Abstract
OSA is a treatable sleep disorder that is pervasive among overweight and obese individuals. Current evidence supports a robust association between OSA and insulin resistance, glucose intolerance and the risk of type 2 diabetes, independent of obesity. Up to 83% of patients with type 2 diabetes suffer from unrecognized OSA and increasing severity of OSA is independently associated with poorer glucose control. Evidence from animal and human models that mimic OSA supports a potential causal role for OSA in altered glucose metabolism. Robust prospective and randomized clinical trials are still needed to test the hypothesis that effective treatment of OSA may prevent the development of type 2 diabetes and its complications, or reduce its severity. Type 2 diabetes is occurring at alarming rates worldwide and despite available treatment options, the economic and public health burden of this epidemic remains enormous. OSA might represent a novel, modifiable risk factor for the development of prediabetes and type 2 diabetes.
Keywords: Obstructive sleep apnea, diabetes, prediabetes, glucose tolerance, insulin resistance, CPAP, cardiovascular, metabolic, glycemic control
Obstructive sleep apnea (OSA) is a chronic sleep disorder, affecting 24% of men and 9% of women in the general population [1]. OSA prevalence appears to increase steadily with advancing age and men are at 2-3 fold greater risk for OSA compared to women [2]. Notably, OSA is pervasive among overweight and obese individuals, who represent about two thirds of the U.S. adult population today. Recent estimates of the prevalence of OSA in obese adults aged 30 to 69 years have ranged from 11 to 46% in women and 33 to 77% in men [3]. In longitudinal analyses, weight gain predicts increased OSA incidence and severity [4] [5].
Despite the demonstrated efficacy of lifestyle interventions and the availability of multiple pharmacological treatment options, the economic and public health burden of diabetes remains enormous [6]. Prediabetes is defined by elevated glucose levels not sufficient to meet the diagnostic criteria for diabetes. Specifically, prediabetes refers to either impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) in response to oral glucose [7]. Both IFG and IGT represent an insulin resistant state and are major risk factors for progression to overt diabetes [8]. Both Type 2 diabetes and prediabetes are associated with increased risk for cardiovascular disease [8-10].
OSA is well recognized as an important mediator of adverse cardiovascular outcomes [11-13]. Over the past few years, there has been a growing interest in the adverse metabolic consequences associated with OSA [14-18]. Numerous studies have shown robust associations between OSA and insulin resistance, glucose intolerance and type 2 diabetes, independently of obesity. In this article, we will review the current evidence that links OSA to type 2 diabetes and prediabetes, and summarize the studies on the impact of treatment of OSA on glucose metabolism. We will also briefly discuss the potential mechanisms by which OSA may contribute to the development of insulin resistance and glucose intolerance.
1. OSA: Definition, Diagnosis and Treatment
OSA is characterized by repetitive upper airway closures or partial collapses that occur during sleep. These respiratory disturbances have two main consequences: 1) intermittent hypoxia which is characterized by cyclic hypoxic episodes alternating with periods of normoxia 2) transient arousals which restore airflow but lead to sleep fragmentation and poor sleep quality. Clinical characteristics of OSA may variably include daytime symptoms such as excessive sleepiness, fatigue, impaired concentration and attention, dry mouth, morning headaches, depressed mood and personality changes; and nighttime symptoms such as snoring, observed apneas, restless sleep, insomnia and nocturia. Notably, women with OSA are more likely to present with “non-classical symptoms” including insomnia, fatigue or mood disturbances, rather than snoring or daytime sleepiness, thus there may be a gender bias in the diagnosis and treatment of OSA [19-21].
Laboratory full night polysomnography (PSG) is the gold standard method for the diagnosis of OSA. Polysomnography is a non-invasive technique that involves overnight monitoring of several physiological variables including electroencephalography, eye movements, muscle tone as well as respiratory effort, airflow and oxygen saturations. An apnea is defined as the complete cessation of airflow for a minimum of 10 seconds. The definition of a hypopnea includes a reduction of airflow that is associated with either an oxygen desaturation (of at least 3% or 4%) or an arousal [22]. OSA is diagnosed when the apnea-hypopnea index (AHI), i.e. the total number of obstructive apneas and hypopneas per hour of sleep, is greater than 5. The severity of OSA is graded according to commonly used clinical criteria as mild (AHI > 5 but less than 15), moderate (AHI > 15 but less than 30), or severe (AHI ≥ 30). Recent guidelines recommend the use of unattended portable home monitoring as an alternative to laboratory-based PSG for the diagnosis of OSA in selected patients with a high pretest probability of moderate to severe OSA [23]. Surveys such as the Berlin questionnaire [24] and Multivariable Apnea Prediction Index [25] are easy-to-use tools for the clinician to screen for OSA.
OSA treatment is recommended in patients with an AHI greater than 15 or in those with an AHI greater than 5 who have daytime sleepiness or cardiovascular disease. Continuous positive airway pressure (CPAP) is the treatment of choice for OSA [26]. CPAP provides a “pneumatic splint” and keeps the upper airway open during sleep. The optimal CPAP pressure setting is individually determined for each patient during an overnight laboratory titration study. CPAP can be applied using a variety of interfaces including nasal masks, oronasal (full face) masks or nasal pillow interfaces. Despite its demonstrated high efficacy, a significant proportion of individuals with OSA (ranging from 46 to 83%) are non-adherent with CPAP [27]. Indeed, maximizing CPAP adherence continues to be a major challenge for the successful treatment of OSA. Individual patient characteristics, the degree of OSA severity, technical aspects, and psychological and social factors have all been examined as potential predictors of CPAP adherence [27, 28]. Weight loss is an effective approach to reduce the severity of OSA [5] Alternative therapies, such as oral appliances and surgical options, are available for specific sub-groups of patients with OSA [29].
2. OSA and Type 2 Diabetes
a. Prevalence of OSA in Type 2 Diabetics
When the prevalence of OSA in patients with type 2 diabetes was assessed by methods other than the gold standard full PSG (e.g, limited PSG or overnight oximetry), estimates have been highly variable, ranging from 2 to 70% [30-33]. One study using limited PSG found a higher OSA prevalence in diabetics as compared to non-diabetics, but both groups had a very low prevalence [32].
