Abstract
Background and objectives: Immunosuppressive medications are essential in preventing kidney transplant rejection. Continuous insurance coverage for outpatient immunosuppressive medications remains a major issue. The objective of this study was to establish the prevalence and consequences of cost-related immunosuppressive medication nonadherence.
Design, setting, participants, & measurements: A descriptive survey of all U.S. kidney transplant programs (n = 254) was conducted. The response rate for the survey exceeded 99%. The main outcome measures included the following: transplant recipient concerns related to medication costs, ability to pay for medications, medication nonadherence and its consequences, and failure of transplant centers to place patients on the transplant waiting list.
Results: Continuous insurance coverage for outpatient immunosuppressive drugs is a problem having potentially grave consequences for the majority of kidney transplant recipients. More than 70% of kidney transplant programs report that their patients have an extremely or very serious problem paying for their medications. About 47% of the programs indicate that more than 40% of their patients are having difficulty paying for their immunosuppressive medications. In turn, 68% of the programs report deaths and graft losses attributable to cost-related immunosuppressive medication nonadherence. Some of the problems identified here are more significant for adult than pediatric patients.
Conclusions: The prevalence and consequences of cost-related immunosuppressive medication nonadherence among kidney transplant recipients have now been documented. The results presented here should serve as the necessary impetus for the development of health care policies supporting Medicare coverage of immunosuppressive medications for the life of the transplanted kidney.
For over a quarter century, insurance coverage and reimbursement for outpatient immunosuppressive medications has been a serious issue for kidney transplant recipients (1–15). Initial concerns were raised in relationship to the cost of cyclosporine after Food and Drug Administration approval in 1983 and were subsequently addressed by the Task Force on Organ Transplantation in 1985 (1,2).
Between 1986 and 2000, modest changes were made in Medicare coverage and reimbursement policies pertaining to immunosuppressive medications (7–12). At this time, Medicare covers 80% of the cost of immunosuppressive medications for 36 months after transplantation (for those whose Medicare entitlement is on the basis of end-stage renal disease). In 1999, Congress authorized an extension of Medicare coverage for immunosuppressive medication to lifetime, but only for a minority of patients: those who were eligible for Medicare because of age or disability (10,11).
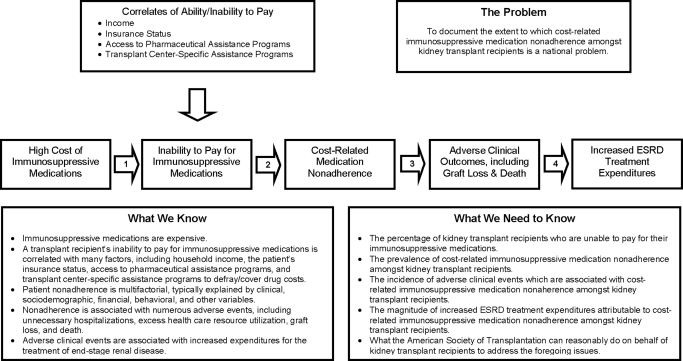
Over the past several years, immunosuppressive drug coverage legislation has again been introduced (16,17). Figure 1 captures the essence of the public policy dilemma Congress has faced.
Figure 1.
Causes, correlates, and consequences of cost-related immunosuppressive medication nonadherence.
Neither kidney transplantation nor dialysis cures ESRD. Kidney transplantation is, however, more cost-effective than maintenance dialysis yet, because some patients are unable to afford their immunosuppressive medications, premature and avoidable graft loss occurs (1,2,10,11,18–21). Therefore, compelling arguments have been made for lifetime coverage of immunosuppressive medications (7,8,10–13,15).
Whereas the economic implications of graft loss have been well-documented, virtually no nationally representative contemporary data have been available to characterize the prevalence of immunosuppressive medication-related problems that kidney-transplant recipients experience (7–11,22–25). Consequently, the efforts of legislators to expand Medicare coverage for immunosuppressive medications have remained unpersuasive.
