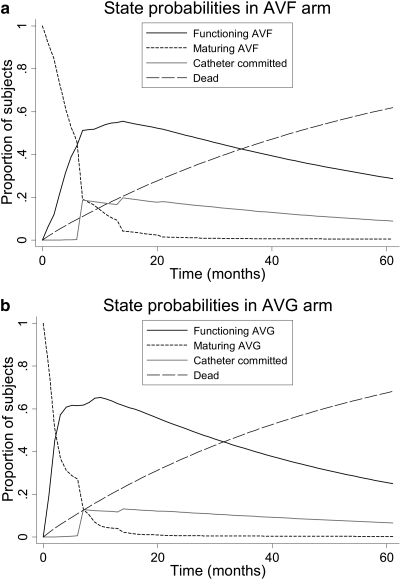
State probabilities for AVF and AVG arms are depicted in Figure 2. Overall, peak prevalence in the functioning access state was greater and was reached more rapidly in the AVG arm but also waned more rapidly such that the prevalence of functioning access was essentially equivalent to that of AVFs at later time points. Overall, a greater proportion of patients in the AVF arm were catheter-committed throughout the period of simulation.
Figure 2.
Proportion of subjects in each state over study time. State probabilities in (a) the AVF arm and (b) the AVG arm.
In the base-case, mean (SEM) overall survival was 39.2 (0.8) months and 36.7 (1.0) months in the AVF and AVG arms, respectively: difference (95% confidence interval [CI]) 2.6 (1.8, 3.3) months (P < 0.001) favoring AVFs (Table 3). Quality-adjusted survival was 36.1 (0.8) QALMs and 32.5 (0.9) QALMs for AVFs and AVGs, respectively: difference (95% CI) 3.6 (2.8, 4.3) QALMs (P < 0.001) favoring AVFs (Table 3).
Table 3.
Mean overall survival and QALM for AVFs and AVGs
| Overall Survival (months) | Quality-Adjusted Survival (QALMs) | |
|---|---|---|
| AVF | 39.2 (0.8) | 36.1 (0.8) |
| AVG | 36.7 (1.0) | 32.5 (0.9) |
| Crossover | 38.8 (0.8) | 35.4 (0.8) |
Estimates presented as mean (SEM).
One- and Two-Way Sensitivity Analyses
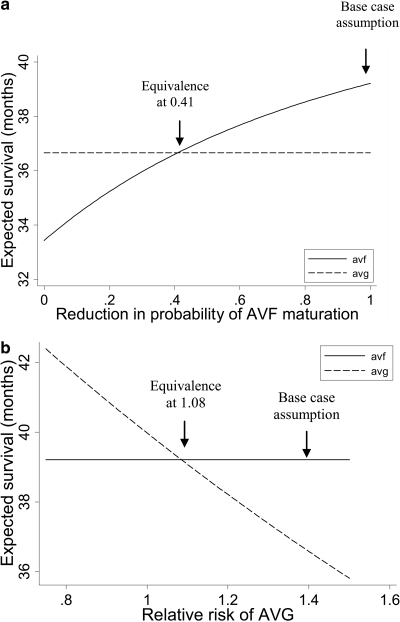
In practice, patients referred for AVGs tend to be poorer vascular access candidates than those referred for AVFs (11). Considering that decision analyses function at the population level (e.g., compare outcomes between the scenario in which all patients receive AVFs and that in which all patients receive AVGs), estimates may have been biased in favor of AVFs. Therefore, we conducted one-way sensitivity analyses to examine how much lower AVF maturation rates would have to be among patients who actually received AVGs to achieve equivalent outcomes. As depicted in Figure 3a, the probability of AVF maturation each month would have to be reduced to 41% of the observed level to create equivalence in overall survival between the AVF and AVG arms. Findings were similar for quality-adjusted survival (equivalence at 43%; data not shown).
Figure 3.
One-way sensitivity analyses. (a) Effects of varying the likelihood of AVF maturation on overall survival. (b) The effect of varying the relative risk of mortality with functioning AVG on overall survival.
