Abstract
Central to the DNA damage response (DDR) is the highly conserved Mre11 complex consisting of Mre11, Rad50 and Nbs1. The Mre11 complex acts as a sensor of DNA double-strand breaks (DSBs) and regulates the signal transduction cascades that are triggered following damage detection.1 Rare human genetic instability syndromes such as Ataxia-telangiectasia (A-T) and Nijmegen Breakage Syndrome (NBS) have underscored the importance of the DSB response in the suppression of tumorigenesis, as well as other severe pathologies affecting the development of both the immune system and the central nervous system. Using murine models of the human diseases, we have investigated the role of the Mre11 complex, and other modulators of the DSB response, in tumor suppression.2,3 We found that the checkpoint kinase Chk2 is crucial for the suppression of a diverse array of tumor types in Mre11 complex mutants and uncovered multiple roles for the Mre11 complex in apoptotic signaling in parallel to Chk2.4,5
Keywords: Mre11, Rad50, Nbs1, Chk2, Brca1, cancer, apoptosis
Introduction
The DNA damage response (DDR) promotes genome stability by triggering cellular programs that control cell cycle progression, regulate DNA repair pathways, and in some cases, induce apoptosis. Genomic instability, defined here as chromosomal breaks and rearrangements, results from DNA replication errors, exposure to reactive oxygen species, oncogene activation, and cellular processes such as antigen receptor gene rearrangements or meiotic recombination. Indices of DDR activation are detectable in pre-malignant tissues from many types of human cancer and the DDR has been proposed to function as an inducible barrier to tumorigenesis.6 Additional evidence for the tumor suppressive role of the DDR comes from the identification of rare human genetic instability syndromes such as Ataxia-telangiectasia (A-T; ATM loss), Nijmegen Breakage Syndrome (NBS; NBS1 hypomorphism), and A-T like disorder (A-TLD; MRE11 hypomorphism), resulting from mutations in genes involved in the DDR.1 The tumor suppressive role of the DDR is also well supported by the generation of numerous murine models.
While genomic instability, checkpoint dysfunction and defective apoptosis are all hallmarks of cancer cells, they are alone insufficient to drive tumorigenesis in the mouse. Mice expressing hypomorphic alleles of Nbs1 or Mre11 (Nbs1ΔB, Nbs1ΔC and Mre11ATLD1) that affect various aspects of the DDR remain free of spontaneous malignancies.2–4 Similarly, mice lacking the checkpoint kinase Chk2 exhibit defective DNA damage induced apoptosis in a wide spectrum of tissues and are not predisposed to spontaneous tumors.7,8 Understanding the complex interplay of the various branches of the DDR in vivo and their relationship to tumor predisposition remains an important challenge for the field. To this end, we have generated mice that mimic many aspects of the human NBS and A-TLD syndromes and analyzed their genetic interactions with mutant alleles of numerous DDR genes involved in apoptosis, DNA repair and checkpoint activation.2–5
The Influence of the Mre11 Complex on ATM Activation and Activity
The Mre11 complex is a DSB sensor and one of the first proteins to accumulate at sites of damage.9 One of the earliest events associated with damage detection by the Mre11 complex is the activation of the central transducing kinase Ataxia-telangiectasia mutated (ATM). The precise mechanism by which the Mre11 complex promotes ATM activation remains elusive but it may include a variety of activities including molecular bridging, DNA end-recognition and processing, modifications in chromatin structure, and direct protein-protein interactions.10
A key aspect of ATM signaling is believed to be its activation of the checkpoint kinase Chk2 in response to DNA damage.11 ATM phosphorylates N-terminal residues, in particular Thr68, which are then recognized by the N-terminal FHA domain of another Chk2 molecule.12 This brings the catalytic domains of Chk2 into proximity allowing trans-autophosphorylation and activation of kinase activity. In cell lacking ATM or expressing hypomorphic Mre11 complex alleles, Chk2 hyperphosphorylation is severely attenuated, suggesting impaired Chk2 kinase activity that has been proposed to underlie some features of the human genetic instability syndromes.1,11
We analyzed the impact of hypomorphic mutations in Mre11 and Nbs1 on the autophosphorylation of ATM-S1987 (S1981 in humans), an early step in ATM activation.13 While mice homozygous for the hypomorphic Nbs1ΔB, Nbs1ΔC and Mre11ATLD1 alleles all exhibited indices of reduced ATM activity, they exhibited a separation of function with regards to ATM activation.5 S1987-phosphorylation was reduced in Mre11ATLD1/ATLD1 mice but a response similar to wild type was observed in cells from both Nbs1ΔB/ΔB and Nbs1ΔC/ΔC mice, as well as the structurally similar Nbs1657Δ5 and Nbs1tr735 alleles reported by others.4,14 As both the Mre11 interaction domain and nuclear localization signals remain intact in these Nbs1 mutant alleles, the nuclear functions of the Mre11 complex, such as ATM activation, may be less severely impaired than in the context of the Mre11ATLD1 that leads to destabilization of all 3 complex members. Alternatively, these alleles may differentially affect other aspects of Mre11 complex function such as protein-protein interactions with or within the Mre11 complex or enzymatic activities.