The American Society of Transplantation, in cooperation with United Network for Organ Sharing (UNOS) and the North American Pediatric Renal Trials and Collaborative Studies, undertook a brief descriptive survey of transplant centers in hopes of establishing the scope and magnitude of the problem with which Congressional leaders have grappled in formulating and passing legislation to address it.
Materials and Methods
The American Society of Transplantation Public Policy Committee took the lead in formulating the survey procedures and the questionnaire, recognizing that nonadherence is a complex multidimensional concept. Thus, for purposes of the survey, nonadherence was defined as patients not taking their maintenance immunosuppressive drugs as prescribed because of difficulties associated with their ability to pay for their medications.
From the outset, the committee's primary goal was to maximize survey participation. This entailed two strategic decisions. First, it was decided that the questionnaire should consist of no more than ten to 15 questions specifically focused on the problem of cost-related immunosuppressive medication nonadherence. Second, it was felt the survey should be descriptive as opposed to explanatory. The committee concluded that subsequent, more detailed surveys should be undertaken in hopes of better understanding preliminary descriptive results.
A survey instrument consisting of 12 closed-ended questions was drafted and subjected to a pretest. The survey procedures were then pilot tested. Few revisions were necessary. The survey completion process was coordinated by the transplant administrators associated with all 254 UNOS-approved transplant centers in the United States. In other words, the survey was not on the basis of a sample of centers. Instead, all transplant centers were asked to participate.
The committee concluded that no single individual was in a position to answer all of the survey questions. Therefore, as deemed appropriate, the transplant administrators were asked to obtain input from transplant physicians, transplant surgeons, social workers, financial coordinators, nurses, and other transplant team members. Those individuals most knowledgeable about each issue were asked to respond accordingly, with the transplant administrator collating and submitting the responses.
An online, commercially available Web-based survey package, SurveyMonkey, was used to create and distribute the survey (26). Survey respondents were provided with a web address that enabled them to access and complete the survey.
To facilitate the survey process, portable document format (PDF) copies of the questionnaire were e-mailed to the transplant administrators, as well as the medical and surgical directors of all kidney transplant programs. This permitted the respondents to distribute and complete a paper copy of the survey as a group effort. Once the data were compiled, the transplant administrators were advised to have a single individual input the responses into SurveyMonkey (26).
The survey materials were distributed by UNOS on June 12, 2009, with an initial deadline of June 26, 2009. The survey deadline was subsequently extended to July 3, 2009, at which time over 99% of the kidney transplant programs had completed the survey. This is exceptional given that a 30% response rate is typical for online surveys (27).
Results
Thirty-one percent of the participating programs were adult (i.e., they only transplanted persons 18 years of age and older). Four percent qualified as pediatric (i.e., they only transplanted patients younger than 18 years of age). The remaining 65% were mixed (i.e., they transplanted both adults and children).
There was considerable variation in the number of transplant recipients followed long-term, with 53.2% of all programs following less than 500 patients. No purely pediatric program followed more than 200 patients, whereas 16.2% of the purely adult programs followed more than 1000 patients. No adult program followed more than 3000 patients, whereas 7% of the mixed programs did so.
The high cost of immunosuppressive drugs is clearly an issue. Over 83% of kidney transplant programs reported that patients frequently contact them with concerns about the high cost of their immunosuppressive medications, although these concerns are more frequently expressed at adult than pediatric programs (86.7% versus 66.7%).
Over 70% of all programs indicate that more than 20% of their patients are encountering difficulties in their ability to pay for their immunosuppressive medications, with the problem being more prevalent at adult than pediatric programs (74.6% versus 55.5%). Over 94% of the programs describe the level of difficulty patients encounter when paying for their immunosuppressive drugs as serious or worse, although the problem is less pronounced at pediatric programs (96.0% versus 88.8%).
The cost of immunosuppressive medications clearly has implications regarding which patients get placed on the kidney transplant waiting list. Overall, 67.3% of the programs indicate that they frequently or occasionally fail to place patients on the waiting list because they may be unable to afford their immunosuppressive medications. This is a much larger issue at adult than pediatric programs (70.7% versus 11.1%).