Similarly, it may be hypothesized that observed differences in mortality between AVFs and AVGs may relate to differences in burden of illness rather than to the direct effect of the access type. Consequently, we conducted one-way sensitivity analyses to examine at what value of relative risk of mortality equivalent outcomes were observed (for AVGs relative to AVFs; base-case scenario 1.39). As depicted in Figure 3b, overall survival was equivalent when the relative mortality risk for AVG recipients was 1.08; quality-adjusted life expectancy equivalence was observed at a relative mortality risk of 0.93 (data not shown).
Additional one-way sensitivity analyses indicate that results were robust with respect to assumptions regarding the relative risk of mortality associated with catheter use, utilities for percutaneous/surgical procedures and catheter states, and cost of hospitalization.
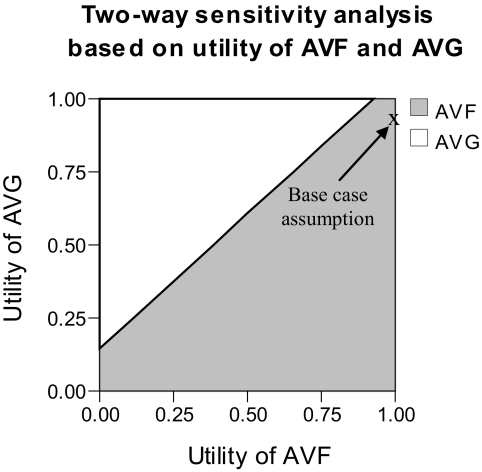
Two-way sensitivity analysis was conducted to examine the influence of assumptions regarding the utility of AVFs and AVGs. Results indicate that the utility of AVGs would have to be between 0.09 and 0.15 higher than that for AVFs for quality-adjusted survival to favor an AVG strategy (Figure 4). Additional two-way sensitivity analyses indicated that results were entirely robust with respect to assumptions made about the probability of continued AVF/AVG patency, the likelihood of successful intervention for loss of AVF/AVG patency, and the probability of attempting a new AVF/AVG after permanent loss of patency.
Figure 4.
Two-way sensitivity analysis based on the utility of AVFs and utility of AVGs (before application of disutilities for percutaneous/surgical interventions and blood stream infections). Outcome is quality-adjusted survival. White areas represent values that favor AVGs; gray areas represent values that favor AVFs. For the model to favor AVGs, the utility for AVGs must be between 0.09 and 0.15 higher than the utility of AVFs.
Crossover Model
Further sensitivity analysis was conducted in which subjects were allowed to crossover from AVFs to AVGs after the first episode of failure to mature or permanent loss of patency (assuming that they were probabilistically determined to have a repeated attempt at arteriovenous access creation). Mean (SEM) overall survival in the crossover arm was 38.8 (0.8) months; quality-adjusted survival was 35.4 (0.8) QALMs (Table 3). Overall survival and quality-adjusted survival was statistically significantly worse in the crossover arm than in the AVF-only arm: differences (95% CIs) were −0.4 (−0.6, −0.3) months (P < 0.001) and −0.6 (−0.8, −0.5) QALMs (P < 0.001), respectively. Conversely, overall and quality-adjusted survival was better in the crossover arm than in the AVG-only arm: differences (95% CIs) 2.1 (1.5, 2.7) months (P < 0.001) and 2.9 (2.3, 3.6) QALMs (P < 0.001), respectively. Allowances for additional attempts at AVF creation (up to four were modeled) before crossover to AVG did not materially alter findings (data not shown).