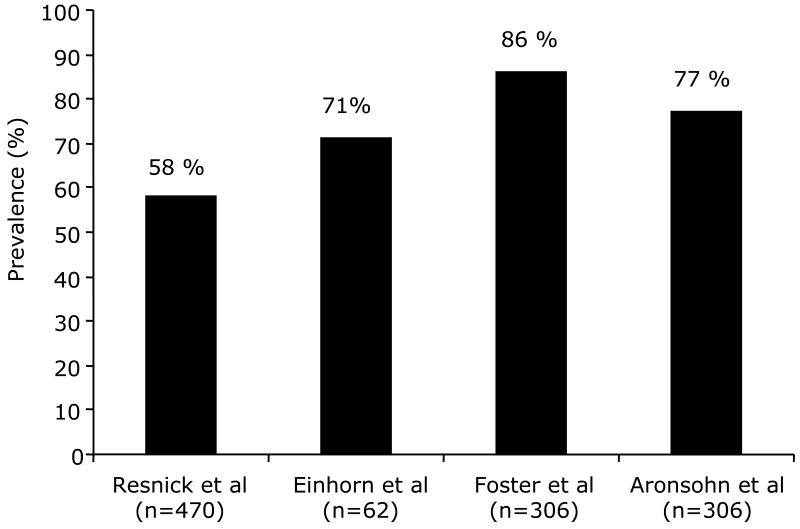
To date, four studies including a total number of nearly 900 type 2 diabetic patients have reported a striking overall prevalence of 73%. [34-37] (Figure 1). The highest estimate was 86%, reported in obese diabetic patients enrolled in the Sleep AHEAD study [37], a multi-center ancillary study of the Look AHEAD trial [37]. The lowest prevalence was estimated at 58% in the Sleep Heart Health Study [34], which included older individuals (with more than half aged over 65 years), used self reported diabetes, and an oxygen desaturation threshold of at least 4% for the definition hypopneas. More recently, Aronsohn et al. reported an OSA prevalence of 77% in 60 type 2 diabetics (mean age: 57 years) using a less stringent cut-off of 3% for oxygen desaturations, and when the dataset was re-analyzed using a stricter 4% criteria this estimate decreased to 58%.
Figure. 1.
The Center for Disease Control estimates that the number of individuals in the U.S. with diagnosed and undiagnosed diabetes approaches 24 million. Considering that the prevalence of OSA by full-night PSG in patients with type 2 diabetes averages 73%, this would then suggest that nearly 17 million diabetics currently suffer from this unrecognized co-morbidity. In the view of these staggering statistics, the International Diabetes Federation has recently released a report highlighting the need for a better understanding of the links between OSA and type 2 diabetes [38].
b. Prevalence and Incidence of Type 2 Diabetes in OSA
The prevalence and incidence of type 2 diabetes in OSA have been assessed in population and clinic-based cohorts with the majority of studies adjusting for shared risk factors such as age, sex, and BMI (Table 1). While a few of these studies relied on self-report or medication use for the diagnosis of diabetes, most have used validated diabetes definitions based on fasting glucose and 2-hr post-challenge glucose levels. Two studies [39, 40] used limited ambulatory PSG with only respiratory monitoring, while all other studies used full PSG for diagnosing OSA.
Table 1.
Studies examining the prevalence and incidence type 2 diabetes (T2DM) in patients with obstructive sleep apnea (OSA)
| Author/year | Sample | OSA diagnosis | Diabetes definition | Adjusted covariates | Main Findings |
|---|---|---|---|---|---|
| Meslier et al.[48] (2003) | 595 men referred to sleep disorders center | AHI ≥ 10 494 with OSA 101 without OSA |
Self-report; Fasting glucose >126 mg/dl; OGTT 2-h post-load glucose ≥ 200 mg/dl |
None | Higher prevalence of T2DM in OSA |
| Reichmuth et al.[39] (2005)* | 1387 (779 men); Wisconsin Sleep Cohort, United States 4-year follow-up (n=987) |
AHI ≥ 5 | Physician diagnosis; Fasting glucose ≥ 126 mg/dl |
Age, sex, body habitus | Higher prevalence of T2DM in moderate-to-severe OSA; Adjusted OR= 2.30 (CI=1.28- 4.11) Higher incidence of T2DM in moderate to severe OSA; Unadjusted OR = 4.06 (CI=1.86-8.85) Adjusted OR = 1.62 (CI=0.67-3.65) |
| Seicean et al.[38] (2008) | 2588 (1196 men); Sleep Heart Health Study, United States |
AHI ≥ 10 1245 with OSA 1343 without OSA |
Fasting glucose ≥126 mg/dl; OGTT 2-h post-load glucose ≥ 200 mg/dl |
Age, sex, BMI, race, waist | Higher prevalence of T2DM in OSA; Adjusted OR = 1.7 (CI=1.1-1.6) |
| Tamura et al.[40] (2008) | 129 Japanese patients with OSA | AHI ≥ 5 | Self-report; Fasting glucose >126 mg/dl; OGTT 2-h post-load glucose ≥ 200 mg/dl |
Sex, BMI | Higher prevalence of T2DM with increasing severity of OSA |
| Ronksley et al.[36] (2009) | 2149 (1346 men); Calgary Health Region, Canada |
AHI ≥ 5 (limited PSG) 1717 with OSA 432 without OSA |
Self-report; Use of diabetes medications |
Age, sex, BMI, neck size, smoking status | Higher prevalence of T2DM in severe OSA; Adjusted OR=2.18 (CI=1.22-3.89) |
| Mahmood et al.[41] (2009) | 1008 (468 men); referred to sleep disorders center |
AHI ≥ 5 745 with OSA 263 without OSA |
Medical chart review; Self-report; Use of diabetes medications |
Age, sex, race, BMI, oxygen parameters | Higher prevalence of T2DM in OSA; Unadjusted OR=1.8 (CI=1.3-2.6) Adjusted OR=1.3 (0.9-2.0) |
| Marshall et al.[35] (2009) * | 399 (294 men); Busselton Health Study, Australia 4-year follow-up |
AHI ≥ 5 (limited PSG) 94 with OSA 278 without OSA |
Physician diagnosis; Use of diabetes medications; Fasting glucose ≥ 126 mg/dl; |
