Abstract
Stroke is the leading cause of disability in developed countries and the third cause of mortality. Up to 15-30% of ischemic strokes are caused by cardiac sources of emboli being associated with poor prognosis and high index of fatal recurrence. In order to establish an adequate preventive strategy it is crucial to identify the cause of the embolism. After a complete diagnostic workup up to 30% of strokes remain with an undetermined cause, and most of them are attributed to an embolic mechanism suggesting a cardiac origin.
There is no consensus in the extent and optimal approach of cardiac workup of ischemic stroke. Clinical features along with brain imaging and the study of the cerebral vessels with ultrasonography or MRI/CT based angiography can identify other causes or lead to think about a possible cardioembolic origin.
Atrial fibrillation is the most common cause of cardioembolic stroke. Identification of occult atrial fibrillation is essential. Baseline ECG, serial ECG(’s), cardiac monitoring during the first 48 hours, and Holter monitoring have detection rates varying from 4 to 8% each separately. Extended cardiac monitoring with event loop recorders has shown higher rates of detection of paroxysmal atrial fibrillation.
Cardiac imaging with echocardiography is necessary to identify structural sources of emboli. There is insufficient data to determine which is the optimal approach. Transthoracic echocardiography has an acceptable diagnostic yield in patients with heart disease but transesophageal echocardiography has a higher diagnostic yield and is necessary if no cardiac sources have been identified in patients with cryptogenic stroke with embolic mechanism.
Keywords: Ambulatory electrocardiography, atrial fibrillation, cardiac workup, cardioembolic stroke, cryptogenic stroke, echocardiography, electrocardiography, stroke.
INTRODUCTION
Stroke is the major cause of serious long-term disability and the third cause of death in developed countries [1]. More than 5 million people die of stroke every year and 1 out of 6 survivors will suffer another stroke in the next 5 years, the majority of them during the first year. Stroke rates in the USA reach 700 000 cases/year with 170 000 dying after consequence of its effects [2]. Incidence rates in European countries are around 1.5-2/1000 cases per year [3,4]. The recurrence rate can be as high as 30% and the mortality of recurrences is two-fold [5,6].
Ischemic stroke accounts for about 70-80% of all strokes and is caused by embolic or thrombotic occlusions in the cerebral vessels. Embolic occlusions can be of arterial or cardiac origin. The etiology of ischemic stroke is mainly due to large artery atherosclerosis, from cardiac sources of embolism in approximately 30% of cases and small vessel disease leading to lacunar infarction, but a significant number of cases remain undetermined after a diagnostic approach [7].
Strokes due to cardiac embolism are generally associated with poor prognosis. Cardioembolic stroke has been associated with short and long-term recurrence, higher in-hospital mortality and a higher index of fatal recurrence versus other causes of stroke [7-9].
Identification of the specific cause is crucial in order to choose the most optimal preventive strategy and these vary for the different subtypes of ischemic stroke.
Depending on the extent of diagnostic evaluation and the criteria employed, 15-30% of ischemic strokes can be attributed to an identifiable source of cardiac embolism.
Searching for cardiac sources of stroke is a crucial part of the urgent ischemic stroke evaluation as it will change treatment decisions. There is a considerable disagreement among experts regarding the extent of cardiac testing in stroke patients. There is insufficient data to support the optimal cost-effective approach.
Even after a complete diagnostic workup up to 30% of all strokes remain classified as undetermined or cryptogenic. Most of these have embolic features [10] and an occult cardiac source is suspected in most cases.
PATHOPHYSIOLOGY AND MECHANISMS
It is important to differentiate ischemic stroke from hemorrhagic stroke. Ischemic stroke is originated by an interruption of cerebral blood flow as a result of an arterial occlusion, while the rupture of a blood vessel causes hemorrhagic stroke. Ischemic stroke is by far the most common and its underlying mechanisms and causes are multiple.
The brain receives 15% of cardiac output and is extremely sensitive to ischemia. There is general agreement that embolism from arterial, cardiac or unknown sources is the most frequent mechanism although thrombosis, in small vessel disease originating lacunar infarction and hemodynamic infarctions due to hypoperfusion in border artery territories are common and accepted mechanisms.
Material dislodged in close proximity to great vessels can travel to the cerebral arteries and occlude cerebral vessels with abrupt neurological manifestations. The embolic material is thrombus and tends to occlude cerebral artery stems, while emboli of small size and different compositions like fat, air or cholesterol crystals tend to travel to smaller terminal branches leading to watershed infarction.
DIAGNOSTIC PROCEDURE
Ischemic stroke is a heterogeneous disease with different mechanisms and etiologies and specific treatments. Identification of the right cause is essential in order to prepare an adequate preventive strategy.