Following ATM activation, the Mre11 complex promotes ATM activity on many of its substrates. In this regard, Mre11 complex hypomorphic alleles differentially affect the modification of ATM substrates. Consistent with the lower levels of activated ATM in IR treated Mre11ATLD1/ATLD1 cell cultures, we observed reduced phosphorylation of all ATM substrates analyzed to date, including Chk2, SMC1, BID and p53 on the ATM consensus site Ser18 (Ser15 in humans). Although not as severe as the defect in Atm−/−, the stabilization of p53 levels following DNA damage was attenuated in Mre11ATLD1/ATLD1 thymocytes.5 Defective Chk2, SMC1 and BID phosphorylation was observed in Nbs1ΔB/ΔB thymocytes, but the phosphorylation and stability of p53 was similar to wild type. The C-terminal Nbs1ΔC/ΔC mutant showed a more circumscribed defect, displaying normal modification of Chk2 and p53, but severely attenuated phosphorylation of SMC1 and BID.4 These data suggest that the Mre11 complex plays an important role in mediating interactions between activated ATM and a subset of its substrates, with the C-terminus of Nbs1 playing an essential role in some of these transactions. ATM no doubt makes multiple contacts with the Mre11 complex, as well as other DDR proteins, that tightly regulate the activation and activity of ATM on its many targets.
The Mre11 Complex and ATM Regulate p53 and Apoptosis in Parallel to Chk2
As Chk2 is required for damage-induced apoptosis in multiple tissues, we analyzed apoptosis in the Mre11 complex mutants, Mre11ATLD1 and Nbs1ΔB, that displayed deficient Chk2 hyperphosphorylation in response to damage. Similar to thymocytes from Atm−/− or Chk2−/− animals, apoptosis was attenuated in Mre11ATLD1/ATLD1. In Nbs1ΔB/ΔB we observed levels of apoptosis comparable to that of wild type, indicating that despite the lack of hyperphosphorylation, Chk2 remained functional for apoptosis. Surprisingly, we found that Chk2 influenced apoptosis independently of either the Mre11 complex or ATM, as more severe defects in apoptosis were observed in either Mre11ATLD1/ATLD1 Chk2−/− or Atm−/− Chk2−/− thymocytes than any of the single mutants.4,5 This indicated that Chk2 was active in promoting apoptosis in the absence of ATM or efficient ATM activation (as in Mre11ATLD1/ATLD1). Corresponding effects on p53 stability were also observed, as p53 levels were almost undetectable in Mre11ATLD1/ATLD1 Chk2−/− cell cultures compared to the intermediate defect in p53 stabilization observed in either of the single mutants.
While apoptosis was not impaired in thymocytes from Nbs1ΔB/ΔB mice, we found that the C-terminus of Nbs1, that has been shown to be important for some interactions between the Mre11 complex and ATM, was required for an efficient apoptotic response in Nbs1ΔC/ΔC. The precise ATM targets that promote apoptosis via the C-terminus of Nbs1 remain unclear but the reduced levels of Nbs1 C-terminus present in Nbs1ΔB/ΔB cells indicates that a low threshold of this domain may be sufficient for this function.