Cost-Utility Analysis
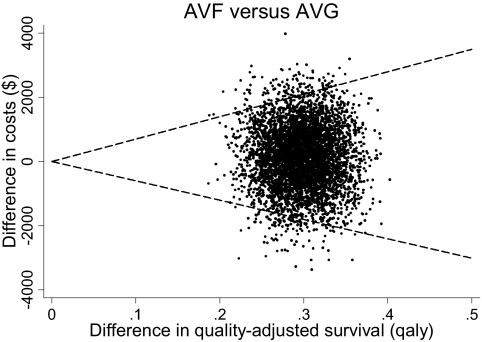
Component cost estimates are provided in Table 1b. On cost-utility analysis, no dominant strategy was identified. The incremental cost-effectiveness ratio (95% CI) comparing the AVF to the AVG strategy was $446 (−6023, 6994)/quality-adjusted life year (meaning that AVFs provide for better quality-adjusted survival at an average cost of $446 for each year of perfect health saved; Figure 5). On sensitivity analysis that also considered crossover, the incremental cost-effectiveness ratios (95% CIs) in moving from AVG-only → crossover → AVF-only were $363 (−6027, 6842) and $823 (−6083, 7731)/quality-adjusted life year saved, respectively.
Figure 5.
Cost-utility plane comparing AVFs to AVGs. Estimates from individual estimates are indicated by points; the 95% confidence bounds are indicated by dashed lines.
Discussion
Vascular access is a HD patient's lifeline and Achilles heel. Using decision and cost-utility analysis, we examined the influence of selection of arteriovenous access type on overall survival, quality-adjusted survival, and in terms of cost-effectiveness. Whereas the study confirmed that AVF use was associated with statistically significantly greater overall (2.6 months) and quality-adjusted (3.6 QALMs) survival (Table 3), the observed differences were much less pronounced than might be expected on the basis of the existing literature. Nonetheless, the observed cost per quality-adjusted life year saved was modest; even the upper bound of the 95% CI ($6994) falls well below traditional thresholds ($50,000 to $75,000).
Prior studies of the relative merits of AVFs versus AVGs have tracked outcomes beginning at the time of first access cannulation (3–8,23). As such, data from these studies do not reflect morbidity and mortality incurred as accesses mature or upon failed attempts at access placement. Considering that AVFs mature more slowly and less reliably than do AVGs (9–13), it is not surprising that the associated survival observed in this study (which considers perimaturational events) is more modest than that observed in prior reports, attributable to increased catheter exposure in the AVF arm.
Sensitivity analyses demonstrated that our results were robust with respect to assumptions made about the probability of continued AVF/AVG patency, the likelihood of achieving assisted patency after mechanical failure or malfunction of AVFs/AVGs, the relative risk of death associated with catheter usage, the disutility of access-related procedures, and the utility of catheter use.
Conversely, sensitivity analysis demonstrated that results were sensitive to assumptions made about the relative likelihood of hypothetical AVF maturation among patients who received AVGs. This finding has important implications for future research in that it suggests that development of accurate prediction rules might enable improvement in patient outcomes via selective placement of AVGs among patients at high risk for primary AVF failure and provides benchmark levels at which AVG becomes the preferred strategy. One such clinical prediction rule has been previously published (10) but was developed in a Canadian population, which was younger and had lower representation of blacks, diabetics, and comorbid coronary and peripheral arterial disease than does the U.S. incident HD population. Additional work in this area, with greater generalizability to the U.S. population, is needed. Alternatively, patients at high-risk for AVF maturation failure might be selectively routed toward peritoneal dialysis while awaiting AVF maturation, thus avoiding exposure to attendant risks associated with central venous catheters.
In addition, our data suggest that findings were sensitive to assumptions regarding the relative mortality risk of AVG relative to AVF. Existing data are derived from observational studies (3–8). Considering that patients receiving AVGs tend to have greater burdens of comorbid illness, reported estimates are likely influenced by residual confounding. Although issues of cost and equipoise make it unlikely that the mortality differences will be measured in the context of a clinical trial, our findings suggest that additional study, at a minimum using more sophisticated methods of statistical adjustment, is warranted.