Patient nonadherence to immunosuppressive medications is related to cost and ability to pay (Table 1). Over 43% of all programs report that more than 10% of their patients are not taking their immunosuppressive drugs as prescribed because of difficulties associated with their ability to pay for them. Yet again, this is a more significant issue at adult than at pediatric programs (46.6% versus 11.1%).
Table 1.
Patients not taking their immunosuppressive drugs as prescribed due to difficulty paying for them
| Percentage of Patients | All Programs (%) | Adult Programs (%) | Pediatric Programs (%) |
|---|---|---|---|
| 0 to 5% | 29.3 | 28.0 | 77.8 |
| 6 to 10% | 26.7 | 25.3 | 11.1 |
| 11 to 20% | 26.7 | 32.0 | 0.0 |
| 21 to 40% | 13.3 | 9.3 | 11.1 |
| 41 to 60% | 3.1 | 4.0 | 0.0 |
| 61 to 80% | 0.4 | 1.3 | 0.0 |
| 81% or more | 0.4 | 0.0 | 0.0 |
The survey question was: What percentage of kidney transplant recipients followed at your center are not taking their maintenance immunosuppressive drugs as prescribed because of difficulties associated with their ability to pay for their medications?
The failure of patients to take immunosuppressive drugs as prescribed can have devastating consequences (7–13,15,22–25). However, whereas over 68% of the participating centers reported deaths and graft losses directly attributable to cost-related immunosuppressive medication nonadherence, neither of these problems were reported by the pediatric only programs.
The loss of insurance coverage for immunosuppressive medications at 3 years post-transplant is troubling (7,8,10–12). All of the problems described above were found to be more significant among patients who had lost coverage (Table 2). With the exception of deaths and graft losses, the findings followed a similar pattern at pediatric and adult programs. However, the pediatric percentages were smaller, implying that the problems were not as serious.
Table 2.
Prevalence of various problems after the loss of Medicare coverage for immunosuppressive medications at 3 years post-transplant
| Problems | All Programs (%) | Adult Programs (%) | Pediatric Programs (%) |
|---|---|---|---|
| Complaints about the “high cost” of immunosuppressive drugs | 75.0 | 84.0 | 75.0 |
| Difficulty paying for immunosuppressive drugs | 87.3 | 88.0 | 62.5 |
| Failure to take immunosuppressive drugs as prescribed | 64.5 | 69.3 | 37.5 |
| Deaths or graft losses due to cost-related immunosuppressive medication nonadherence | 49.5 | 50.7 | 0.0 |
| In our opinion, none of the foregoing problems are more prevalent | 6.8 | 5.3 | 12.5 |
The survey question was: Of the problems mentioned in this survey, which of the following, if any, appear to be more prevalent amongst kidney transplant recipients who have lost their Medicare coverage for immunosuppressive medications at 3 years post-transplant?
Insurance coverage for immunosuppressive medications is available through a patchwork of public and private sources, with many patients having more than a single source of coverage (Table 3). There are noteworthy differences between adult and pediatric programs.
Table 3.
Sources of coverage for maintenance immunosuppressive drugs/medications
| Source of Coverage | All Programs (%) | Adult Programs (%) | Pediatric Programs (%) |
|---|---|---|---|
| Medicare | 63.9 | 68.5 | 34.0 |
| Medicaid | 30.2 | 29.8 | 42.3 |
| Private insurance | 30.3 | 29.0 | 31.0 |
| Self-funded (out-of-pocket) | 6.4 | 5.6 | 1.7 |
| Military program | 4.5 | 4.5 | 1.0 |
| Other state health insurance program | 11.8 | 14.3 | 42.4 |
| Pharmaceutical assistance program (company-sponsored) | 14.1 | 11.8 | 11.0 |
| Complete write-off (no formal coordinated assistance program) | 1.9 | 1.2 | 0.0 |
| Another source of payment not listed here | 6.3 | 5.7 | 1.3 |
The survey question was: What percentage of kidney transplant recipients followed at your center have their maintenance immunosuppressive drugs/medications paid for by any of the following sources? (Patients may have more than one source of coverage.) Because patients may have more than one source of coverage, the cumulative percentages shown in the table exceed 100%.
