Abstract
Background and objectives: Cinacalcet is effective in reducing serum parathyroid hormone (PTH) in patients with secondary hyperparathyroidism. However, it has not been proven whether parathyroid gland size predicts response to therapy and whether cinacalcet is capable of inducing a reduction in parathyroid volume.
Design, setting, participants, & measurements: This 52-week, multicenter, open-label study enrolled hemodialysis patients with moderate to severe secondary hyperparathyroidism (intact PTH >300 pg/ml). Doses of cinacalcet were adjusted between 25 and 100 mg to achieve intact PTH <180 pg/ml. Ultrasonography was performed to measure the parathyroid gland size at baseline, week 26, and week 52. Findings were also compared with those of historical controls.
Results: Of the 81 subjects enrolled, 56 had parathyroid glands smaller than 500 mm3 (group S) and 25 had at least one enlarged gland larger than 500 mm3 (group L). Treatment with cinacalcet effectively decreased intact PTH by 55% from baseline in group S and by 58% in group L. A slightly greater proportion of patients in group S versus group L achieved an intact PTH <180 pg/ml (46 versus 32%) and a >30% reduction from baseline (88 versus 78%), but this was not statistically significant. Cinacalcet therapy also resulted in a significant reduction in parathyroid gland volume regardless of pretreatment size, which was in sharp contrast to historical controls (n = 87) where parathyroid gland volume progressively increased with traditional therapy alone.
Conclusions: Cinacalcet effectively decreases serum PTH levels and concomitantly reduces parathyroid gland volume, even in patients with marked parathyroid hyperplasia.
Secondary hyperparathyroidism (SHPT) is a common complication of chronic kidney disease, characterized by parathyroid hyperplasia and persistently elevated levels of parathyroid hormone (PTH) (1,2). Parathyroid hyperplasia can be divided into two types with different morphologic features: diffuse and nodular hyperplasia (3). Nodular hyperplasia is a more advanced type of hyperplasia and is associated with more marked proliferation and greater resistance to medical therapy (4).
Until recently, calcitriol and other vitamin D analogs have been the cornerstone of secretory PTH suppression in patients with chronic kidney disease; however, these agents also enhance the intestinal absorption of calcium and phosphate and result in elevations in serum calcium and phosphate concentrations (5). Moreover, the response to vitamin D sterols is substantially reduced once parathyroid hyperplasia has progressed to the advanced nodular form, presumably because of the reduced expression of calcium-sensing receptors (CaSR) and vitamin D receptors (VDR) (6–10). Several clinical studies suggest that parathyroid gland volume in excess of 500 mm3, as evaluated by ultrasonography, appears to be a useful indicator of responsiveness to vitamin D therapy (11–13). This concept is supported by the observation that surgically removed parathyroid glands weighing >500 mg usually exhibit nodular formations (3). Interestingly, vitamin D therapy may also induce regression of parathyroid hyperplasia (14), but such an effect may be less likely in more advanced stages (15).
Cinacalcet hydrochloride, a calcimimetic agent that acts as an allosteric modulator of the CaSR, is a new option for the therapeutic control of SHPT. A large number of clinical trials have shown that treatment with cinacalcet effectively reduces PTH levels in patients with SHPT that is refractory to vitamin D therapy (16–21). More recent studies suggest that combined therapy with cinacalcet and low doses of vitamin D sterols could be an effective strategy to treat SHPT while adequately maintaining acceptable levels of calcium and phosphorus (22–24). However, there is controversy as to whether cinacalcet is capable of controlling parathyroid hyperfunction in patients with marked parathyroid hyperplasia (25,26). In addition, although experimental studies suggest that regression of parathyroid hyperplasia could be induced by calcimimetics (27,28), this possibility has not been adequately explored in the clinical setting (26,29).
Therefore, the purpose of this study was twofold: (1) to elucidate whether parathyroid gland size could be used as an indicator of response to cinacalcet therapy and (2) to examine whether cinacalcet reduces parathyroid gland volume in patients with moderate to severe SHPT.
Materials and Methods
Study Population
Patients were considered for the study if they were 18 years of age or older and had required maintenance dialysis for at least 16 weeks. The main eligibility criteria were serum intact PTH >300 pg/ml, confirmed within a 30-day screening period, and serum calcium >9.0 mg/dl. Exclusion criteria included a history of parathyroidectomy or an unstable medical condition during the previous 30 days. The study was conducted in accordance with the principles of the Declaration of Helsinki, and all patients provided written informed consent. The study protocol was reviewed and approved by the institutional review board at each study site. This study is registered with the Cochrane Renal Group Registry, no. CRG020800134.
Study Design
This was a multicenter, open-label study conducted at eight centers in Japan between January 2008 and August 2009. The study period consisted of a 30-day screening period, a 12-week dose-titration phase, a 26-week maintenance phase, and a 14-week efficacy-assessment phase. Patients were divided into two groups according to the parathyroid gland volume at baseline: group S with all glands smaller than 500 mm3 and group L with one or more enlarged glands larger than 500 mm3. All study subjects received cinacalcet hydrochloride (Regpara; Kyowa Hakko Kirin, Co., Ltd., Tokyo, Japan) in an attempt to achieve target ranges specified in the Japanese Society for Dialysis Therapy (JSDT) guideline (calcium, 8.4 to 10.0 mg/dl; phosphorus, 3.5 to 6.0 mg/dl; intact PTH, 60 to 180 pg/ml) (30).