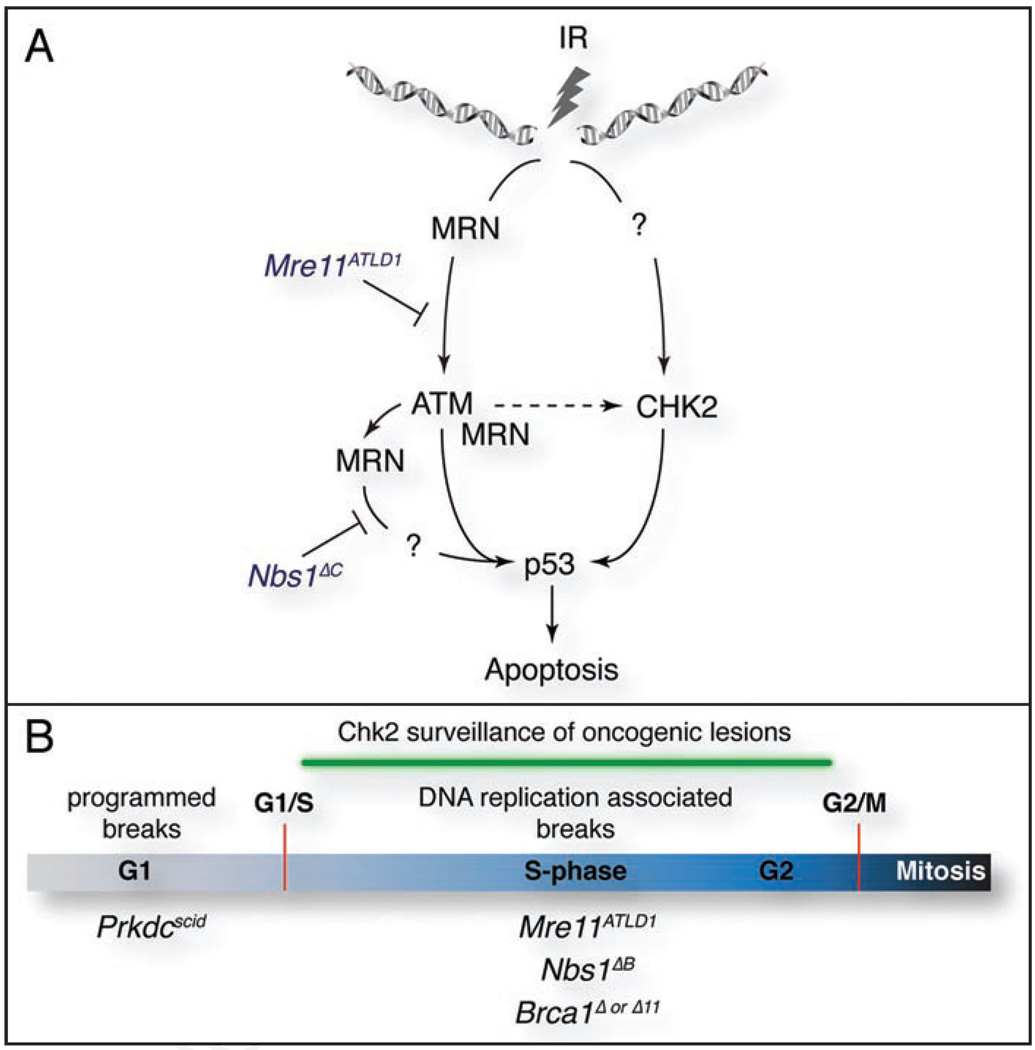
Our data has elucidated multiple roles for the Mre11 complex in apoptosis; activation of ATM and the facilitation of ATM activity through the C-terminus of Nbs1 (Fig. 1A).4,5 In addition, these data show unequivocally that Chk2 can promote apoptosis in the absence of either its hyperphosphorylation or the ATM protein. Phosphorylation of Chk2 appears to be required for apoptosis as complementation of Chk2−/− cells with an allele containing 7 N-terminal SQ/TQ residues mutated to alanine did not restore apoptotic function.8 Additional kinases, including ATR and DNA-PKcs, have been demonstrated to modify Chk2 in vitro and ATR displayed a different preference for phosphorylation sites in the N-terminus of Chk2 than ATM.15,16 Thus, it is plausible that some redundancy exists with regards to Chk2 phosphorylation and that these alternative modes of activation may not cause the mobility shift that is readily observed in the presence of ATM. Further work remains to identify the precise phosphorylation status of Chk2 and the proteins required for its activation in the absence of ATM. Moreover, the substrates of Chk2 that influence apoptosis remain largely unclear. Modification of p53 is no doubt an important aspect of Chk2’s function but it is unlikely to be the sole target important for apoptosis.11
Figure 1.