Age, sex, BMI, waist, mean blood pressure, HDL cholesterol | Higher prevalence of T2DM in moderate-to-severe OSA; Unadjusted OR=4.37 (CI=1.12-17.12) Adjusted OR=1.98 (0.41-9.55) Higher incidence of T2DM in moderate to severe OSA; Unadjusted OR = 11.20 (CI=1.88-66.75) Adjusted OR = 13.45 (CI=1.59-114.11) |
| Botros et al.[37] (2009) * | 544 individuals; VA Connecticut Sleep Center, United States 2.7 year follow-up |
AHI ≥ 8 (full PSG) 402 with OSA 142 without OSA |
Physician diagnosis; Fasting glucose ≥ 126 mg/dl; |
Age, sex, race, BMI, baseline fasting glucose, weight change | Higher incidence of T2DM with increasing severity of OSA; Adjusted HR per quartile of OSA severity=1.43 (CI=1.10-1.86) |
includes prospective analysis; Bold type indicates non-significant findings, OR=odds ratio; CI= 95% confidence interval; HR=hazard ratio
In cross-sectional analyses, seven of eight studies [40-43] have demonstrated a significantly higher prevalence of diabetes in patients with OSA as compared to those without OSA. Some studies have also found a significant dose-response relationship between the severity of OSA and the prevalence of diabetes [40, 43, 44]. In two studies [39, 45], the higher odds ratio of self-reported diabetes in patients with OSA as compared to those without OSA was no longer significant after adjusting for potential confounders. In another study [40], only severe OSA group had significantly higher adjusted odds of self-reported diabetes, and interestingly this independent association was observed exclusively in patients who reported excessive sleepiness. Whether daytime sleepiness, which is commonly but not universally associated with OSA, is a relevant factor in the link between OSA and diabetes remains unclear. Further studies using objective measures of sleepiness and more rigorous assessments of diabetes are warranted to better understand the links between OSA and diabetes in sleepy versus non-sleepy individuals.
Importantly, three of these eight cross-sectional studies have also included longitudinal follow-ups, which may provide evidence regarding the direction of causality in the link between OSA and the development of diabetes. In the longitudinal analysis of the Wisconsin Sleep Cohort [43], OSA was found to be a risk factor for incident diabetes over a 4-year follow-up period, but this association was no longer significant after adjustment for age, sex and body habitus. The Busselton Health Study [39] found a significant independent association between moderate to severe OSA and incident diabetes over a 4-year follow-up period, but the sample size was small and there were only few incident cases of diabetes, which resulted in a wide confidence interval. Finally, the study by Botros et al. [41] reported an independent association between OSA and incident diabetes, after adjusting for various confounders as well as the weight change over a mean follow-up period of 2.7 years.
In summary, the current evidence suggests that type 2 diabetes is more prevalent among patients with OSA compared to those without OSA, and this association appears to be independent of shared risk factors. However, more data are needed to conclude unequivocally that OSA represents an independent risk for the development of diabetes over time. Thus, future studies from large longitudinal cohorts are clearly needed to assess the role of OSA as a potential risk factor for diabetes.
c. Impact of untreated OSA on glycemic control in type 2 diabetics
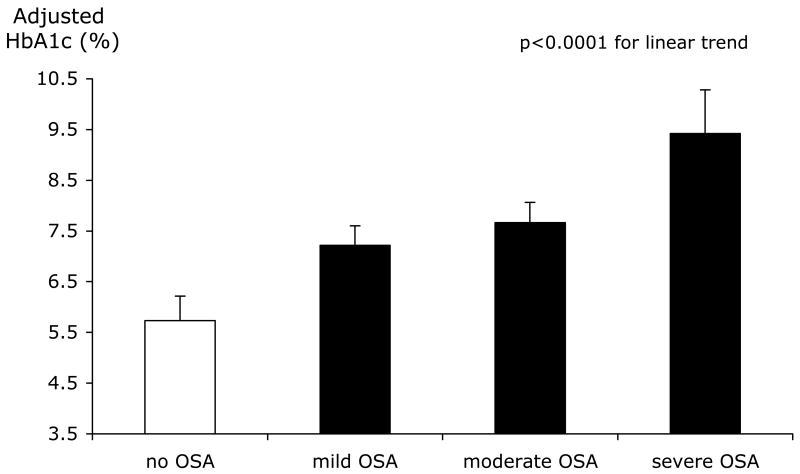
In a recent study, Aronsohn and colleagues [36] performed in-laboratory full-night PSG and measured hemoglobin A1c (HbA1c) levels in 60 patients with physician-diagnosed diabetes. The authors showed that increasing severity of OSA was associated with poorer glucose control, after controlling for age, sex, race, BMI, number of diabetes medications, level of exercise, years of diabetes and total sleep time. Compared to patients without OSA, the adjusted mean HbA1c was increased by 1.49% in patients with mild OSA, 1.93% in patients with moderate OSA, and 3.69% in patients with severe OSA (Figure 2). These effect sizes are comparable, if not exceeding to those of widely used hypoglycemic medications, and thus support the hypothesis that reducing the severity of OSA may be an important therapeutic approach to optimize glucose control.
Figure. 2.