The initial dose of cinacalcet was 25 mg, given orally, once daily. The doses were increased to the next sequential daily dose in the series 25, 50, 75, and 100 mg after at least a 3-week interval if their intact PTH level was >180 pg/ml at the previous study visit, unless serum calcium was <8.4 mg/dl. If a patient's intact PTH level dropped below 60 pg/ml or serum calcium dropped below 7.5 mg/dl, the dose was reduced to the next lowest dose level. The dose of vitamin D sterols were increased only if the serum calcium remained below 8.4 mg/dl or if hypocalcemic symptoms persisted despite an increase in the dose of calcium carbonate to 3000 mg daily. Reductions in the doses of vitamin D sterols were permitted in cases in which the serum calcium was >10.0 mg/dl. No restrictions were imposed on the dose or type of phosphate binders used.
Biochemical Determinations
Blood samples were collected at the start of the dialysis session following the longest interdialytic period. Serum levels of intact PTH, calcium, and phosphorus were measured at local laboratories. Serum intact PTH levels were determined using an electrochemiluminescence immunoassay (Elecsys PTH; Roche Diagnostics, Mannheim, Germany). Serum calcium levels were corrected for albumin concentration using the modified Payne method (31).
Parathyroid Gland Imaging
High-resolution ultrasonography was performed by experienced technicians in each center at baseline, week 26, and week 52, according to a standardized protocol using a 7.5 or 10 MHz linear array transducer. Parathyroid gland volume was estimated using the ellipsoid formula (π/6 × a × b × c, where a, b, and c represent the diameters of the gland in three dimensions).
Study End Points
The primary study end point was the proportion of patients with a mean intact PTH level <180 pg/ml during the efficacy-assessment phase. Secondary end points included the proportion of patients with a >30% reduction from baseline in intact PTH levels, the percent change from baseline in intact PTH, calcium, and phosphorus concentrations, and the change from baseline in parathyroid gland volume, as evaluated by ultrasonography. Safety was evaluated by reports of adverse events.
Historical Controls
The results of this prospective trial were also compared with those of historical controls who had been treated with traditional therapy alone. Patients who met the same eligibility criteria as trial participants and had undergone ultrasonography twice at an interval of 1 year between 2000 and 2008 were recruited as historical controls through a retrospective chart review.
Statistical Analysis
All values are expressed as mean ± SD or mean ± SEM, as indicated. Baseline laboratory and parathyroid gland size measurements were obtained and patient characteristics were recorded during the screening period. A comparison of baseline data between groups was performed with χ2 tests and one-way ANOVA followed by the Bonferroni post hoc test. Mean values for intact PTH, calcium, and phosphorus during the efficacy-assessment phase were used to evaluate efficacy. Patients who withdrew from the study during the dose-titration phase were considered to not have met either the primary or the secondary end points. For patients who did not have any values measured during the efficacy-assessment phase, the last-observation-carried-forward method was used. Comparisons of proportions between groups were performed with the Fisher exact test. For comparison of paired proportions, the McNemar test was used. Logistic regression analysis was used to examine whether the presence of at least one enlarged gland was a predictor of response to cinacalcet therapy. Differences in parathyroid gland volume at baseline and at week 26 and week 52 were analyzed on the basis of the as-treated analysis, using repeated measures ANOVA followed by the Bonferroni post hoc test. Absolute changes in parathyroid gland volume from baseline to week 52 in cinacalcet trial participants were compared with those in historical controls using t test. The likelihood of achieving a >30% reduction from baseline in total parathyroid volume was compared between the cinacalcet group and historical controls using logistic regression analyses, stratified by age, sex, duration of dialysis, baseline biochemical variables, the presence of enlarged glands, or the use of intravenous vitamin D. Pearson correlation coefficient analyses were used to examine the relationships between each parameter. A 10-μg dose of maxacalcitol was considered to be equal to 1.5 μg of calcitriol, and all results for injectable vitamin D sterol dosages are presented as calcitriol equivalents. P < 0.05 was considered statistically significant. All analyses were performed using Dr. SPSS II for Windows, version 11.01 J (SPSS Japan, Tokyo, Japan).
Results
Study Population
A total of 81 patients were enrolled in the prospective trial: 56 had parathyroid glands smaller than 500 mm3 (group S) and 25 had at least one enlarged gland larger than 500 mm3 (group L). For the historical control group, 87 patients were identified from a chart review: 65 had small glands only (group HS) and 22 had one or more enlarged glands (group HL). Demographic characteristics and baseline laboratory values according to parathyroid gland size are presented in Table 1. The baseline demographics and renal history did not differ significantly between groups. Nearly all patients were receiving vitamin D sterols and phosphate binders, with little difference between groups in the types of agents used. At baseline, patients in group L and group HL had significantly higher calcium and intact PTH levels compared with those in group S and group HS, respectively. The proportion of patients achieving the target calcium level was not significantly greater in group S than in group L (75 versus 56%; P = 0.08), whereas that of phosphorus did not differ between the two groups (54 versus 52%; P = 0.54).
Table 1.