Among all programs, Medicare was the major source of coverage (63.9%), followed by private insurance (30.3%), and Medicaid (30.2%). Transplant programs reported that they essentially wrote off immunosuppressive medications costs for approximately 2% of their patients because they had no source of coverage.
Pediatric programs reported that patients were most likely covered by other state health insurance programs (42.4%), Medicaid (42.3%), Medicare (34.0%), and private insurance (31.0%). Meanwhile, the major sources of coverage reported by adult programs were as follows: Medicare (68.5%), Medicaid (29.8%), and private insurance (29.0%).
Discussion
This is the first systematic, quantitative, national survey conducted to ascertain the prevalence and consequences of cost-related immunosuppressive medication nonadherence among kidney transplant recipients. It has established critical parameters where none have previously existed.
The coverage-related problems identified here affect the vast majority of kidney transplant recipients. Fortunately, some progress has been made since 1985, when the Task Force on Organ Transplantation concluded that 25% of kidney transplant recipients essentially had no insurance coverage for immunosuppressive medications (1,2).
Today, well over 100,000 people are living with a functioning kidney transplant (28,29). Given the results of this survey, a large number of these individuals are at risk for cost-related immunosuppressive medication nonadherence, potentially leading to death and graft loss. In addition, otherwise eligible patients are not being placed on the kidney transplant waiting list. These problems are identical to those identified by Congress when it passed the National Organ Transplantation Act in 1984 and by the Institute of Medicine in its comprehensive analysis of the Medicare End-Stage Renal Disease Program in 1991 (7,8,30).
The findings of the survey further indicate that some of the problems related to insurance coverage for immunosuppressive medications are more significant for adult than for pediatric patients. This may be due to the fact that, historically, state-level programs have evolved to better meet the needs of children relative to adults (31). However, given prevailing economic circumstances and systemic fiscal distress at both the federal and the state levels, at least some of these programs are now in jeopardy, potentially having the same grave consequences for children that adult patients have experienced.
The economic sequelae of graft losses have been addressed and underscored in a variety of contexts, including Congressional hearings (7–12,15). When a kidney transplant fails, there are three possible outcomes: death, return to dialysis, or retransplantation. As one might expect, death is typically the least expensive outcome, because no further treatment costs are incurred. In reality, however, most graft failure patients eventually return to dialysis or are retransplanted, both of which have staggering adverse economic consequences, consequences that could have otherwise been avoided with adequate coverage for immunosuppressive medications.
In the year a kidney transplant recipient's graft fails, third party payers experience an average annual expense of $82,765 (32). If the patient returns to dialysis, the average annual expense is $70,581, and, if the patient is retransplanted, the average cost is $106,373. However, annual third party reimbursements for a patient who has a functioning kidney transplant average $16,844, making transplantation the most economically desirable long-term option.
Furthermore, the foregoing figures do not take into account other remarkable differences between dialysis and transplantation. For example, on virtually every dimension that has been quantified, including quality of life, morbidity, health services utilization, return to work in adults, and cognitive development and growth in children, successful kidney transplantation is the superior treatment modality for end-stage kidney disease (33–39).
Thus, on the basis of the expenses incurred and the outcomes achieved, kidney transplantation is far more cost-effective than kidney dialysis, making it the treatment modality preferred by the majority of end-stage renal disease patients, their families, and their physicians (7–11,18–20). Unfortunately, long-standing perverse Medicare coverage policies associated with immunosuppressive medications not only jeopardize patient lives, they seriously compromise the cost-effective delivery of health care to patients with kidney failure (7–11).