The Mre11 complex and Chk2 in apoptosis and tumor suppression. (A) The Mre11 complex (MRN) functions at multiple stages in apoptotic signaling; the activation of ATM, which is impaired by the Mre11ATLD1 allele, and the promotion of ATM activity by the C-terminal domain of Nbs1 that is deleted in the Nbs1ΔC allele. ATM phosphorylates Chk2 and this requires the Mre11 complex, but Chk2 promotes apoptosis in the absence of ATM, defining a parallel pathway. 4,5 (B) The influence of the Mre11 complex and Chk2 on tumorigenesis. Alleles that impair the metabolism of DNA replication associated breaks in S and G2 (Mre11ATLD1, Nbs1ΔB or Brca1Δ or Δ11) predispose tumors in the absence of Chk2.5,25,26 DSBs arising from the deficient repair of programmed breaks, as in DNA-PKcs deficient mice (Prkdcscid), are not sufficient to promote tumorigenesis in the absence of Chk2.5 The G1/S and G2/M checkpoints are indicated in red.
The DDR in the Suppression of Spontaneous Tumors
Despite attenuating many aspects of ATM dependent signaling, the hypomorphic alleles of Mre11 and Nbs1 were insufficient to predispose tumorigenesis. We previously investigated their genetic interactions with the tumor suppressor p53.3 Loss of p53 leads to the rapid development of T-cell lymphoma, as well as other types of tumors at a lower frequency, and p53+/− animals develop tumors over a longer latency period with a high percentage displaying loss of heterozygosity (LOH).17 The tumor predisposition of Nbs1ΔB/ΔB p53−/− or Mre11ATLD1/ATLD1 p53−/− double mutant animals was not significantly different than that of p53−/− alone. However, we found that both Nbs1ΔB and Mre11ATLD1 reduced the latency of tumors in p53+/− mice.3 We have proposed that the genomic instability resulting from these hypomorphic Mre11 complex alleles results in cell death in S and G2/M when p53 surveillance is inoperative and the checkpoint defects and enhanced genomic stability promote mitotic recombination and more rapid LOH at the p53 locus.18
As the Chk2 orthologue Rad53 functions primarily during S-phase, we were interested to determine if the loss of Chk2 would interact genetically with Mre11 complex mutants that manifested cell cycle checkpoint defects and genomic instability in the S and G2 phases of the cell cycle.2,3,19 Loss of Chk2 resulted in apoptotic defects in Nbs1ΔB/ΔB and increased the severity of those already present in Mre11ATLD1/ATLD1. Consistent with previous observations, loss of Chk2 exacerbated the intra-S phase checkpoint defect of Nbs1ΔB/ΔB cell cultures but did not significantly alter the G2/M checkpoint defects, chromosome stability, or damage sensitivity of Mre11 complex mutants.5,20 Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− animals more closely recapitulated the phenotypes of Atm−/− or p53−/− animals, as they displayed checkpoint dysfunction and severe apoptotic defects, but surprisingly they were not prone to rapid lymphomas.5 These animals did however develop a broad spectrum of aggressive tumors following a longer latency period of 12–18 months. As few differences were observed between Nbs1ΔB/ΔB Chk2−/− and Mre11ATLD1/ATLD1 Chk2−/− animals with regards to tumor latency or spectrum, it is unlikely that the influence of the Mre11 complex on apoptosis was of crucial importance for tumor suppression. Other aspects of Mre11 complex function, such as the regulation of the G2/M checkpoint, may play a more important role in suppressing oncogenic lesions in the absence of Chk2. As Chk2 did not strongly impair any aspects of the DDR except apoptosis, this function is likely the key to Chk2’s role as a tumor suppressor. Interestingly we did not observe mutations in p53 or ARF in any of the tumors we analyzed from these mice, indicating that loss of Chk2 reduces the selective pressure to attenuate these pathways.