Demographics, baseline laboratory values, and parathyroid gland size
| Characteristic | Cinacalcet Trial Participants |
Historical Controls |
P | ||
|---|---|---|---|---|---|
| Group S (n = 56) | Group L (n = 25) | Group HS (n = 65) | Group HL (n = 22) | ||
| Age (years) | 62 ± 11 | 66 ± 12 | 61 ± 10 | 60 ± 7 | 0.11 |
| Gender (%) | |||||
| men | 64 | 60 | 63 | 64 | 0.99 |
| women | 36 | 40 | 37 | 36 | |
| Duration of dialysis (months) | 194 ± 108 | 162 ± 79 | 204 ± 117 | 229 ± 89 | 0.18 |
| Primary cause of renal failure (%) | |||||
| glomerulonephritis | 63 | 80 | 60 | 86 | 0.35 |
| diabetes | 18 | 8 | 11 | 0 | |
| hypertension | 2 | 4 | 3 | 5 | |
| pyelonephritis | 4 | 4 | 0 | 0 | |
| polycystic kidney disease | 4 | 0 | 3 | 0 | |
| others | 7 | 4 | 17 | 9 | |
| unknown | 4 | 0 | 6 | 0 | |
| Use of vitamin D sterols (%) | 93 | 84 | 94 | 100 | 0.19 |
| maxacalcitol | 48 | 52 | 45 | 68 | 0.29 |
| calcitriol | 23 | 20 | 8 | 0 | 0.014 |
| oral vitamin D | 21 | 12 | 42 | 32 | 0.018 |
| Use of phosphate binders (%) | 91 | 96 | 88 | 86 | 0.62 |
| calcium carbonate | 59 | 40 | 82 | 64 | 0.001 |
| sevelamer hydrochloride | 68 | 72 | 49 | 36 | 0.015 |
| Dialysate calcium (%) | |||||
| 2.5 mEq/L | 62 | 52 | 23 | 5 | <0.001 |
| 3.0 mEq/L | 38 | 48 | 77 | 95 | |
| Intact PTH (pg/ml) | 508 ± 187 | 765 ± 468 | 554 ± 292 | 726 ± 270 | <0.001 |
| Serum calcium (mg/dl) | 9.7 ± 0.5 | 10.0 ± 0.5 | 9.3 ± 0.8 | 9.9 ± 0.3 | <0.001 |
| Serum phosphorus (mg/dl) | 6.0 ± 1.1 | 6.0 ± 1.6 | 6.3 ± 1.2 | 6.0 ± 1.2 | 0.55 |
| Parathyroid gland volume (mm3) | |||||
| total volume | 377 ± 240 | 1479 ± 1029 | 343 ± 268 | 1076 ± 349 | <0.001 |
| volume of the largest gland | 231 ± 130 | 1065 ± 1034 | 216 ± 154 | 667 ± 181 | <0.001 |
Plus-minus values are mean ± SD.
Ninety-six percent of patients in group S and 92% of those in group L completed the 12-week dose-titration phase; 91 and 80%, respectively, completed the 26-week maintenance phase; and 88 and 76%, respectively, completed the 14-week efficacy-assessment phase. Reasons for early discontinuation for patients in group S versus those in group L included the following: adverse events (4 versus 12%), administrative decisions (4 versus 8%), death (4 versus 0%), and loss to follow-up (2 versus 4%). None of the deaths were considered related to treatment.
Achievement of Intact PTH Targets
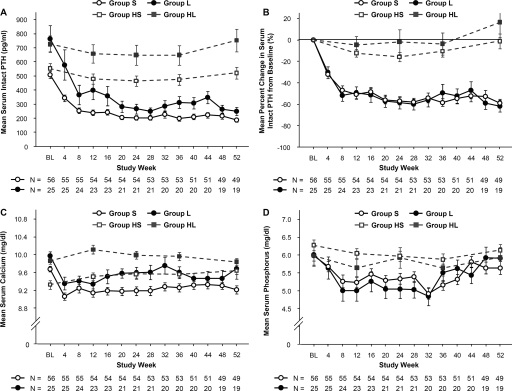
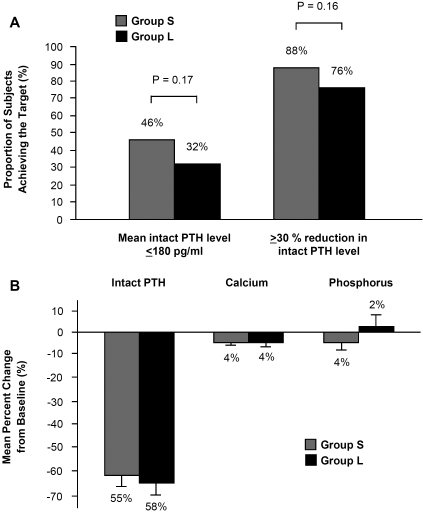
Mean intact PTH levels declined progressively both in group S and in group L during the first 8 weeks and reached a plateau by the end of the study (Figure 1A). During the efficacy-assessment phase, 46% of patients in group S and 32% of those in group L reached the primary end point of a mean intact PTH level <180 pg/ml; this difference was not statistically significant (P = 0.17; Figure 2A). The percent change in intact PTH levels was comparable between the two groups throughout the study (Figure 1B). During the efficacy-assessment phase, mean intact PTH decreased by 55% from baseline in group S and by 58% in group L (Figure 2B). The proportions of patients whose intact PTH levels decreased by >30% did not differ between the two groups (88 versus 76%; P = 0.16; Figure 2A). Logistic-regression analysis showed that the presence of one or more enlarged glands at baseline did not predict either the likelihood of achieving the primary end point (odds ratio, 0.54; 95% confidence interval, 0.20 to 1.46) or the secondary end point of a reduction in mean intact PTH level by >30% (odds ratio, 0.45; 95% confidence interval, 0.13 to 1.52).