Given the foregoing, the results presented here indicate that current Medicare coverage policy as it pertains to outpatient immunosuppressive medications should be changed. Unfortunately, this did not occur with the recent passage of the Patient Protection and Affordable Care Act. Nonetheless, Congressional leaders are fully aware of the situation and continue to valiantly and persistently struggle to pass legislation intended to align patient interests with clinical practice in an effort to meet societal objectives (16,17). Whereas efforts thus far have failed, it is not due to a lack of effort but rather because the prevalence and consequences of cost-related medication nonadherence among kidney transplant recipients have never been definitively established. The scope of the problem has heretofore been elusive. The results presented here eliminate this knowledge gap, are extremely persuasive, and should serve as the necessary impetus for the development of health care policies supporting Medicare coverage of immunosuppressive medications for the life of the transplanted kidney.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Task Force on Organ Transplantation: Report to the Secretary and the Congress on Immunosuppressive Therapies. Rockville, MD, Office of Organ Transplantation, Health Resources and Services Administration, Department of Health and Human Services, October 1985 [Google Scholar]
- 2. Task Force on Organ Transplantation: Organ Transplantation: Issues and Recommendations. Rockville, MD, Office of Organ Transplantation, Health Resources and Services Administration, Department of Health and Human Services, April 1986 [Google Scholar]
- 3. Kolata G: Drug transforms transplant medicine. Science 221: 40–42, 1983 [DOI] [PubMed] [Google Scholar]
- 4. Macek C: Cyclosporine's acceptance heralds new era in immunpharmacology. JAMA 250: 449–455, 1983 [DOI] [PubMed] [Google Scholar]
- 5. Evans RW: Kidney transplantation and cyclosporine. N Engl J Med 311: 127, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Evans RW, Manninen DL: Economic impact of cyclosporine in transplantation. Transplant Proc 20[Suppl 3]: 49–62, 1988 [PubMed] [Google Scholar]
- 7. Rettig RA, Levinsky NG. eds: Kidney Failure and the Federal Government, Washington, D.C., National Academy Press, 1991 [PubMed] [Google Scholar]
- 8. Levinsky NB, Rettig RA: The Medicare End-Stage Renal Disease Program: A report from the Institute of Medicine. N Engl J Med 324: 1143–1148, 1991 [DOI] [PubMed] [Google Scholar]
- 9. U.S. Congress, Office of Technology Assessment: Outpatient Immunosuppressive Drugs Under Medicare. Report No. OTA-H-452 Washington, D.C., U.S. Government Printing Office, September 1991 [Google Scholar]
- 10. Kasiske BL, Cohen D, Lucey MR, Neylan JF: Payment for immunosuppression after organ transplantation. JAMA 283: 2445–2450, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Field MJ, Lawrence RL, Zwanziger L. eds: Extending Medicare Coverage for Preventive and Other Services, Washington, D.C., National Academy Press, 2000 [PubMed] [Google Scholar]
- 12. Ekstrand L: End-Stage Renal Disease: Characteristics of Kidney Transplant Recipients, Frequency of Transplant Failures, and Cost to Medicare. Report No. GAO-07-1117 Washington, D.C., United States Government Accountability Office, 2007 [Google Scholar]
- 13. Bennett WM: Extension of Medicare immunosuppressive drug benefits: A “nobrainer” that did not happen. Clin J Am Soc Nephrol 5: 743, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Becker BN: A conflict of responsibility: No patient left behind. Clin J Am Soc Nephrol 5: 744–745, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Cohen DJ, Murphy B: Drug coverage for transplantation turns into political football: Big business trumps patients. Clin J Am Soc Nephrol 5: 746–747, 2010 [DOI] [PubMed] [Google Scholar]
- 16. S. 565 Comprehensive Immunosuppressive Drug Coverage for Kidney Transplant Patients Act of 2009 (Introduced in the Senate). March 10, 2009. Available at: http://thomas.loc.gov/ and enter Bill No. S. 565 Accessed March 13, 2010