Surprisingly, an earlier study involving 279 diabetic patients, reported no significant associations between OSA and HbA1c values [35]. This negative finding may be partly due to the fact that only about 22% of the sample underwent full PSG and the duration of sleep recording was reported to be as low as 4 hours. In contrast, Aronsohn et al used a minimum PSG recording time of 7 hours, and noted that when only the first 4 hours of recording was analyzed, the robust relationship between OSA severity and HbA1c was no longer apparent. Thus, obtaining PSG recordings longer than the commonly used minimum of 4 hours may be an important factor for the assessment of the associations between OSA and type 2 diabetes.
d. Effects of CPAP treatment of OSA on glycemic control in type 2 diabetics
To date, six studies, which include a total of 120 patients, have examined the effects of CPAP treatment of OSA on measures of glucose control in type 2 diabetes (Table 2) [30, 46-50]. Babu et al. [48] investigated 25 obese diabetic patients and demonstrated beneficial effects of 3 months of CPAP use on HbA1c and post-prandial glucose levels. Other studies reported improvements in nighttime glucose levels during just one night of CPAP therapy [49] and also during 5 weeks of CPAP use [47]. Two earlier studies, which included only a total of 19 subjects [30, 50], showed no change in HbA1c levels, but reported improvements in insulin sensitivity, as assessed by gold standard hyperinsulinemic eugylcemic clamp studies, after 3 to 4 months of CPAP use. The only randomized controlled study was published by by West et al. [46] and included 20 of the 42 obese diabetic patients randomized to the active CPAP arm, found no effect of active CPAP on HbA1c levels or insulin sensitivity, but reported significant improvements in measures of excessive daytime sleepiness. The latter finding raises the possibility that the effects of CPAP may vary according to the measured outcome. Of note, in this randomized controlled study, the average nightly CPAP use was only ∼ 3.3 hrs. By contrast, the positive study by Babu et al., found that in patients who used CPAP for more than 4 hrs per night (averaging ∼ 6.6 hrs/night) the reduction in HbA1c levels was strongly correlated with CPAP use. In an observational cohort study, among patients with moderate-to-severe OSA (AHI>20), the regular use of CPAP as determined by physician follow-up, was associated with a significant attenuation of incident diabetes even after adjusting for subsequent weight loss during an average of 2.7 year follow-up period [41].
Table 2.
Studies examining the effects of continuous positive airway pressure (CPAP) treatment of obstructive sleep apnea (OSA) on measures of glucose metabolism in patients with type 2 diabetes
| Author/Year | Sample | Measures of Glucose Metabolism | Treatment duration | CPAP adherence (h/night) | Main findings |
|---|---|---|---|---|---|
| Brooks et al.[26] (1994) | 10 | Hyperinsulinemic euglycemic clamp | 4 months | Not reported | No change in HbA1c Improvement in insulin sensitivity |
| Harsch et al. [46] (2004) | 9 | Hyperinsulinemic euglycemic clamp, HbA1c | 3 months | ∼ 5.8 hours | No change in HbA1c Improvement in insulin sensitivity |
| Babu et al.[44] (2005) | 25 | Continuous glucose monitoring, HbA1c | ∼ 12 weeks | ∼ 4.2 hours | Improvements in HbA1c and postprandial glucose levels |
| West et al.[42] (2007) | 20 (CPAP) 22 (sham CPAP) | Hyperinsulinemic euglycemic clamp, HOMA, HbA1c | 3 months | ∼ 3.3 hours | No change on HbA1c or insulin sensitivity |
| Pallayova et al.[45] (2008) | 14 | Continuous glucose monitoring | 1 night | Not reported | Improvement in nocturnal glucose levels |
| Dawson et al. [43] (2008) | 20 | Continuous glucose monitoring, HbA1c | ∼ 5 weeks | ∼ 5.8 hours | Improvement in glucose levels during sleep No change in HbA1c |
In summary, the findings from studies that examined the response to CPAP treatment are inconsistent, and the discrepancies between studies could be partly explained by differences in sample size and duration of therapy, lack of objective adherence data, and the possibility of changes in body composition over the study period. The negative findings do not rule out the possibility that OSA causes diabetes. More rigorous CPAP studies with a randomized placebo controlled design will be required to confirm the hypothesis that glucose control may improve after effective treatment of OSA. The threshold for OSA severity associated with a beneficial effect of CPAP on glycemic control and the nightly duration of CPAP treatment that is required to improve glucose control in diabetic patients remain also to be determined.
3. OSA and Prediabetes
a. Insulin resistance and glucose intolerance in patients with OSA
Numerous population and clinic-based cross-sectional studies have consistently found a robust independent association between the presence and severity of OSA and insulin resistance and glucose intolerance. A comprehensive review of the details of all these studies is beyond the scope of this article, but has been the topic of recent reviews [14-18]. Notably, the majority of these studies were conducted in men. The most commonly used markers of severity of OSA were AHI and the frequency and the degree of intermittent hypoxia. To estimate insulin sensitivity, the majority of the studies used fasting insulin levels and/or homeostatic model assessment (HOMA) index, i.e. the normalized product of fasting glucose and insulin. A number of studies have used fasting blood glucose and oral glucose tolerance test (OGTT) to assess glucose tolerance, where IFG was defined by fasting plasma glucose levels of 100 to 125 mg/dL and IGT was defined by 2-hour post-load glucose levels of 140 to 199 mg/dL.
The prevalence of prediabetes, as defined by the presence of either IFG and/or IGT have been found to be significantly higher in OSA patients than those without OSA and the estimates have ranged between 20 and 37% [42, 44, 51, 52]. In a recent cross-sectional analysis of over 2500 non-diabetic individuals from the Sleep Heart Health Study [42], the presence of OSA was associated with significantly higher odds of IFG and IGT after adjusting for age, sex, race, BMI and waist circumference. The magnitude of these associations was similar in non-overweight and overweight individuals [42].
In an earlier study, in 595 men who were referred to a sleep clinic, the increasing severity of OSA was associated with worsening glucose tolerance and insulin resistance, independently of age and BMI [51]. An earlier report from the Sleep Heart Healthy study also showed that the severity of OSA (as measured by AHI and the frequency of oxygen destaurations) was independently associated with both fasting and 2-hour post-load glucose levels during an OGTT [53]. In a population-based sample of 400 females, those with severe OSA had significantly lower insulin sensitivity than those without OSA, and the AHI was associated with increasing fasting and 2-hour post load insulin levels during an OGTT, after controlling for age, waist-hip ratio and other confounders [54].