Ischemic stroke is mainly classified into 5 main subtypes: atherotrombotic, cardioembolic, lacunar, uncommon and cryptogenic stroke.
An arterial embolic occlusion, from an arterial stenosis originated by great vessel disease, usually causes atherotrombotic stroke although hemodynamic stroke caused by hypoperfusion in watershed territories and occlusion by thrombosis can also occur. Cardioembolic stroke is caused by emboli from a cardiac or aortic source. Lacunar stroke is caused by occlusion by lipohyalinosis of terminal penetrant arteries, small vessel disease. Stroke from uncommon cause is another subtype originated by less frequent disorders such as arterial dissection, coagulopathy, disinmune disorders, drug abuse, etc. Ultimately stroke is classified as cryptogenic or undetermined when no cause is identified after a thorough study.
The first step after a clinically defined stroke is to rule out a brain hemorrhage with brain imaging techniques such as magnetic resonance imaging (MRI) or computed tomography (CT). After the confirmation of an ischemic stroke all the clinical and complementary studies are directed to find out the cause of the stroke.
In many patients medical history, risk factor profile, physical exploration, and basic explorations (ECG and thorax x-ray) may already indicate or suggest some specific causes such as the presence of carotid bruits originated by a carotid stenosis, rheumatic or prosthetic valve disease and atrial fibrillation.
If no etiology is found the next step is usually directed towards identifying arterial causes of embolism using ultrasound techniques, transcranial Doppler (TCD) and cervical arteries Doppler (CD), color coded transcranial or cervical arteries duplex (TCCD, CCD) or angiography, usually MRI or CT based, of the intracranial and cervical vessels.
When an embolic source is suspected and great vessel disease has been ruled out, it is common practice to start cardiac explorations to identify a cardiac source of stroke. The depth of these studies varies among centers and remains a subject of debate among the different fields dedicated to the study of stroke.
DIAGNOSIS OF CARDIOEMBOLIC STROKE
Diagnosis of cardioembolic infarction depends on suspicion by clinical, neuroimaging and laboratory features (Table 1).
Table 1.
Features Suggestive of Cardioembolic Stroke
| Clinical |
| Sudden onset to maximal deficit |
| Rapid regression of symptoms |
| Visual field defect, neglect or aphasia |
| Concomitant palpitations or oppressive chest pain |
| MRI or CT |
| Simultaneous or sequential infarcts in different arterial territories |
| Hemorrhagic transformation |
| Hyperdense artery sign in absence of arterial pathology |
| Ultrasound |
| Occlusion of the carotid artery by a mobile thrombus |
| Early recanalisation of an arterial occlusion |
| Microembolism (HITS) in both middle cerebral arteries |
| Laboratory |
| Elevation of troponins or cardiac enzymes |
| Brain natriuretic peptide |
The definite diagnosis of cardioembolic stroke relies on identification of cardiac sources of emboli caused by abnormalities of cardiac rhythm or structure using electocardiographic monitoring and cardio-aortic imaging.
CLINICAL SYMPTOMS AND SIGNS
Cardiac emboli can travel along to the intracranial vessels and due to their variable size cause either massive infarcts by occlusion of proximal arteries, small superficial infarcts in distal arterial territories, single large deep infarcts or multiple infarcts in different arterial territories.
Clinical presentation is characterized by sudden neurological deficits maximal at onset due to abrupt interruption of blood flow. While a stuttering course has usually been attributed to atherothrombotic stroke, cardioembolic emboli can have a progressive course in at least one-fifth of cases given that emboli can recanalise, move and fragment after initial impaction. Noncardioembolic strokes can appear with sudden deficits in two-fifths of cases [11,12]. Rapid regression of symptoms (the spectacular shrinking syndrome) reflecting early recanalisation has also been related to cardioembolic stroke [13].
Some neurological syndromes such as Wernicke’s aphasia, global aphasia without hemiparesis, Wallenberg’s syndrome, cerebellar infarcts, top-of-the basilar artery syndrome have been commonly associated with cardiac embolism [14]. Visual-field abnormalities, neglect and aphasia are clinical deficits more frequent in cardioembolic stroke [15]. Neurological deficits and syndromes may indicate embolic mechanisms but all have suboptimal discriminatory capacity [14].
NEUROIMAGING
Brain imaging is key to identify cerebral infarcts and to distinguish hemorrhagic stroke. Imaging techniques of the brain parenchyma are useful to rule out lacunar stroke caused by small vessel disease. Neuroimaging can help to diagnose cardioembolic stroke by revealing characteristic patterns of ischemic lesions associated to an embolic mechanism from a cardiac source.