Figure 1.
Mean (±SEM) serum intact PTH (A), mean (±SEM) percent change in the PTH level from baseline at each time point (B), mean (±SEM) serum calcium (C), and mean (±SEM) serum phosphorus (D). BL, baseline.
Figure 2.
(A) Proportion of patients with serum intact PTH <180 pg/ml or >30% reduction in the PTH level from baseline. (B) Mean (±SEM) percent change from baseline to assessment values for intact PTH, calcium, and phosphorus.
Other Biochemical Parameters
After cinacalcet initiation, mean calcium levels in both groups transiently declined and remained stable throughout the study, with slightly higher levels in group L than in group S (Figure 1C). Serum phosphorus levels decreased slightly during the first 8 weeks and gradually increased thereafter in both groups (Figure 1D). The percent changes in serum calcium and phosphorus levels from baseline during the efficacy-assessment phase were comparable between group S and group L (Figure 2B). The proportion of patients achieving the target calcium and phosphorus levels remained unchanged from baseline in both groups: 84% of patients in group S achieved the calcium target, compared with 56% in patients in group L (group S versus group L; P = 0.009), and 59% of patients in group S achieved the phosphorus target, compared with 60% in patients in group L (group S versus group L, P = 0.56). The proportion of subjects who achieved simultaneous control of PTH, calcium, and phosphorus was significantly higher in group S than in group L (23 versus 4%; P = 0.029).
Effect of Cinacalcet on Parathyroid Gland Volume
A total of 213 parathyroid glands were detected by ultrasonography at baseline. Among these, 179 glands in 65 patients were followed at 26-week intervals until the end of the study. Nine glands disappeared during the study. Overall, treatment with cinacalcet was associated with significant reductions in the total volume of each patient's glands: mean ± SD at baseline, week 26, and week 52 were 649 ± 490 mm3, 552 ± 468 mm3, and 527 ± 462 mm3, respectively (P = 0.003). The percent change from baseline at week 52 in total gland volume was significantly correlated with reduced intact PTH levels (r = 0.30, P = 0.02). There was also a significant correlation between the absolute change in total gland volume and the absolute change in intact PTH levels (r = 0.37, P = 0.002).
The volume of each parathyroid gland also decreased significantly during the study: mean ± SD at baseline, week 26, and week 52 were 236 ± 272 mm3, 200 ± 266 mm3, and 191 ± 252 mm3, respectively (P < 0.001). With stratification by baseline gland volume, significant volume reductions were observed both in glands with baseline volume >500 mm3 (780 ± 357 mm3, 627 ± 468 mm3, and 571 ± 453 mm3 at baseline, week 26, and week 52, respectively; P = 0.01) and in glands with baseline volume <500 mm3 (152 ± 116 mm3, 134 ± 128 mm3, and 132 ± 129 mm3 at baseline, week 26, and week 52, respectively; P = 0.02; Figure 3). At baseline, there was a significant correlation between total gland volume and intact PTH levels (r = 0.23, P = 0.04), but this correlation disappeared at the end of the study. Exclusion of the disappeared glands during the study did not change these results.
Figure 3.
Mean (±SEM) parathyroid gland volume, estimated by ultrasonography, before and after 26 and 52 weeks of treatment with cinacalcet, stratified according to the baseline gland volume. BL, baseline.
Medication Use
At the end of the study, 96% of subjects in group S and 89% of those in group L received daily doses of either 25 or 50 mg of cinacalcet. The mean ± SD dose of cinacalcet in group L was slightly, but not significantly, higher than that in group S (36.7 ± 14.5 mg/d versus 42.8 ± 20.9 mg/d; P = 0.19; Table 2). At baseline, the mean ± SD dose of intravenous vitamin D sterols, expressed as calcitriol equivalents, was significantly higher in group L compared with that in group S (2.18 ± 1.07 μg/d versus 3.06 ± 1.65 μg/d; P < 0.01), and the use and dose of vitamin D sterols remained relatively unchanged in both groups throughout the study. The use of calcium carbonate in group S increased gradually throughout the study, whereas such changes were transient among patients in group L. The dose of calcium carbonate remained constant in both groups. The proportion of patients receiving sevelamer hydrochloride remained constant in both groups during the study, with a slight decrease in the mean daily dose.
Table 2.