More recently, Punjabi and colleagues measured insulin sensitivity in 118 nondiabetic individuals using a frequently sampled intravenous glucose tolerance test [55]. Compared to normal subjects, those with mild, moderate, and severe OSA showed a 26.7, 36.5 and 43.7% decrease in insulin sensitivity, respectively, after adjusting for age, sex, race and percent body fat. [55]. While these cross-sectional studies have controlled for various adiposity measures, the amount of visceral fat remains an important confounding factor in the link between OSA and alterations in glucose metabolism. To address this issue, Kono and colleagues studied 42 lean men with OSA and 52 controls who were matched for age, gender, BMI, and visceral fat. In the absence of confounding effects of visceral adiposity, OSA was found to be associated with insulin resistance (assessed by HOMA index) and higher fasting glucose levels [52].
Overall, the current evidence from cross-sectional studies strongly supports an independent association between OSA and insulin resistance and glucose intolerance. Adiposity, in particular visceral adiposity, could still be a major confounder in these associations. Future studies with prospective and interventional designs are needed to further address the direction of causality.
b. Effects of CPAP treatment of OSA on insulin sensitivity and glucose tolerance
A total of 21 studies have examined the effects of CPAP therapy on glucose tolerance and/or insulin sensitivity in non-diabetic patients with OSA (Table 3). The majority of the studies were conducted in obese men. While 9 studies reported positive findings [56-64], 12 were negative [65-76]. Insulin sensitivity was estimated by fasting HOMA index [57-59, 61-63, 67, 72, 73, 75, 76] in the majority of studies (11 out of 21) while a few studies used the gold standard hyperinsulinemic euglycemic clamp technique [60, 64, 68, 70]. Only 2 studies have used the clinical standard method of OGTT to assess glucose tolerance [58, 68]. The average CPAP treatment period ranged between one night and 6 months, with one study reporting findings after 2.9 years of follow-up period. Importantly, only 13 of the 21 studies [50, 56-58, 60, 62, 63, 68, 70, 73-76] reported objective data on CPAP adherence, and the mean reported adherence was ∼5 hours/night.
Table 3.
Studies examining the effects of continuous positive airway pressure (CPAP) treatment on measures of glucose metabolism in patients with obstructive sleep apnea (OSA)
| Author/Year | Sample | Average BMI (kg/m2) | Measures of Glucose Metabolism | Treatment duration | Average CPAP adherence (h/night) | Main findings |
|---|---|---|---|---|---|---|
| Positive studies | ||||||
| Harsch et al.[61] (2004) | 40 (34 men) | ∼ 33 | Hyperinsulinemic euglycemic clamp | 3 months | ∼5.2 | Insulin sensitivity improved after 2 days and remained stable after 3 months |
| Lindberg et al.[60] (2006) | 28 men | ∼ 29 | HOMA, fasting insulin | 6 months | ∼5.2 | Improvement in insulin sensitivity and fasting insulin after 2 weeks of CPAP |
| Barcelo et al.[59] (2008) | 44 men | ∼ 29 | HOMA | 3 months | ∼5.6 | Improvement in insulin sensitivity only in patients with daytime sleepiness (n=22) |
| Dorkova et al.[58] (2008) | 32 (27 men) | ∼ 35 | HOMA | 8 weeks | Not reported | Improvement in insulin sensitivity in those who used CPAP ≥ 4 h/night (n=16) |
| Schahin et al.[57] (2008) | 9 patients | ∼ 34 | Hyperinsulinemic euglycemic clamp | 2.9 years | ∼5.2 | Improvement in insulin sensitivity |
| Cuhadaroglu et al.[56] (2009) | 31 (27 men) | ∼ 32 | HOMA | 8 weeks | Not reported | Improvement in insulin sensitivity |
| Henley et al.[55] (2009) | 15 men | ∼ 36 | HOMA, OGTT | 3 months | ∼5.3 | Improvement in insulin sensitivity Reduced fasting and post-load glucose |
| Steiroploulos et al.[54](2009) | 56 (50 men) | ∼ 36 | HbA1c, fasting glucose, HOMA | 6 months | ∼2.7 | Decrease in HbA1c in those who used CPAP ≥ 4 h/night (n=21) |
| Lam et al.[53] (2010) | 61 men | ∼ 28 | Short Insulin Tolerance Test | 3 months | ∼ 4.9 | Improvement in insulin sensitivity in those who received therapeutic CPAP (n=31) |
| Negative studies | ||||||
| Saini et al.[63] (1993) | 8 men | ∼ 33 | Profiles of glucose and insulin at night | 1 night | Not reported | No change in nocturnal glucose and insulin |
| Cooper et al.[62] (1995) | 6 men | ∼ 38 | Profiles of glucose and insulin at night | 1 night | Not reported | No change in nocturnal glucose and insulin |
| Stoohs et al.[68] (1996) | 5 patients | Not reported | Fasting glucose and insulin | 2 months | Not reported | Increase in fasting and nocturnal glucose No change in fasting and nocturnal insulin |
| Saarlainen et al.[67] (1997) | 7 (6 men) | ∼ 34 | Hyperinsulinemic euglycemic clamp | 3 months | ∼5.6 | No change in insulin sensitivity |
| Ip et al.[66] (2000) | 9 patients | Not reported | Fasting glucose and insulin | 6 months | Not reported | No change in fasting glucose and insulin |
| Smurra et al.[65] (2001) | 16 men | ∼30 | Hyperinsulinemic euglycemic clamp, OGTT | 2 months | ∼ 6.4 | No change in insulin sensitivity and glucose tolerance |
| Czupryniak et al.[64] (2005) | 9 patients | ∼35 | HOMA, continuous glucose monitoring, fasting insulin | 1 night | Not reported | Increased nocturnal glucose No change in insulin resistance |
| Trenell et al.[73] (2007) | 29 patients | ∼35 | HOMA | 3 months | ∼6 (n=19) ∼2 (n=10) | No change in insulin sensitivity |
| Coughlin et al.[72] (2007) | 34 men | ∼36 | Fasting glucose and insulin, HOMA | 6 weeks | ∼3.9 | No change in glucose and insulin sensitivity with CPAP compared to sham CPAP |
| Vgontzas et al.[71] (2008) | 16 men | ∼38 | Fasting glucose and insulin | 3 months | ∼4.6 | No change in fasting glucose and insulin |
| Carneiro et al.[70] (2009) | 7 men | ≥ 40 | HOMA | 3 months | ∼6.6 | No change in insulin sensitivity |
| Murri et al.[69] (2009) | 78 (67 men) | ∼32 | HOMA | 4 weeks | Not reported | No change in insulin sensitivity |
HOMA=Homeostatic model assessment; HbA1c: Hemoglobin A1c; OGTT: Oral glucose tolerance test
Only two studies have used a randomized controlled design [56, 75]. In the first study [75], Coughlin and colleagues found no significant difference in insulin sensitivity (as assessed by HOMA) after 6 weeks of therapeutic CPAP versus sham-CPAP in 34 obese men (average BMI=36 kg/m2), but the average CPAP use was only 3.9 hours/night. Notably, despite the relatively low average CPAP use, there was a significant improvement in blood pressure [75], suggesting that the amount and the duration needed to reverse cardiovascular and metabolic abnormalities may differ. The second study [56] involved 61 men with lesser degrees of obesity (average BMI=28 kg/m2) and found an improvement in insulin sensitivity (as assessed by insulin tolerance test) in patients who received therapeutic CPAP as early as one week after treatment, and this improvement in insulin sensitivity was maintained at 3 months in those who were overweight.