Cardiac emboli often occlude middle-large size arteries and multiple vascular territories. Hemorrhagic transformation of an ischemic infarct and early recanalisation of an arterial occlusion are typical characteristics related to cardiac embolisms [16,17].
Simultaneous or sequential strokes in different arterial territories, especially if bihemispheric, combining anterior and posterior circulation or with concurrent systemic embolism are highly suggestive features of a cardiac source of embolism [11,14,18]. The identification of cortical involvement is characteristic of an embolic mechanism.
MRI has demonstrated to be clearly superior to CT in identifying ischemic lesions not visible on CT and has the capacity to detect cerebral ischemia within minutes of onset by diffusion-weighted sequences (DWI) [19]. MRI is superior to CT in detecting cortical involvement and multiple ischemic lesions correlating with cardioembolic stroke [18].
Cerebral ischemia with hemorrhagic transformation has been associated to cardioembolic stroke. Hemorrhagic transformation occurs in up to 71% of cardioembolic strokes and 91% of hemorrhagic infarcts are caused by a cardiac embolism [16,20]. Hemorrhagic transformation has been traditionally explained by recanalisation of an occluded vessel to a damaged ischemic tissue and vessel walls. Recanalisation after 6 hours of ischemia and the detection of microbleeds on gradient-echo T2-weighted MRI are predictors of hemorrhagic transformation [17,21].
The presence of the hyperdense cerebral artery sign on non-contrast CT scanning (Fig. 1) [22], or the corresponding hyperintense artery sign on MRI [23], originated by an occluding thrombus, suggests the diagnosis of an embolic arterial occlusion that may be of cardiac origin if no arterial pathology is detected.
Fig. (1).
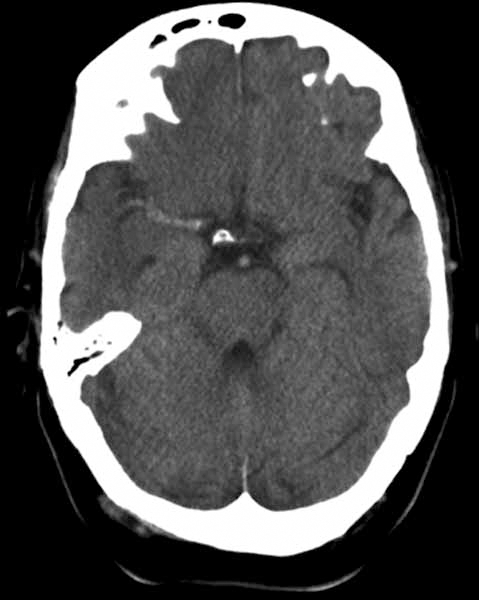
Hyperdense artery sign on CT scan showing a thrombus in the middle cerebral artery.
Early noninvasive vascular imaging of the cerebral and cervical arteries by ultrasonography or MRI/CT angiography can help to impugn a cardiac source of emboli. The detection of oscillating, homogenous, elastic mass-echos by CCD in an occlusion of the internal carotid artery suggests a thrombus of cardiac origin [24]. TCD has shown the capacity to detect early recanalisation of a stenotic or occluded artery in the territory of the infarct correlating with an embolic thrombus [25].
TCD allows a first line non-invasive diagnosis of a right-to-left shunt (RLS) caused by a patent foramen ovale (PFO) by detecting bubble signals in the middle cerebral artery (Fig. 2) after the injection of agitated saline in the antecubital vein [26]. The magnitude of the RLS could have prognostic implications [27].
Fig. (2).
Right-to-left shunt detected by TCD with microbubbles in the middle cerebral artery.
TCD can detect high intensity transient signals (HITS) indicating microembolism from an embolic condition. HITS have a strong correlation with arterial or cardiac sources of emboli in the acute phase of stroke and have an independent predictive value of poor outcome [28,29]. However HITS are more reliable from a carotid than from a cardiac source of embolism where they tend to disappear soon after the embolic event and do not have a clear relation with the embolic risk [30].
LABORATORY TESTS
There are no diagnostic laboratory tests to identify cardioembolic stroke. Cardiac enzyme and troponins are routinely recommended in the acute phase of stroke in order to detect an underlying acute coronary syndrome [31]. Several efforts have been conducted towards the research of plasma biomarkers to diagnose the different subtypes of stroke and Brain Natriuretic Peptide (BNP) has been correlated with cardioembolic stroke [32,33].
ELECTROCARDIOGRAPHIC MONITORING
Atrial fibrillation (AF) is the most common cause of cardioembolic stroke. Valvular AF has higher embolic risk than nonvalvular AF. Paroxysmal AF has a similar stroke risk than continuous AF. Suffering a stroke caused by lone AF boosts annual stroke risk from 0.5% to 12%.