Use of cinacalcet hydrochloride and concomitant medications for secondary hyperparathyroidism
| Group S |
Group L |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 56) | Week 12 (n = 55) | Week 26 (n = 53) | Week 52 (n = 49) | Baseline (n = 25) | Week 12 (n = 24) | Week 26 (n = 21) | Week 52 (n = 19) | |
| Cinacalcet hydrochloride (mg/d) | 29.3 ± 9.9 | 32.7 ± 13.2 | 36.7 ± 14.5 | 28.7 ± 10.0 | 35.7 ± 19.9 | 42.8 ± 20.9 | ||
| Intravenous vitamin D sterols | ||||||||
| subjects (%) | 71 | 65 | 62 | 65 | 72 | 54 | 52 | 68 |
| calcitriol dose equivalentsa (μg/wk) | 2.18 ± 1.07 | 2.28 ± 1.16 | 2.32 ± 1.25 | 2.39 ± 1.33 | 3.06 ± 1.65 | 3.11 ± 1.37 | 2.86 ± 1.30 | 3.32 ± 2.00 |
| Oral vitamin D sterol use (%) | 21 | 25 | 25 | 29 | 12 | 21 | 29 | 32 |
| Calcium carbonate | ||||||||
| subjects (%) | 59 | 73 | 79 | 82 | 40 | 75 | 62 | 53 |
| calcium carbonate dose (mg/d) | 2506 ± 1067 | 2737 ± 1144 | 2779 ± 1370 | 2755 ± 1408 | 2030 ± 868 | 2044 ± 805 | 2023 ± 831 | 2150 ± 1156 |
| Sevelamer hydrochloride | ||||||||
| subjects (%) | 68 | 64 | 64 | 65 | 72 | 67 | 67 | 63 |
| sevelamer hydrochloride dose (mg/d) | 3026 ± 1048 | 3100 ± 980 | 2977 ± 1019 | 2930 ± 1069 | 3167 ± 1222 | 2878 ± 1224 | 2942 ± 1351 | 2625 ± 1517 |
Plus-minus values are mean ± SD.
1.5 μg of calcitriol = 10 μg of maxacalcitol.
Safety
A total of 39% of patients in group S and 52% of those in group L experienced at least one adverse event (Table 3). The most common adverse events were stomach discomfort, nausea, and vomiting, all of which tended to occur more frequently in group L than in group S. Gastrointestinal events were typically of mild to moderate severity, but 5% of patients discontinued cinacalcet use because of these adverse effects. Hypocalcemia (serum calcium <7.5 mg/dl) occurred exclusively in patients belonging to group S (7%), and one patient was withdrawn from the study. There were three deaths during the study, but none were attributed to cinacalcet treatment.
Table 3.
Adverse events in >3% of patients
| Events | All patients (n = 81), % | Group S (n = 56), % | Group L (n = 25), % |
|---|---|---|---|
| At least one adverse event | 43 | 39 | 52 |
| Stomach discomfort | 16 | 13 | 24 |
| Nausea | 9 | 4 | 20a |
| Vomiting | 5 | 2 | 12 |
| Diarrhea | 5 | 4 | 8 |
| Muscle cramp | 4 | 4 | 4 |
| Hypocalcemia | 4 | 7 | 0 |
P < 0.05 versus group S.
Comparison with Historical Controls
Mean intact PTH, calcium, and phosphorus levels in historical controls remained relatively unchanged during the retrospective period, both in group HS and in group HL (Figure 1). At the end of follow-up, only 8% of patients in group HS achieved an intact PTH <180 pg/ml whereas no patients achieved this in group HL.
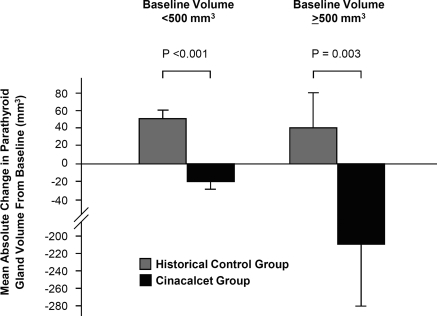
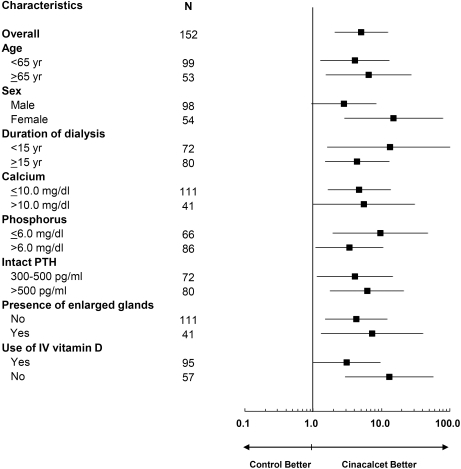
The mean ± SD volume of each parathyroid gland in historical controls increased significantly in glands with baseline volume <500 mm3 (133 ± 135 mm3 and 183 ± 229 mm3 at baseline and week 52, respectively; P < 0.001) and not significantly in glands with baseline volume >500 mm3 (655 ± 172 mm3 and 695 ± 275 mm3 at baseline and week 52, respectively; P = 0.31) during the 52-week observation period. These changes were in sharp contrast to those of cinacalcet trial participants in whom parathyroid gland volume progressively decreased regardless of pretreatment parathyroid size (group S versus group HS, P < 0.001; group L versus group HL, P = 0.003; Figure 4). The likelihood of achieving a >30% reduction in total gland volume was greater among cinacalcet-treated patients than historical controls (odds ratio, 5.05; 95% confidence interval, 2.07 to 12.31) and was consistent across subgroups stratified by age, sex, duration of dialysis, baseline biochemical variables, the presence of enlarged glands, or the use of intravenous vitamin D (Figure 5).
Figure 4.
Mean (±SEM) absolute change in parathyroid gland volume from baseline to week 52 in historical controls and cinacalcet trial participants.
Figure 5.
Stratified analysis of the likelihood of achieving a >30% reduction in total gland volume in cinacalcet-treated patients as compared with historical controls.