Of note, these analyses were limited to the consideration of arteriovenous access creations occurring at the time of/after dialysis initiation. Limitations in available data precluded incorporation of pre-emptive access placement attempts. It would stand to reason that absent the need for bridge catheter exposure after unsuccessful/prolonged maturational attempts, pre-emptive access placement should further favor AVFs. However, until data on the likelihood and timing of pre-emptive AVF/AVG maturation become known, the magnitude of this benefit cannot be formally established. Nonetheless, our findings do pertain to most dialysis patients who (unfortunately) initiate dialysis before any attempt at arteriovenous access creation.
This study has several important strengths. First, we incorporated for the first time the effects of perimaturational events into the overall risk calculus regarding choice of arteriovenous access type. Second, use of the FMCNA cohort to derive critical transitional probabilities ensures that consistent methods were applied to AVF and AVG arms, and that differences in source populations were minimized to the degree possible. In particular, use of this cohort precluded the need to derive estimates from AVF and AVG cohorts that were separated by continent and by decades. Third, overall survival estimates for our model (36 to 39 months) were in line with published estimates (1,24), as were the cumulative probabilities and timing of AVF and AVG maturation (9,11,25,26), which offers a degree of reassurance that the form and assumptions of our model were reasonable. Of note, a recent randomized trial has suggested the cumulative maturation probability of AVGs may be as low as 50% among patients with a mean dialysis vintage of 24 months, approximately half of whom were undergoing repeat arteriovenous access placement (27). Although direct comparison to the AVF group considered here (which consisted of strictly incident patients) is not advisable, it serves to highlight that these findings should not be extrapolated beyond the incident HD population in which they were derived.
Limitations of this study should also be mentioned. First, as noted above, estimates used to populate the model were derived from observational data and thus may be subject to residual confounding, including confounding by indication (e.g., on the basis of vascular suitability or comorbid disease burden). Multiple sensitivity analyses were conducted to examine the potential effects of confounded component data on our results. Second, because of data limitations, we were unable to account for morbidity and costs incurred in the context of collateral vein ligation, radiologic evaluations without interventions, or endovascular salvage procedures for accesses that were failing to mature (data on the frequency of such procedures are lacking in the literature). Considering that such procedures are more common among patients with attempted AVFs, this may have biased estimates of quality-adjusted survival and cost utility in favor of AVFs. Third, existing data were insufficient to consider particular anatomical locations and access procedures, and so comparisons between AVGs and transposed brachiobasilic AVFs (which is an important clinical consideration for patients who are marginal fistula candidates) cannot be made. Fourth, it has been recommended that in marginal access candidates, a distal AVG be created as a first arteriovenous access to facilitate development of proximal veins for subsequent AVF creation (1). Unfortunately, data on the efficacy of this approach on improving secondary AVF maturation are lacking in the literature, and thus informed consideration was not possible here. Such studies are needed. Finally, it should be noted that decision analysis is not a substitute for well done clinical trials (which may fall short in terms generalizability and of the number of variables that can simultaneously be considered) but should nonetheless be considered as part of the evidence base upon which clinical decisions are made.
In conclusion, our findings suggest that preferential placement of AVFs versus AVGs among patients initiating HD without a maturing or functional arteriovenous access was associated with modest but statistically significant improvements in overall and quality-adjusted survival. Further studies are needed to identify patients at high risk of primary AVF failure who might benefit from preferential placement of AVGs, to clarify the magnitude of the casual association between AVGs (versus AVFs) and mortality, and to better explore alternative (hybrid) approaches to successful arteriovenous access placement and utilization.
Disclosures
E.L. and W.W. are employees of FMCNA.