Given the stroke risk and the clear benefit of warfarin over aspirin for secondary prevention, identification of atrial fibrillation, especially when occult, is warranted in the cardiac workup of ischemic stroke.
Electrocardiogram (ECG)
Most clinical practice guidelines include a standard 12-lead ECG in the immediate evaluation of acute ischemic stroke and transient ischemic attack (TIA) [31]. Cardiac abnormalities either acute, like coexisting acute coronary syndrome, or reflecting chronic cardiac disease are prevalent in the acute phase of stroke.
ECG can diagnose the cause of stroke by identifying AF, coexisting acute myocardial infarction (MI) or chronic cardiac disease that may predispose to embolic sources. ECG is also necessary to detect common cardiac complications such as myocardial ischemia or cardiac arrhythmias in the acute phase of stroke.
ECG abnormalities are reported in 75-90% of stroke patients [34]. The most common findings include repolarization and ischemic-like ECG changes. Most of these findings represent preexisting cardiac disease. After excluding those patients with underlying heart disease there is still up to one third of patients showing abnormalities like QT-prolongation or nonspecific ST changes. The sensibility to detect ECG abnormalities is high but the specificity to detect acute myocardial infarction is very low [34].
Initial ECG has a detection rate for AF varying from 5 to 25%, usually due to patients with already known AF [35]. Detection of new-onset AF is more infrequent, with rates around 4.8%. Douen et al. has shown that performance of serial ECG within the first 72 hours of acute stroke can increase detection almost 3-fold, half of the new-onset AF being detected on initial ECG and the rest on serial ECG assessments. This result is even more relevant as Holter monitoring was only able to detect AF in 50% of the new-onset cases [36]. This result could be explained by the delay of 4.75 days in Holter monitoring.
ECG changes suggestive of acute MI such as ST changes may be a potential etiology. Left ventricular thrombus is found in 5% of patients with acute MI, in 11.5% of patients with anterior MI compared to 2.3% of MI in other sites [37]. The risk of cardioembolism can be as high as 6% in the next 6 to 12 months [38].
Some ECG changes such as unrecognized MI, p-wave abnormalities and left ventricular hypertrophy have been associated with increased risk of stroke. Ventricular arrhythmias, concurrent MI and a prolonged QT interval have been associated with increased mortality in stroke patients [39].
Continuous Monitoring
Paroxysmal AF and continuous AF present a comparable risk of stroke. ECG may not detect transient arrhythmias. Clinical practice guidelines recommend continuous cardiac rhythm monitoring for hospitalized patients with acute stroke [31]. Telemetry monitoring during 48 hours in acute ischemic stroke patients detects between 4 to 8’4% of new onset paroxysmal AF not previously diagnosed by history or routine ECG [40,41].
Although the rate of diagnosis of new-onset AF by telemetry may be low, continuous cardiac monitoring has prognostic implications in all stroke patients. Between 60-65% of patients will develop conduction or rhythm abnormalities. Multivariate analysis shows that AF, atrioventricular block, ST-changes and inverted T-waves predict 3 month mortality independent of stroke severity, disability and age [42].
Holter Monitoring
Holter monitors are portable devices that record continuous data from 2 or 3 ECG leads during 24 or 48 hours. There is no clear recommendation on routine Holter monitoring for unselected patients. There is considerable controversy regarding the use of routine Holter monitoring. Low detection rates and cost-effectiveness are arguments against its recommendation in routine workup in stroke units [43,44].
A recent review including studies with 736 participants analyzed Holter monitoring in acute ischemic stroke with a global rate of new-onset AF detection of 4.6% [45]. Reasons for low detection rates might be the inclusion of unselected patients and the variable duration and timing of monitoring. The results of the different studies reviewed suggest that early and more prolonged monitoring might improve detection rates [45].
Holter monitoring can also be useful to identify the subgroup of patients with characteristics associated with occult AF where extended cardiac monitoring could be effective. Wallman et al. described that >70 premature atrial beats per 24 hours predicts a 26% detection of AF when monitoring is extended to 7 days [46].
Event Recorders
Event loop recorders are external ambulatory devices that allow up to 30 days of cardiac rhythm recording.
Two prospective studies evaluated event recorders for diagnosing paroxysmal AF in stroke patients. Barthelemy et al. evaluated 60 consecutive stroke patients, 28 of them without etiology after the standard diagnostic procedures including Holter monitoring, event recorders revealed paroxysmal AF in 14.3% of them [47]. The duration of monitoring in this study ranged from 24 to 162 hours. Jaboudon et al. included 149 consecutive patients with 7-day ambulatory ECG monitoring detecting paroxysmal AF in 5.7% of them [48].