Discussion
In this multicenter, open-label study, treatment with cinacalcet effectively reduced serum PTH levels in hemodialysis patients with moderate to severe SHPT, even in those who had markedly enlarged parathyroid glands. This treatment resulted in a rapid decline in serum PTH levels by >50% in both groups with or without enlarged glands, and this response was sustained over 52 weeks. The proportion of patients who achieved intact PTH <180 pg/ml was slightly greater in group S compared with group L, but this was not statistically significant. We also observed a significant reduction in parathyroid gland volume during treatment with cinacalcet regardless of pretreatment size. Analysis of additional historical controls showed that in patients with SHPT the volume of parathyroid gland progressively increased with traditional therapy alone, which further highlights the effect of cinacalcet to induce a reduction in parathyroid gland volume.
It is well known that, in patients with SHPT, the presence of parathyroid enlargement is a major determinant of response to treatment with vitamin D sterols (11–13). This finding is attributed to the observation that most of the enlarged glands removed from severe SHPT patients exhibit a nodular pattern of hyperplasia and decreased VDR expression (6,9,10), which could render the parathyroid glands less responsive to the biologic actions of vitamin D. In contrast, the present study showed that treatment with cinacalcet, an allosteric activator of the CaSR, is effective in suppressing PTH secretion even in patients with enlarged glands, in which the expression of CaSR is likely to be markedly decreased (7–9). This is not, however, surprising because experimental studies have shown that CaSR signaling to suppress PTH secretion is largely preserved in vitro, even in the setting of reduced CaSR expression (32). Cinacalcet therapy has also been shown to upregulate the expressions of VDR (33) and CaSR (34), which may facilitate the inhibitory effects of vitamin D sterols and cinacalcet on PTH secretion in these patients.
In this study, we also observed a significant reduction in parathyroid gland volume concomitantly with PTH suppression during treatment with cinacalcet. Such a reduction in parathyroid gland volume has also been reported by Meola et al. (26). However, contrary to their results, the current study demonstrated that a reduction in parathyroid gland volume by cinacalcet could occur even in enlarged glands with a baseline volume larger than 500 mm3, further supporting the effectiveness of cinacalcet in advanced parathyroid hyperplasia. There are two possible mechanisms for the reduction in parathyroid gland volume during therapy with cinacalcet. One possibility is a reduction in cell volume in response to decreased demand for PTH synthesis. Support for this hypothesis was provided by an experimental study by Chin et al., which showed that, in uremic rats treated with calcimimetics, the reduction in parathyroid volume was attributable solely to a decrease in volume of the parathyroid cells (27). Although cell hypertrophy is considered to play only a minor role compared with cell proliferation in the pathogenesis of parathyroid enlargement (1), the observed degree of reduction in parathyroid size in the current study could be sufficiently explained by a reduction in cell hypertrophy. The other possibility is a reduction in cell number as a result of increased apoptosis. The effects of cinacalcet on parathyroid cell apoptosis, however, appear to be inconsistent depending on the experimental model. Mizobuchi et al. showed that high concentrations of calcimimetics induce apoptosis in parathyroid cells from uremic rats in vitro (34), whereas several investigators were not able to detect apoptotic parathyroid cells in vivo in calcimimetic-treated uremic rats (35,36). In clinical settings, the use of cinacalcet for treating SHPT has been linked with increased oxyphil/chief cell ratios (37) and cystic degeneration (26). The question of whether these morphologic changes were caused by the apoptosis of parathyroid cells is intriguing and worthy of further investigation.
The results of the current trial highlight the effectiveness of cinacalcet in patients with advanced parathyroid hyperplasia. This supports the notion that cinacalcet therapy can be an alternative to parathyroidectomy for these patients. However, it should be noted that, in our study, the proportion of subjects who achieved simultaneous control of PTH, calcium, and phosphorus were significantly lower in patients with enlarged glands compared with those without. Adverse gastrointestinal events also occurred more frequently in patients with enlarged glands. Given that some patients develop resistance to cinacalcet therapy and need to be referred for surgery (37,38), the observed difficulty in managing mineral metabolism and the limited use of cinacalcet because of safety concerns might imply future resistance to cinacalcet in these patients. Studies of much longer duration will be required to examine this possibility.
The strength of this study lies in its prospective design, long follow-up period, uniform data acquisition, and standardized ultrasonography protocol. Furthermore, it should be acknowledged that our study protocol permitted alteration of vitamin D dosage only on the grounds of patient safety, resulting in a relatively constant dosage of vitamin D sterols during the study period. This enabled us to isolate the effects of cinacalcet on parathyroid hyperplasia from those of vitamin D sterols. Our results, therefore, support the hypothesis that cinacalcet directly induces a reduction in parathyroid gland volume, although this effect might be partly mediated by cinacalcet-induced upregulation of parathyroid VDR (33).
There are, however, several limitations that should be considered. First, historical controls were used to evaluate the effect of cinacalcet on parathyroid gland volume. This could result in biases in patient selection and data collection, and thus it can be argued that certain baseline differences may have favored the cinacalcet-treated group. Second, the sample size was small, and the distribution of subjects between groups was unbalanced. These limitations may have resulted in low statistical power to detect differences between groups. Third, the study protocol was designed according to the JSDT guideline for the management of SHPT (30); thus, the cinacalcet dosage was increased to achieve intact PTH levels <180 pg/ml, a relatively lower value compared with that of previous studies (18–20) and the target range of the KDIGO guideline (39). Such aggressive suppression of PTH secretion might have facilitated the effect of cinacalcet on parathyroid gland volume in our study, but further studies are needed to examine whether treatment to achieve this level of PTH results in improved outcome. Finally, the evaluation of parathyroid gland volume was performed solely by neck ultrasonography. Interoperator coefficients of variation were not studied and are therefore unavailable. According to the reported sensitivity of ultrasonography in SHPT (40), a certain number of parathyroid glands, particularly small ones, might be missed by ultrasound study. It is also possible that a few patients with an ectopic, enlarged parathyroid gland were misclassified into group S or group HS.