Acknowledgments
This work was supported by DK079056 from the NIH/NIDDK (to S.M.B.). The authors thank Dr. Henry Glick (University of Pennsylvania) for his valuable assistance in deriving CIs for incremental cost-effectiveness ratios.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. National Kidney Foundation Kidney Disease Outcomes Quality Initiative Guidelines: Clinical Practice Guidelines and Clinical Practice Recommendations: Bethesda, MD, National Kidney Foundation, 2006 [Google Scholar]
- 2. Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall T, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on vascular access. Nephrol Dial Transplant 22[Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW: Predictors of early mortality among incident U.S. hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2: 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Ishani A, Collins AJ, Herzog CA, Foley RN: Septicemia, access and cardiovascular disease in dialysis patients: The USRDS Wave 2 study. Kidney Int 68: 311–318, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Astor BC, Eustace JA, Powe NR, Klag MJ, Fink NE, Coresh J: Type of vascular access and survival among incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol 16: 1449–1455, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Gulati S, Sahu KM, Avula S, Sharma RK, Ayyagiri A, Pandey CM: Role of vascular access as a risk factor for infections in hemodialysis. Ren Fail 25: 967–973, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK: Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int 60: 1443–1451, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Powe NR, Jaar B, Furth SL, Hermann J, Briggs W: Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int 55: 1081–1090, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI: Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH: The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 47: 415–421, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K: Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis 42: 1000–1012, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Chemla ES, Morsy MA: Is basilic vein transposition a real alternative to an arteriovenous bypass graft? A prospective study. Semin Dial 21: 352–356, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Foley RN, Chen SC, Collins AJ: Hemodialysis access at initiation in the United States, 2005 to 2007: Still “catheter first”. Hemodial Int 13: 533–542, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Wasse H, Kutner N, Zhang R, Huang Y: Association of initial hemodialysis vascular access with patient-reported health status and quality of life. Clin J Am Soc Nephrol 2: 708–714, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brothers TE, Cox MH, Robison JG, Elliott BM, Nietert P: Prospective decision analysis modeling indicates that clinical decisions in vascular surgery often fail to maximize patient expected utility. J Surg Res 120: 278–287, 2004 [DOI] [PubMed] [Google Scholar]
- 17. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, 2009 [Google Scholar]
- 18. Scheetz MH, Bolon MK, Postelnick M, Noskin GA, Lee TA: Cost-effectiveness analysis of an antimicrobial stewardship team on bloodstream infections: A probabilistic analysis. J Antimicrob Chemother 63: 816–825, 2009 [DOI] [PubMed] [Google Scholar]
- 19. U.S. Department of Health and Human Services Centers for Medicare and Medicaid Services. Available at: http://www.medicarenhic.com/ne_prov/fees/January%202010%20ASP%20Price%20File.pdf Accessed February 16, 2010
- 20. Chand DH, Teo BW, Fatica RA, Brier M: Influence of vascular access type on outcome measures in patients on maintenance hemodialysis. Nephrol Clin Pract 108: c91–c98, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Berman SJ, Johnson EW, Nakatsu C, Alkan M, Chen R, LeDuc J: Burden of infection in patients with end-stage renal disease requiring long-term dialysis. Clin Infect Dis 39: 1747–1753, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH: Healthcare-associated bloodstream infection: A distinct entity? Insights from a large U.S. database Crit Care Med 34: 2588–2595, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Reed SD, Friedman JY, Engemann JJ, Griffiths RI, Anstrom KJ, Kaye KS, Stryjewski ME, Szczech LA, Reller LB, Corey RG, Schulman KA, Fowler VG: Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 26: 175–183, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Xue JL, Dahl D, Ebben JP, Collins AJ: The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis 42: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Huber TS, Carter JW, Carter RL, Seeger JM: Patency of autogenous and polytetrafluoroethylene upper extremity arteriovenous hemodialysis accesses: A systematic review. J Vasc Surg 38: 1005–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ; CIMINO study group: Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 3: 714–719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, Dember LM, Himmelfarb J, Gassman JJ, Greene T, Radeva MK, Davidson IJ, Ikizler TA, Braden GL, Fenves AZ, Kaufman JS, Cotton JR, Jr, Martin KJ, McNeil JW, Rahman A, Lawson JH, Whiting JF, Hu B, Meyers CM, Kusek JW, Feldman HI: Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med 360: 2191–2201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajan DK, Bunston S, Misra S, Pinto R, Lok CE: Dysfunctional autogenous hemodialysis fistulas: outcomes after angioplasty—Are there clinical predictors of patency? Radiology 232: 508–515, 2004 [DOI] [PubMed] [Google Scholar]