Prolonged monitoring has shown to improve the rates of detection [46]. Tayal et al. evaluated retrospectively 56 consecutive patients with cryptogenic stroke or TIA with a Mobile Cardiac Outpatient Telemetry (MCOT) device during 21 days resulting in an AF detection rate of 23% [49].
Implantable monitoring devices offer the advantage of more prolonged monitoring, up to 14 months, revealing a high incidence of recurrent AF [50,51] but to date we have not found any studies evaluating stroke patients.
An interesting study used a modified automatic sphygmomanometer to detect recurrent atrial fibrillation as an ambulatory self-screening method with sensitivity near 100% and specificity near 91% [52].
Improving Paroxysmal AF Detection
Patient selection for cardiac monitoring is mandatory to improve cost-effectiveness of prolonged monitoring (Table 2). Longer monitoring should be limited to patients with a high suspicion of cardioembolic stroke.
Table 2.
Rates of New-Onset Atrial Fibrillation Detection
| Test | Rate % | Duration |
|---|---|---|
| Initial ECG | 4.8 | |
| Serial ECG | 5.5 | 72h |
| Holter | 4.6 | 24h |
| Telemetry | 4- 8.4 | 48h |
| Event loop recorders | 5.7 | 24h |
| or other ambulatory | 14.3 | 4 days |
| devices | 23 | 21 days |
Suissa et al. have recently published a predictive score to identify AF in patients who have suffered a stroke, Score for the Targeting of Atrial Fibrillation (STAF). The score calculates the sum of 4 items: age >62 (2 points), National Institutes of Health Stroke Scale (NIHSS) >8 (1 point), left atrial dilatation (2 points), absence of symptomatic intra or extracranial stenosis > 50% or clinic-radiological lacunar syndrome (3 points). A STAF score >5 identified patients with AF with 89% sensitivity and 88% specificity [53].
Cryptogenic stroke with different arterial territories affected on neuroimaging, dilated left atrium in echocardiography, >70 premature atrial beats per 24 hours in Holter monitoring [46] and increased age suggest an occult AF as a cause of stroke and should warrant longer periods of monitoring.
CARDIAC IMAGING
In many patients history, physical examination and ECG may reveal a cardiac source of emboli. If embolic stroke is suspected and extra and intracranial cerebral vessel imaging have not revealed an arterial ipsilateral stenosis and routine ECG and 48 hour telemetry have not detected AF, cardiac imaging is necessary to detect cardiac sources of emboli originated by abnormalities of cardiac structure (Table 3).
Table 3.
Cardiac Sources and Embolic Risk
| High risk | Low or uncertain risk |
| Atrial | Interatrial septal abnormalities |
| - Atrial fibrillation | - Patent Foramen Ovale |
| - Atrial flutter | -Atrial-septal aneurysm |
| - Sick sinus syndrome | |
| - Left atrial thrombus | Pulmonary arteriovenous malformation |
| Valvular | Spontaneous echo contrast (“smoke”) |
| - Mitral valve stenosis | |
| - Prosthetic cardiac valve | Mitral valve prolapse |
| - Left ventricular thrombus | Mitral annular calcification |
| - Acute myocardial infarction | Aortic valve sclerosis/stenosis |
| - Dilated cardiomyopathy | |
| Valvular strands | |
| Vegetations | |
| - Infective endocarditis | |
| - Marantic endocarditis | |
| Complex aortic arch atheroma | |
| Tumours | |
| - Myxoma | |
| - Papillary fibroellastoma | |
| - Mestastasic tumours |
Left heart thrombus is considered a high-risk source and requires blood stasis to develop. Left atrial thrombus (LAT) is usually observed in AF or mitral stenosis. LAT can also develop in myocardial or valvular disease [54]. Left ventricular thrombus is more frequently associated to MI. A meta-analysis calculated a risk of stroke after MI of 12.2 per 1000 MI at 30 days [55]. Ventricular aneurysms develop mural thrombi with persistent embolic risk [56]. Dilated cardiomyopathy has an annual risk of embolisation of 1-3.5% rising to 9% after suffering a stroke [57]. Intracavitary thrombus conveys a high risk of embolisation and favours early anticoagulation [38].
Vegetations related to infective or non-infective endocarditis are also high-risk conditions. Infective endocarditis has a 15-20% incidence of ischemic stroke, with higher risk during the first week or with mitral involvement [58]. Marantic or non-bacterial thrombotic endocarditis is usually associated to neoplastic, immunological or other debilitating diseases with a high risk of embolism [59].