Harsch and colleagues [64] performed hyperinsulinemic euglycemic clamp in 40 patients with OSA, and found that CPAP improved insulin sensitivity even after 2 days of therapy. The improvement persisted at 3-months, particularly in those with a BMI <30 kg/m2. The same group of investigators also reported a long-term positive effect of CPAP on insulin sensitivity and glucose tolerance at 2.9 years in a small number of patients [60]. In contrast, an earlier study [68] using hyperinsulinemic euglycemic clamp and OGTT in 16 obese men found no change in insulin sensitivity or glucose tolerance after 2 months of CPAP. A few studies reported a positive effect of CPAP on insulin sensitivity exclusively in subgroups of patients, specifically those who used CPAP for more than 4 hours/night [57, 61] or those with excessive daytime sleepiness [62].
In conclusion, there is still controversy as to whether CPAP treatment of OSA improves insulin resistance and/or glucose intolerance. The current evidence supports the hypothesis that the degree of obesity and the amount of CPAP use may be important predictors of metabolic response to CPAP. Large scale randomized controlled trials with robust assessments of insulin sensitivity and glucose tolerance will be required to fully determine the effects of CPAP treatment of OSA on measures of glucose metabolism. Future studies are also needed to investigate the optimal duration and the amount of CPAP use that is needed to improve metabolic outcomes. Such interventional studies would be essential to address the causality between OSA and alterations in glucose metabolism.
4. Mechanistic Pathways Linking OSA to Insulin Resistance and Glucose Intolerance
The potential mechanisms linking OSA and alterations in glucose metabolism are likely to be multiple. OSA is intrinsically associated with chronic intermittent hypoxia and sleep fragmentation, both of which could potentially be detrimental to glucose metabolism via intermediate mechanisms including activations of sympathetic nervous system, hypothalamic-pituitary axis and inflammatory pathways [16].
Animal models mimicking OSA have used intermittent hypoxia stimuli when rodents are most likely to be asleep. Electroencephalogram tracings that were recorded only in one of these studies have showed that hypoxia stimuli were accompanied by arousals, suggesting that sleep fragmentation, and thus altered sleep quality, is also an intrinsic feature of these hypoxia models. Intermittent hypoxia resulted in sympathetic activation and hypertension [77, 78] impaired glucose homeostasis [79] and insulin resistance in both lean [80] and obese [81] rodents. In healthy humans, only one study used intermittent hypoxia selectively during sleep to mimic OSA and found that 2 to 4 weeks of exposure to intermittent hypoxia (30 cycles per hour, oxygen saturation range: 95-85%) resulted in increased morning blood pressure [82], but glucose metabolism was not assessed in this study. Another study that exposed healthy subjects to 5 hours of intermittent hypoxia or normoxia - but during wakefulness - found decreased insulin sensitivity as assessed by ivGTT [83]. In a prospective study involving about 4000 middle-aged individuals, nocturnal intermittent hypoxia was associated with an increased risk of developing type 2 diabetes after a 3-year median follow-up period [84]. Two studies have used acoustic stimuli to induce sleep fragmentation (without changing sleep duration) in healthy humans and examined its effects on glucose metabolism by ivGTT. In the first study by Tasali et al. [85], all night selective suppression of deep sleep (i.e. slow wave sleep) for 3 consecutive nights resulted in about 25% decrease insulin sensitivity without adequate compensatory increase in insulin secretion, leading to reduced glucose tolerance and increased diabetes risk. In the second study, non-selective fragmentation of sleep across all stages for 2 nights was associated with a decrease in insulin sensitivity and non-insulin dependent glucose disposal [86]. Notably, the non-selective fragmentation in the second study was also associated with marked reductions in slow wave sleep, whereas other sleep stages were only modestly affected. Both studies were associated with increases in daytime sympathetic activity as assessed by heart rate variability [85, 86].
In summary, evidence from animal and human models that mimics OSA, supports a potential causal role for OSA in insulin resistance, glucose intolerance and increased diabetes risk. The relative independent contributions of the two main characteristics of OSA, namely intermittent hypoxia and sleep fragmentation on glucose metabolism remain to be determined.
5. Practice Points
Laboratory full night polysomnography (PSG) is the gold standard method for the diagnosis of OSA.
Unattended portable home PSG monitoring could be used as an alternative to laboratory-based PSG for the diagnosis of OSA in selected patients with a high pretest probability of moderate to severe OSA
Surveys such as the Berlin questionnaire and Multivariable Apnea Prediction Index are easy-to-use tools for the clinician to screen for OSA.