Not All Breaks are Created Equal
To determine if Chk2 was a general suppressor of oncogenesis in the presence of DSBs, we analyzed the effects of Chk2 loss in DNA-PKcs deficient severe combined immunodeficient (Scid; Prkdcscid) animals that harbor stabilized DSBs in G1/G0 phase lymphocyte populations.21 DNA-PKcs deficiency impairs the functions of the Artemis nuclease that is required for the resolution of coding joint hairpins during V(D)J recombination, a prerequisite for the subsequent repair of coding joints by non-homologous end-joining (NHEJ) pathways.22 These persistent breaks activate p53, resulting in the attrition of lymphocytes and the resulting Scid phenotype.23,24 Loss of p53 partially rescues lymphocyte progression, as significant numbers of T and B-cells are evident, but Prkdcscid/scid p53−/− mice develop T and B cell lymphomas more rapidly than p53−/− single mutants.23,24 We observed no rescue of lymphoid cellularity and no predisposition to tumors in Prkdcscid/scid Chk2−/− mice. This indicated that p53-dependent pathways that respond to these G1/G0 breaks remained functional in the absence of Chk2 and suggested that Chk2 suppresses oncogenic lesions associated with DNA replication, such as those that arise as a result of Mre11 complex hypomorphism or deficiencies in Brca1 (Fig. 1B).5,25,26
Previous studies have demonstrated that the p53-dependent G1/S checkpoint, and more specifically p21, suppressed genomic instability and delayed lymphoma progression when p53-dependent apoptosis was compromised.27 We have proposed that the G1/S checkpoint, that is intact in all of the Chk2 double mutants we have analyzed to date, may be of vital importance for suppressing early lymphomas in these animals.5 This checkpoint would prevent programmed DNA breaks from entering S-phase where they would be replicated or exposed to additional modes of DSB repair that could promote the genesis of oncogenic lesions.
Together, our data has revealed unexpected complexity in IR induced apoptosis and demonstrated that the Mre11 complex and Chk2 synergize to prevent tumorigenesis.4,5 Precisely what aspects of DDR signaling are important for tumor suppression remains unclear. Further experiments using additional mutants, such as Nbs1ΔC/ΔC that isolates intra-S phase checkpoint dysfunction from an intact G2/M checkpoint, will be useful for identifying the roles of particular Mre11 complex functions in tumor suppression and allow us to gain a better understanding of how the Mre11 complex and Chk2 collaborate to suppress tumorigenesis. As a central node in the DDR, determining the functions of the Mre11 complex and ATM that are crucial for preventing malignancy remains an important and formidable challenge for future studies.