Prosthetic valves either biological or mechanical have a 1 to 4% annual risk of embolisation, being this risk higher in mitral prosthetic valves, 2 to 3.5% annually, and amplified by AF [60].
Complex aortic arch atheroma (CAA) protruding ≥4mm has a relative risk of recurrent stroke of 1.6 to 4.3, and the risk increases if the plaque is ulcerated, non-calcified or mobile [61].
Other findings such as PFO and atrial septal aneurysm (ASA), pulmonary shunt, spontaneous echo contrast (SEC), valvular strands, valvular calcification and mitral valve prolapse presumably have a lower risk and are classified as medium or uncertain risk cardiac sources of emboli [38].
Echocardiography
There is controversy regarding the indication and the optimal echocardiographic approach in the cardiac workup of ischemic stroke. The European Stroke Organization guidelines recommend the use of echocardiography in selected patients while the American Stroke Association guidelines do not make any clear recommendation on the use of echocardiography [62,31].
There are pros and cons to TTE and TEE. TTE is easier to perform, not invasive, widely available and cheaper than TEE. However it is clearly less sensitive for the detection of unknown cardiac sources of emboli. TEE has the disadvantage of being invasive, more expensive and requires more training to be performed adequately.
TEE identifies possible cardiac sources of embolism in more than 50% of patients without clinically known heart disease [63]. TTE with agitated saline-injection may reach a 25% rate of success but drops to 10% without saline-injection. The sensibility for the detection of left ventricular thrombi is similar but the detection of LAT, PFO and vegetations favors TEE [38].
New techniques such as second-harmonic are improving the sensitivity of TTE reaching 62.5% compared to the 90% of TTE diagnosing PFO. However, PFO can be diagnosed by TCD non-invasively with agitated-saline injection detecting RLS with comparable diagnostic accuracy to TEE [64, 65].
TEE is considered safe with a 0.025 complication rate [66] but cases of paradoxical air emboli have been published [67] and the risks of hypotension during the procedure should not be underestimated in acute stroke patients [39].
Optimal Diagnostic Approach
There is no data to support an optimal approach in the echocardiographic evaluation of stroke patients. Few studies evaluate patient selection in order to improve the diagnostic and therapeutic yield of echocardiography.
While the superiority of TEE is clear some authors focus on the impact in clinical decisions. The diagnosis of thrombus, infectious endocarditis and cardiac tumours will have therapeutic implications but other conditions such as PFO, ASA, CAA and SEC have uncertain treatment strategies.
The presence of heart disease may be a major conditioning as TTE has superior yield close to 25% if heart disease is present [38]. While age could be used to select patients, several studies have concluded that it should not be used as an exclusion criterion in the selection of patients for TEE [68].
Strandberg et al. evaluated 441 unselected stroke patients with TEE detecting a cardiac source of embolism in 56% of the patients. After excluding patients with known heart disease or AF, analysis of the patients in sinus rhythm and without heart disease revealed that 8% of them suffered a change of therapy due to TEE results [69].
De Bruijn et al. evaluated 231 cryptogenic stroke patients with TTE and TEE. TEE detected a cardiac source of emboli with indication for oral anticoagulation in 16% of the patients [68].
Harloff et al. included 503 consecutive patients with acute brain ischemia. All patients received routine diagnostics and TEE. The patients were classified by TOAST criteria into atherotrombotic, cardioembolic, small vessel disease, stroke of other known etiology and undetermined or cryptogenic stroke. In patients with large artery, small vessel and cardiembolic stroke or stroke of other known causes TEE had a low therapeutic yield of 3% while in patients with cryptogenic stroke TEE revealed a cardiac source which prompted anticoagulant treatment in 30% of the patients [70].
These results support the use of TEE in patients who are candidates for oral anticoagulants after routine diagnostics cannot find an etiology. Arguably in these studies either no details are given about the findings that led to anticoagulation therapy or the findings that prompted a change do not have a clear recommendation for anticoagulation. The therapeutic yield of TEE is a matter of debate and needs more data to recommend an optimal approach.
CONCLUSIONS
The diagnosis of cardioembolic stroke relies on the detection of cardiac sources of embolism and the absence of other more plausible etiologies. There are no clear recommendations on the optimal and most cost-effective approach although the high prevalence of the disease and its prognostic implications warrants a cautious evaluation.
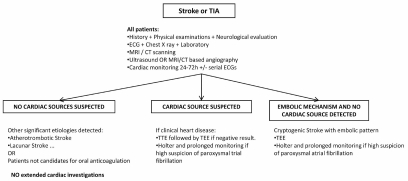
We propose a systematic approach based on general accepted practice with slight modifications based on articles reviewed and expert opinion (Fig. 3) [31, 39, 62].