OSA treatment is recommended in patients with an AHI greater than 15 or in those with an AHI greater than 5 who have daytime sleepiness or cardiovascular disease. CPAP is the treatment of choice for OSA.
Given its exceedingly high prevalence, a systematic evaluation of OSA is warranted in patients with type 2 diabetes.
Current evidence strongly supports an independent association between OSA and insulin resistance and glucose intolerance, but causality remains to be determined.
6. Research Agenda
The role of OSA in the management of type 2 diabetes and prediabetes is in urgent need of further rigorous assessment.
The question of whether OSA represents an independent risk for the development of prediabetes and type 2 diabetes over time, remains to be investigated by prospective studies of sufficient duration and power.
Large scale randomized controlled trials of CPAP treatment of OSA with well-validated assessments of insulin sensitivity and glucose tolerance are needed.
The sufficient amount and duration of CPAP that is required to improve glucose metabolism remains to be determined.
The vast majority of studies of the relationship between OSA and glucose regulation have been conducted in males. There is a need to evaluate this relationship in women, in whom OSA may be underdiagnosed and undertreated.
Acknowledgments
The authors would like to acknowledge the grant support from the National Institutes of Health P01 AG11412, R01 HL086459, and RC1HL100046-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165:2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 6.CDC Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 7.Standards of medical care in diabetes--2009. Diabetes Care. 2009;32 1:S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 9.Kashyap SR, Defronzo RA. The insulin resistance syndrome: physiological considerations. Diab Vasc Dis Res. 2007;4:13–19. doi: 10.3132/dvdr.2007.001. [DOI] [PubMed] [Google Scholar]
- 10.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 12.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 13.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 15.Tasali E, Mokhlesi B, Van Cauter E. Obstructive Sleep Apnea and Type 2 Diabetes: Interacting Epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 16.Jun J, Polotsky VY. Metabolic consequences of sleep-disordered breathing. ILAR J. 2009;50:289–306. doi: 10.1093/ilar.50.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–260. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 18.Punjabi, Polotsky VY. Disorders of glucose metabolism in sleep apnea. Journal of Applied Physiology. 2005;99:19998–12007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 19.Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing. Clin Chest Med. 2004;25:257–268. doi: 10.1016/j.ccm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med. 2004;98:984–989. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Young T, Hutton R, Finn L, et al. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med. 1996;156:2445–2451. [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. 2007. [Google Scholar]
- 23.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 24.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. [see comments] Annals of Internal Medicine. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–166. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 26.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007;132:1057–1072. doi: 10.1378/chest.06-2432. [DOI] [PubMed] [Google Scholar]
- 27.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver TE. Adherence to positive airway pressure therapy. Curr Opin Pulm Med. 2006;12:409–413. doi: 10.1097/01.mcp.0000245715.97256.32. [DOI] [PubMed] [Google Scholar]
- 29.Sanders M. Sleep Breathing Disorders. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: W.B Saunders Company; 2005. pp. 969–1157. [Google Scholar]
- 30.Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: Effects of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–1685. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 31.Elmasry A, Lindberg E, Berne C, et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study. J Intern Med. 2001;249:153–161. doi: 10.1046/j.1365-2796.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 32.West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax. 2006;61:945–950. doi: 10.1136/thx.2005.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsumata K, Okada T, Miyao M, Katsumata Y. High incidence of sleep apnea syndrome in a male diabetic population. Diabetes Res Clin Pract. 1991;13:45–51. doi: 10.1016/0168-8227(91)90032-9. [DOI] [PubMed] [Google Scholar]
- 34.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–709. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 35.Einhorn D, Stewart DA, Erman MK, et al. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract. 2007;13:355–362. doi: 10.4158/EP.13.4.355. [DOI] [PubMed] [Google Scholar]
- 36.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181:507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster GD, Sanders MH, Millman R, et al. Obstructive Sleep Apnea among Obese Patients with Type 2 Diabetes. Diabetes Care. 2009 doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw JE, Punjabi NM, Wilding JP, et al. Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract. 2008;81:2–12. doi: 10.1016/j.diabres.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Marshall NS, Wong KK, Phillips CL, et al. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5:15–20. [PMC free article] [PubMed] [Google Scholar]
- 40.Ronksley PE, Hemmelgarn BR, Heitman SJ, et al. Obstructive sleep apnoea is associated with diabetes in sleepy subjects. Thorax. 2009;64:834–839. doi: 10.1136/thx.2009.115105. [DOI] [PubMed] [Google Scholar]
- 41.Botros N, Concato J, Mohsenin V, et al. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–1127. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31:1001–1006. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 43.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura A, Kawano Y, Watanabe T, Kadota J. Relationship between the severity of obstructive sleep apnea and impaired glucose metabolism in patients with obstructive sleep apnea. Respir Med. 2008;102:1412–1416. doi: 10.1016/j.rmed.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Mahmood K, Akhter N, Eldeirawi K, et al. Prevalence of type 2 diabetes in patients with obstructive sleep apnea in a multi-ethnic sample. J Clin Sleep Med. 2009;5:215–221. [PMC free article] [PubMed] [Google Scholar]
- 46.West SD, Nicoll DJ, Wallace TM, et al. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawson A, Abel SL, Loving RT, et al. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4:538–542. [PMC free article] [PubMed] [Google Scholar]