- 17. H.R. 1458 Comprehensive Immunosuppressive Drug Coverage for Kidney Transplant Patients Act of 2009 (Introduced in the House). March 12, 2009. Available at http://thomas.loc.gov/ and enter Bill No. H.R. 1458 Accessed March 13, 2010
- 18. Evans RW, Kitzmann DJ: An economic analysis of kidney transplantation. Surg Clin North Am 78: 149–174, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Eggers P: Comparison of treatment costs between dialysis and transplantation. Semin Nephrol 12: 284–289, 1992 [PubMed] [Google Scholar]
- 20. Perovic S, Jankovic S: Renal transplantation vs hemodialysis: Cost-effectiveness analysis. Vojnosanit Pregl 66: 639–644, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Klarenbach S, Manns B: Economic evaluation of dialysis therapies. Semin Nephrol 29: 524–532, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Paris W, Dunham S, Sebastian A, Jacobs C, Nour B: Medication nonadherence and its relation to financial restriction. J Transpl Coord 9: 149–152, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Butkus DE, Dottes AL, Meydrech EF, Barber WH: Effect of poverty and other socioeconomic variables on renal allograft survival. Transplantation 72: 261–266, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Rodrigue JR, Reed AI, Nelson DR, Jamieson I, Kaplan B, Howard RJ: The financial burden of transplantation: a single-center survey of liver and kidney transplant recipients. Transplantation 84: 295–300, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Hansen R, Seifeldin R, Noe L: Medication adherence in chronic disease: Issues in posttransplant immunosuppression. Transplant Proc 39: 1287–1300, 2007 [DOI] [PubMed] [Google Scholar]
- 26. The SurveyMonkey Web Site Home page. Available at: http://www.SurveyMonkey.com Accessed on January 31, 2010
- 27. Instructional Assessment Resources: Survey response rates. Available at: http://www.utexas.edu/academic/diia/assessment/iar/teaching/gather/method/survey-Response.php?task=research Accessed January 31, 2010
- 28. Table 5.16 Prevalence of People Living with a Functioning Transplant at End of Year: Kidney Recipients, 1998 to 2006. OPTN/SRTR Annual Data Report, 2008. Available at http://www.ustransplant.org/annual_reports/current/516_ki.pdf Accessed January 31, 2010
- 29. U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009; Volume Three Reference Tables on ESRD in the United States, page 518, Table D 9 [Google Scholar]
- 30. National Organ Transplantation Act Public Law 98-507, October 19, 1984 [Google Scholar]
- 31. Iglehart JK: Expanding coverage for children: The Democrats' power and SCHIP reauthorization. N Engl J Med 360: 855–857, 2009 [DOI] [PubMed] [Google Scholar]
- 32. U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009; Volume Three Reference Tables on ESRD in the United States, pages 737–745, Tables K. 4-K 12 [Google Scholar]
- 33. Evans RW, Manninen DL, Garrison LP, Jr., Hart LG, Blagg CR, Gutman RA, Hull AR, Lowrie EG: The quality of life of patients with end-stage renal disease. N Engl J Med 312: 553–559, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Evans RW, Manninen DL, Garrison LP, Jr., Hart LG: Special Report: Finding from the National Kidney Dialysis and Kidney Transplantation Study. HCFA Publication No. 03230 Baltimore, MD, Health Care Financing Administration, 1987 [Google Scholar]
- 35. Joseph JT, Baines LS, Morris MC, Jindal RM: Quality of life after kidney and pancreas transplantation: A review. Am J Kidney Dis 42: 431–445, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Fine RN: Growth following solid-organ transplantation. Pediatr Transplant 6: 47–52, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Goldstein SL, Gerson AC, Furth S: Health-related quality of life for children with chronic kidney disease. Adv Chronic Kidney Dis 14: 364–369, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Burra P, De Bona M: Quality of life following organ transplantation. Transpl Int 20: 397–409, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Hamiwka LA, Cantell M, Crawford S, Clark CG: Physical activity and health related quality of life in children following kidney transplantation. Pediatr Transplant 13: 861–867, 2009 [DOI] [PubMed] [Google Scholar]