Fig. (3).
Diagnostic algorithm for cardiac workup of ischemic stroke.
Patient selection is the clue for a cost-effective approach to the diagnosis of cardioembolic stroke.
All acute ischemic stroke patients must receive a complete cardiovascular history and physical examination with accurate cardiac evaluation in order to detect already known or unknown underlying heart disease. A proper neurological evaluation should reveal the suspicion of embolic stroke.
All stroke patients should be evaluated with initial ECG and receive serial ECG and 48 hour telemetry to identify potential sources of embolism. ECG and telemetry are cost-effective in all stroke patients as they also have prognostic implications detecting cardiac complications in the acute phase of stroke.
Neuroimaging, preferably with MRI, may help reveal patterns of ischemia suggestive of embolic stroke of cardiac origin.
Ultrasonography of the intracranial and extracranial arteries or MRI or CT based angiography must be done in all stroke patients to rule out large vessel disease. TCD may be used first-line to diagnose RLS. HITS monitoring may be useful in selected patients.
There is no reason for further cardiological studies in those patients not eligible for oral anticoagulation. Patients with lacunar stroke or with symptomatic ≥50% ipsilateral artery stenosis may not need extended cardiac monitoring or imaging. Patients with symptomatic intracranial stenosis should be reevaluated later in time to rule out recanalisation suggestive of an embolic thrombus.
All patients with undetermined stroke after these studies with an embolic pattern should receive TTE followed by TEE if the previous is non-diagnostic. In patients without previous heart disease, with RLS on TCD or in young patients where other sources of emboli such as cardiac tumours or PFO are more relevant it may be more cost-effective to directly proceed with TEE.
Patients with no etiology and with evidence of embolic stroke where paroxysmal AF is suspected should receive prolonged rhythm monitoring with Holter monitors and if the suspicion is high because of age, neuroimaging results, echocardiographic and Holter findings, prolonged cardiac rhythm monitoring with other devices should be considered.
More research with prospective data is needed to evaluate an appropriate cost-effective approach.
REFERENCES
- 1.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: cosponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. Heart Disease and Stroke Statistics-2003 Update. Dallas,TX : American Heart Association; 2002. [Google Scholar]
- 3.Kolominsky-Rabas PL, Sarti C, Heuschmann PU, et al. A prospective community-based study of stroke in Germany – the Erlangen Stroke Project (ESPro): incidence and case fatality at 1,3, and 12 months. Stroke. 1998;29:2501–6. doi: 10.1161/01.str.29.12.2501. [DOI] [PubMed] [Google Scholar]
- 4.Di Carlo A, Inzitari D, Galati F, et al. A prospective community-based study of stroke in Southern Italy: the Vibo Valentia incidence of stroke study (VISS). Methodology, incidence and case fatality at 28 days, 3 and 12 months. Cerebrovasc Dis. 2003;16:410–7. doi: 10.1159/000072565. [DOI] [PubMed] [Google Scholar]
- 5.Prencipe M, Culasso F, Rasura M, et al. Long term prognosis after a minor stroke: 10-year mortality and major stroke recurrence rates in a hospital-based cohort. Stroke. 1998;29:126–32. doi: 10.1161/01.str.29.1.126. [DOI] [PubMed] [Google Scholar]
- 6.Burn J, Dennis M, Bramford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project. Stroke. 1994;25:333–7. doi: 10.1161/01.str.25.2.333. [DOI] [PubMed] [Google Scholar]
- 7.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundorfer B, Heuschemann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria. Incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–40. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson S-E, Olsson J-E. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis. 2001;12:171–80. doi: 10.1159/000047700. [DOI] [PubMed] [Google Scholar]
- 9.Arboix A, García-Eroles L, Massons J, Oliveres M. Predictive clinical factors of in-hospital mortality in 231 consecutive patients with cardioembolic cerebral infarction. Cerebrovasc Dis. 1998;8:8–13. doi: 10.1159/000015809. [DOI] [PubMed] [Google Scholar]