References
- 1.Stracker TH, Theunissen JW, Morales M, Petrini JH. The Mre11 complex and the metabolism of chromosome breaks: the importance of communicating and holding things together. DNA Repair (Amst) 2004;3:845–854. doi: 10.1016/j.dnarep.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Williams BR, Mirzoeva OK, Morgan WF, Lin J, Dunnick W, Petrini JH. A murine model of nijmegen breakage syndrome. Curr Biol. 2002;12:648–653. doi: 10.1016/s0960-9822(02)00763-7. [DOI] [PubMed] [Google Scholar]
- 3.Theunissen JW, Kaplan MI, Hunt PA, Williams BR, Ferguson DO, Alt FW, Petrini JH. Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol Cell. 2003;12:1511–1523. doi: 10.1016/s1097-2765(03)00455-6. [DOI] [PubMed] [Google Scholar]
- 4.Stracker TH, Morales M, Couto SS, Hussein H, Petrini JH. The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature. 2007;447:218–221. doi: 10.1038/nature05740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stracker TH, Couto SS, Cordon-Cardo C, Matos T, Petrini JH. Chk2 suppresses the oncogenic potential of DNA replication-associated DNA damage. Mol Cell. 2008;31:21–32. doi: 10.1016/j.molcel.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 7.Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S, et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 2002;21:5195–5205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrini JH, Stracker TH. The cellular response to DNA double-strand breaks: defining the sensors and mediators. Trends Cell Biol. 2003;13:458–462. doi: 10.1016/s0962-8924(03)00170-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 11.Ahn J, Urist M, Prives C. The Chk2 protein kinase. DNA Repair (Amst) 2004;3:1039–1047. doi: 10.1016/j.dnarep.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Oliver AW, Knapp S, Pearl LH. Activation segment exchange: a common mechanism of kinase autophosphorylation? Trends Biochem Sci. 2007;32:351–356. doi: 10.1016/j.tibs.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 14.Difilippantonio S, Celeste A, Kruhlak MJ, Lee Y, Difilippantonio MJ, Feigenbaum L, et al. Distinct domains in Nbs1 regulate irradiation-induced checkpoints and apoptosis. J Exp Med. 2007;204:1003–1011. doi: 10.1084/jem.20070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Stern DF. Regulation of CHK2 by DNA-dependent protein kinase. J Biol Chem. 2005;280:12041–12050. doi: 10.1074/jbc.M412445200. [DOI] [PubMed] [Google Scholar]
- 16.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attardi LD, Jacks T. The role of p53 in tumour suppression: lessons from mouse models. Cell Mol Life Sci. 1999;55:48–63. doi: 10.1007/s000180050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrini JH, Theunissen JW. Double strand break metabolism and cancer susceptibility: Lessons from the Mre11 complex. Cell Cycle. 2004;3 doi: 10.4161/cc.3.5.835. [DOI] [PubMed] [Google Scholar]
- 19.Paulovich AG, Hartwell LH. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell. 1995;82:841–847. doi: 10.1016/0092-8674(95)90481-6. [DOI] [PubMed] [Google Scholar]
- 20.Falck J, Petrini JH, Williams BR, Lukas J, Bartek J. The DNA damage-dependent intra—S phase checkpoint is regulated by parallel pathways. Nat Genet. 2002;30:290–294. doi: 10.1038/ng845. [DOI] [PubMed] [Google Scholar]
- 21.Roth DB, Menetski JP, Nakajima PB, Bosma MJ, Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992;70:983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- 22.Bassing CH, Alt FW. The cellular response to general and programmed DNA double strand breaks. DNA Repair (Amst) 2004;3:781–796. doi: 10.1016/j.dnarep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Guidos CJ, Williams CJ, Grandal I, Knowles G, Huang MTF, Danska JS. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocytes. Genes & Dev. 1996;10:2038–2054. doi: 10.1101/gad.10.16.2038. [DOI] [PubMed] [Google Scholar]
- 24.Nacht M, Strasser A, Chan YR, Harris AW, Schlissel S, Bronson RT, Jacks T. Mutations in the p53 and SCID genes cooperate in tumorigenesis. Genes & Dev. 1996;10:2055–2066. doi: 10.1101/gad.10.16.2055. [DOI] [PubMed] [Google Scholar]
- 25.McPherson JP, Lemmers B, Hirao A, Hakem A, Abraham J, Migon E, et al. Collaboration of Brca1 and Chk2 in tumorigenesis. Genes Dev. 2004;18:1144–1153. doi: 10.1101/gad.1192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao L, Kim S, Xiao C, Wang RH, Coumoul X, Wang X, et al. ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency. EMBO J. 2006;25:2167–2177. doi: 10.1038/sj.emboj.7601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barboza JA, Liu G, Ju Z, El-Naggar AK, Lozano G. p21 delays tumor onset by preservation of chromosomal stability. Proc Natl Acad Sci USA. 2006;103:19842–19847. doi: 10.1073/pnas.0606343104. [DOI] [PMC free article] [PubMed] [Google Scholar]