- 48.Babu AR, Herdegen J, Fogelfeld L, et al. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 49.Pallayova M, Donic V, Tomori Z. Beneficial effects of severe sleep apnea therapy on nocturnal glucose control in persons with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;81:e8–11. doi: 10.1016/j.diabres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Harsch IA, Schahin SP, Bruckner K, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71:252–259. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 51.Meslier N, Gagnadoux F, Giraud P, et al. Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J. 2003;22:156–160. doi: 10.1183/09031936.03.00089902. [DOI] [PubMed] [Google Scholar]
- 52.Kono M, Tatsumi K, Saibara T, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131:1387–1392. doi: 10.1378/chest.06-1807. [DOI] [PubMed] [Google Scholar]
- 53.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 54.Theorell-Haglow J, Berne C, Janson C, et al. Is obstruction sleep apnea associated with the metabolic syndrome and impaired glucose metabolism. Sleep Medicine. 2006;7 2:S5. [Google Scholar]
- 55.Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 35:138–145. doi: 10.1183/09031936.00047709. [DOI] [PubMed] [Google Scholar]
- 57.Steiropoulos P, Papanas N, Nena E, et al. Markers of glycemic control and insulin resistance in non-diabetic patients with Obstructive Sleep Apnea Hypopnea Syndrome: does adherence to CPAP treatment improve glycemic control? Sleep Med. 2009;10:887–891. doi: 10.1016/j.sleep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Henley DE, Buchanan F, Gibson R, et al. Plasma apelin levels in obstructive sleep apnea and the effect of continuous positive airway pressure therapy. J Endocrinol. 2009;203:181–188. doi: 10.1677/JOE-09-0245. [DOI] [PubMed] [Google Scholar]
- 59.Cuhadaroglu C, Utkusavas A, Ozturk L, et al. Effects of nasal CPAP treatment on insulin resistance, lipid profile, and plasma leptin in sleep apnea. Lung. 2009;187:75–81. doi: 10.1007/s00408-008-9131-5. [DOI] [PubMed] [Google Scholar]
- 60.Schahin SP, Nechanitzky T, Dittel C, et al. Long-term improvement of insulin sensitivity during CPAP therapy in the obstructive sleep apnoea syndrome. Med Sci Monit. 2008;14:CR117–121. [PubMed] [Google Scholar]
- 61.Dorkova Z, Petrasova D, Molcanyiova A, et al. Effects of CPAP on Cardiovascular Risk Profile in Patients with Severe Obstructive Sleep Apnea and Metabolic Syndrome. Chest. 2008;134:686–92. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 62.Barcelo A, Barbe F, de la Pena M, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 2008;63:946–950. doi: 10.1136/thx.2007.093740. [DOI] [PubMed] [Google Scholar]
- 63.Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample--what are the benefits and the treatment compliance? Sleep Med. 2006;7:553–560. doi: 10.1016/j.sleep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 65.Cooper BG, White JES, Ashworth LA, et al. Hormonal and metabolic profiles in subjects with obstructive sleep apnea syndrome and the effects of nasal continuous positive airway pressure (CPAP) treatment. Sleep. 1995;18:172–179. [PubMed] [Google Scholar]
- 66.Saini J, Krieger J, Brandenberger G, et al. Continuous positive airway pressure treatment: Effects on growth hormone, insulin and glucose profiles in obstructive sleep apnea patients. Hormone Metab Res. 1993;25:375–381. doi: 10.1055/s-2007-1002123. [DOI] [PubMed] [Google Scholar]
- 67.Czupryniak L, Loba J, Pawlowski M, et al. Treatment with continuous positive airway pressure may affect blood glucose levels in nondiabetic patients with obstructive sleep apnea syndrome. Sleep. 2005;28:601–603. doi: 10.1093/sleep/28.5.601. [DOI] [PubMed] [Google Scholar]
- 68.Smurra M, Philip P, Taillard J, et al. CPAP treatment does not affect glucose-insulin metabolism in sleep apneic patients. Sleep Med. 2001;2:207–213. doi: 10.1016/s1389-9457(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 69.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 70.Saarelainen S, Lahtela J, Kallonen E. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res. 1997;6:146–147. doi: 10.1046/j.1365-2869.1997.00034.x. [DOI] [PubMed] [Google Scholar]
- 71.Stoohs R, Facchini F, Guilleminault C. Insulin Resistance and Sleep-Disordered Breathing in Healthy Humans. American Journal of Respiratory and Critical Care Medicine. 1996;154:170–174. doi: 10.1164/ajrccm.154.1.8680675. [DOI] [PubMed] [Google Scholar]
- 72.Murri M, Alcazar-Ramirez J, Garrido-Sanchez L, et al. Oxidative stress and metabolic changes after continuous positive airway pressure treatment according to previous metabolic disorders in sleep apnea-hypopnea syndrome patients. Transl Res. 2009;154:111–121. doi: 10.1016/j.trsl.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Carneiro G, Togeiro SM, Ribeiro-Filho FF, et al. Continuous positive airway pressure therapy improves hypoadiponectinemia in severe obese men with obstructive sleep apnea without changes in insulin resistance. Metab Syndr Relat Disord. 2009;7:537–542. doi: 10.1089/met.2009.0019. [DOI] [PubMed] [Google Scholar]
- 74.Vgontzas AN, Zoumakis E, Bixler EO, et al. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest. 2008;38:585–595. doi: 10.1111/j.1365-2362.2008.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coughlin SR, Mawdsley L, Mugarza JA, et al. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 76.Trenell MI, Ward JA, Yee BJ, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9:679–687. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 77.Fletcher EC, Lesske J, Qian W, et al. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 78.Fletcher EC. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep. 2003;26:15–19. doi: 10.1093/sleep/26.1.15. [DOI] [PubMed] [Google Scholar]
- 79.Yokoe T, Alonso LC, Romano LC, et al. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol. 2008;586:899–911. doi: 10.1113/jphysiol.2007.143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iiyori N, Alonso LC, Li J, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–857. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polotsky VY, Li J, Punjabi NM, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–264. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamisier R, Gilmartin GS, Launois SH, et al. A new model of chronic intermittent hypoxia in humans: effect on ventilation, sleep, and blood pressure. J Appl Physiol. 2009;107:17–24. doi: 10.1152/japplphysiol.91165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muraki I, Tanigawa T, Yamagishi K, et al. Nocturnal intermittent hypoxia and the development of type 2 diabetes: the Circulatory Risk in Communities Study (CIRCS) Diabetologia. 53:481–488. doi: 10.1007/s00125-009-1616-0. [DOI] [PubMed] [Google Scholar]
- 85.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]