Conclusions
Cinacalcet is effective in lowering serum PTH levels and concomitantly inducing a reduction in parathyroid gland volume in moderate to severe SHPT, even in patients with enlarged parathyroid glands. Whether the reduction in parathyroid gland volume induced by cinacalcet results in the long-term controllability of SHPT and an improvement in clinical outcomes warrants further investigation.
Disclosures
M.F. has received lecture fees and a grant from Kyowa Hakko Kirin Co., Ltd., Japan, and Chugai Pharmaceutical Co., Ltd., Japan, and was a member of the advisory committee on clinical trials of cinacalcet in Japan. The other authors have no conflicts of interests.
Acknowledgments
The authors thank the following investigators who participated in the study: Dr. Kazuko Itoh, Dr. Kazutaka Matsushita, and Dr. Makoto Sakai. The authors also thank Mr. Kensuke Moriwaki for assistance in the statistical analysis and Mr. Yasushi Shimizu and Ms. Chiemi Suzuki for their valuable technical assistance. This study was supported in part by grants from the ROD-21 Clinical Research Group (to S.N.) and The Kidney Foundation, Japan (JKFB08-29, to A.F.). Preliminary results of this study were presented in part at the World Congress of Nephrology in Milan on May 24, 2009.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Drueke TB: Cell biology of parathyroid gland hyperplasia in chronic renal failure. J Am Soc Nephrol 11: 1141–1152, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Goodman WG, Quarles LD: Development and progression of secondary hyperparathyroidism in chronic kidney disease: Lessons from molecular genetics. Kidney Int 74: 276–288, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Tominaga Y, Tanaka Y, Sato K, Nagasaka T, Takagi H: Histopathology, pathophysiology, and indications for surgical treatment of renal hyperparathyroidism. Semin Surg Oncol 13: 78–86, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Fukagawa M, Nakanishi S, Kazama JJ: Basic and clinical aspects of parathyroid hyperplasia in chronic kidney disease. Kidney Int 70[suppl 102]: S3–S7, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GFM: Meta-analysis: Vitamin D compounds in chronic kidney disease. Ann Intern Med 147: 840–853, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Fukuda N, Tanaka H, Tominaga Y, Fukagawa M, Kurokawa K, Seino Y: Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest 92: 1436–1442, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kifor O, Moore FD, Jr., Wang P, Goldstein M, Vassilev P, Kifor I, Hebert SC, Brown EM: Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab 81: 1598–1606, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E, Drüeke TB: Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int 51: 328–336, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Yano S, Sugimoto T, Tsukamoto T, Chihara K, Kobayashi A, Kitazawa S, Maeda S, Kitazawa R: Association of decreased calcium-sensing receptor expression with proliferation of parathyroid cells in secondary hyperparathyroidism. Kidney Int 58: 1980–1986, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Tokumoto M, Tsuruya K, Fukuda K, Kanai H, Kuroki S, Hirakata H, Iida M: Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int 62: 1196–1207, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Fukagawa M, Kitaoka M, Yi H, Fukuda N, Matsumoto T, Ogata E, Kurokawa K: Serial evaluation of parathyroid size by ultrasonography is another useful marker for the long-term prognosis of calcitriol pulse therapy in chronic dialysis patient. Nephron 68: 221–228, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Okuno S, Ishimura E, Kitatani K, Chou H, Nagasue K, Maekawa K, Izumotani T, Yamakawa T, Imanishi Y, Shoji T, Inaba M, Nishizawa Y: Relationship between parathyroid gland size and responsiveness to maxacalcitol therapy in patients with secondary hyperparathyroidism. Nephrol Dial Transplant 18: 2613–2621, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Tominaga Y, Inaguma D, Matsuoka S, Tahara H, Kukita K, Kurihara S, Onoda N, Tsuruta Y, Tsutsui S, Ohta K, Kuwahara M, Tanaka M, Nishizawa Y: Is the volume of the parathyroid gland a predictor of Maxacalcitol response in advanced secondary hyperparathyroidism? Ther Apher Dial 10: 198–204, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Fukagawa M, Okazaki R, Takano K, Kaname S, Ogata E, Kitaoka M, Harada S, Sekine N, Matsumoto T, Kurokawa K: Regression of parathyroid hyperplasia by calcitriol-pulse therapy in patients on long-term dialysis. N Engl J Med 323: 421–422, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Quarles LD, Yohay DA, Carroll BA, Spritzer CE, Minda SA, Bartholomay D, Lobaugh BA: Prospective trial of pulse oral versus intravenous calcitriol treatment of hyperparathyroidism in ESRD. Kidney Int 45: 1710–1721, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Goodman WG, Hladik GA, Turner SA, Blaisdell PW, Goodkin DA, Liu W, Barri YM, Cohen RM, Coburn JW: The calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol 13: 1017–1024, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Quarles LD, Sherrard DJ, Adler S, Rosansky SJ, McCary LC, Liu W, Turner SA, Bushinsky DA: The calcimimetic AMG 073 as a potential treatment for secondary hyperparathyroidism of end-stage renal disease. J Am Soc Nephrol 14: 575–583, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Block GA, Martin KJ, de Francisco ALM, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drueke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, Roger SD, Husserl FE, Klassen PS, Guo MD, Albizem MB, Coburn JW: Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: A randomized double-blind, multicenter study. J Am Soc Nephrol 16: 800–807, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Fukagawa M, Yumita S, Akizawa T, Uchida E, Tsukamoto Y, Iwasaki M, Koshikawa S: Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant 23: 328–335, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Komaba H, Fukagawa M: Impact of cinacalcet hydrochloride on the achievement of the Japanese Society for Dialysis Therapy (JSDT) guideline targets: A post-hoc analysis of the KRN1493 study. Ther Apher Dial 12[Suppl 1]: S44–S49, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Block GA, Zeig S, Sugihara J, Chertow GM, Chi EM, Turner SA, Bushinsky DA: Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrol Dial Transplant 23: 2311–2318, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Fishbane S, Shapiro WB, Corry DB, Vicks SL, Roppolo M, Rappaport K, Ling X, Goodman WG, Turner S, Charytan C: Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: The ACHIEVE study results. Clin J Am Soc Nephrol 3: 1718–1725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Messa P, Macário F, Yaqoob M, Bouman K, Braun J, von Albertini B, Brink H, Maduell F, Graf H, Frazão JM, Bos WJ, Torregrosa V, Saha H, Reichel H, Wilkie M, Zani VJ, Molemans B, Carter D, Locatelli F: The OPTIMA study: Assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol 3: 36–45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanaka M, Nakanishi S, Komaba H, Itoh K, Matsushita K, Fukagawa M: Association between long-term efficacy of cinacalcet and parathyroid gland volume in haemodialysis patients with secondary hyperparathyroidism. NDT Plus 1: iii49–iii53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meola M, Petrucci I, Barsotti G: Long-term treatment with cinacalcet and conventional therapy reduces parathyroid hyperplasia in severe secondary hyperparathyroidism. Nephrol Dial Transplant 24: 982–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chin J, Miller SC, Wada M, Nagano N, Nemeth EF, Fox J: Activation of the calcium receptor by a calcimimetic compound halts the progression of secondary hyperparathyroidism in uremic rats. J Am Soc Nephrol 11: 903–911, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Drueke TB, Martin D, Rodriguez M: Can calcimimetics inhibit parathyroid hyperplasia? Evidence from preclinical studies. Nephrol Dial Transplant 22: 1828–1839, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Komaba H, Fukagawa M: Regression of parathyroid hyperplasia by calcimimetics—fact or illusion? Nephrol Dial Transplant 24: 707–709, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Guideline Working Group, Japanese Society for Dialysis Therapy: Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial 12: 514–525, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Payne RB, Little AJ, Williams RB, Milner JR: Interpretation of serum calcium levels in patients with abnormal serum proteins. BMJ 4: 643–646, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawata T, Imanishi Y, Kobayashi K, Onoda N, Okuno S, Takemoto Y, Komo T, Tahara H, Wada M, Nagano N, Ishimura E, Miki T, Ishikawa T, Inaba M, Nishizawa Y: Direct in vitro evidence of the suppressive effect of cinacalcet HCl on parathyroid hormone secretion in human parathyroid cells with pathologically reduced calcium-sensing receptor levels. J Bone Miner Metab 24: 300–306, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez ME, Almaden Y, Cañadillas S, Canalejo A, Siendones E, Lopez I, Aguilera-Tejero E, Martin D, Rodriguez M: The calcimimetic R-568 increases vitamin D receptor expression in rat parathyroid glands. Am J Physiol Renal Physiol 292: F1390–F1395, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Mizobuchi M, Hatamura I, Ogata H, Saji F, Uda S, Shiizaki K, Sakaguchi T, Negi S, Kinugasa E, Koshikawa S, Akizawa T: Calcimimetic compound upregulates decreased calcium-sensing receptor expression level in parathyroid glands of rats with chronic renal insufficiency. J Am Soc Nephrol 15: 2579–2587, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Wada M, Furuya Y, Sakiyama J, Kobayashi N, Miyata S, Ishii H, Nagano N: The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. Control of parathyroid cell growth via a calcium receptor. J Clin Invest 100: 2977–2983, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Colloton M, Shatzen E, Miller G, Stehman-Breen C, Wada M, Lacey D, Martin D: Cinacalcet HCl attenuates parathyroid hyperplasia in a rat model of secondary hyperparathyroidism. Kidney Int 67: 467–476, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Lomonte C, Vernaglione L, Chimienti D, Bruno A, Cocola S, Teutonico A, Cazzato F, Basile C: Does vitamin D receptor and calcium receptor activation therapy play a role in the histopathologic alterations of parathyroid glands in refractory uremic hyperparathyroidism? Clin J Am Soc Nephrol 3: 794–799, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cunningham J, Danese M, Olson K, Klassen P, Chertow GM: Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int 68: 1793–1800, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int 76[Suppl 113]: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Vulpio C, Bossola M, De Gaetano A, Maresca G, Bruno I, Fadda G, Morassi F, Magalini SC, Giordano A, Castagneto M. Usefulness of the combination of ultrasonography and 99mTc-sestamibi scintigraphy in the preoperative evaluation of uremic secondary hyperparathyroidism. Head Neck, in press [DOI] [PubMed] [Google Scholar]