- 10.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382–90. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 11.Kittner SJ, Sharkness CM, Price TR, et al. Infarcts with a cardiac source of embolism in the NINCDS Stroke Data Bank: historical features. Neurology. 1990;40:281–4. doi: 10.1212/wnl.40.2.281. [DOI] [PubMed] [Google Scholar]
- 12.Arboix A, Oliveres M, Massons J, Pujades R, Garcia-Eroles L. Early differentiation of cardioembolic from atherothrombotic cerebral infarction: a multivariate analysis. Eur J Neurol. 1999;6:677–83. doi: 10.1046/j.1468-1331.1999.660677.x. [DOI] [PubMed] [Google Scholar]
- 13.Minematsu K, Yamaguchi T, Omae T. Spectacular shrinking deficit: rapid recovery from a major hemispheric syndrome by migration of an embolus. Neurology. 1992;42:157–62. doi: 10.1212/wnl.42.1.157. [DOI] [PubMed] [Google Scholar]
- 14.Bogousslavsky J, Cachin C, Regli F, Despland PA, Van Melle G, Kappenberger L. Cardiac sources of embolism and cerebral infarction-clinical consequences and vascular concomitants: the Lausanne stroke registry. Neurology. 1991;41:855–9. doi: 10.1212/wnl.41.6.855. [DOI] [PubMed] [Google Scholar]
- 15.Kittner SJ, Sharkness CM, Sloan MA, et al. Infarcts with a cardiac source of embolism in the NINDS Stroke Data Bank: neurological examination. Neurology. 1992;42:299–302. doi: 10.1212/wnl.42.2.299. [DOI] [PubMed] [Google Scholar]
- 16.Jörgensen L, Torvik A. Ischaemic cerebrovascular disease in autopsy series: part prevalence, location, pathogenesis and clinical course of cerebral infarcts. J Neurol Sci. 1969;9:285–320. doi: 10.1016/0022-510x(69)90078-1. [DOI] [PubMed] [Google Scholar]
- 17.Molina CA, Montaner J, Abilleira S, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–84. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- 18.Wessels T, Wessels C, Ellsiepen A, et al. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR Am J Neuroradiol. 2006;27:35–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–8. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moulin T, Crepin-Leblond T, Chopard JL, Bogousslavsky J. Hemorrhagic infarcts. Eur Neurol. 1993;34:64–77. doi: 10.1159/000117012. [DOI] [PubMed] [Google Scholar]
- 21.Nighoghossian N, Hermier M, Adeleine P, et al. Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient-echo T2-weighted brain MRI study. Stroke. 2002;33:735–42. doi: 10.1161/hs0302.104615. [DOI] [PubMed] [Google Scholar]
- 22.Pressman BD, Tourje EJ, Thompson Jr. An early CT sign of ischemic infarction: increased density in a cerebral artery. AJR Am J Roentgenol. 1987;149:583–6. doi: 10.2214/ajr.149.3.583. [DOI] [PubMed] [Google Scholar]
- 23.Schellinger PD, Chalela JA, Kang DW, Latour LL, Warach S. Diagnostic and prognostic value of early MR imaging vessel signs in hyperacute stroke patients imaged <3 hours and treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 2005;26:618–24. [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura K, Yasaka M, Minematsu K, et al. Oscillating thromboemboli within the extracranial internal carotid artery demonstrated by ultrasonography in patients with acute cardioembolic stroke. Ultrasound Med Biol. 1998;24:1121–24. doi: 10.1016/s0301-5629(98)00108-2. [DOI] [PubMed] [Google Scholar]
- 25.Segura T, Serena J, Castellanos M, Teruel J, Vilar C, Dávalos A. Embolism in acute middle cerebral artery stenosis. Neurology. 2001;56:497–501. doi: 10.1212/wnl.56.4.497. [DOI] [PubMed] [Google Scholar]
- 26.Teague SM, Sharma MK. Detection of paradoxical cerebral echo contrast embolization by transcranial Doppler ultrasound. Stroke. 1991;22:740–5. doi: 10.1161/01.str.22.6.740. [DOI] [PubMed] [Google Scholar]
- 27.Serena J, Segura T, Perez-Ayuso MJ, Bassaganyas J, Molins A, Dávalos A. The need to quantify right-to-left shunt in acute ischemic stroke: a case-control study. Stroke. 1998;29:1322–8. doi: 10.1161/01.str.29.7.1322. [DOI] [PubMed] [Google Scholar]
- 28.Castellanos M, Serena J, Segura T, Pérez-Ayuso MJ, Silva Y, Dávalos A. Atheroesclerotic aortic arch plaques in cryptogenic stroke: a microembolic signal monitoring study. Eur Neurol. 2001;45:145. doi: 10.1159/000052113. [DOI] [PubMed] [Google Scholar]
- 29.Serena J, Segura T, Castellanos M, Dávalos A. Microembolic signal monitoring in hemispheric acute ischaemic stroke: a prospective study. Cerebrovasc Dis. 2000;10:278–82. doi: 10.1159/000016070. [DOI] [PubMed] [Google Scholar]
- 30.Batista P, Oliveira V, Ferro JM. The detection of microembolic signals in patients at risk of recurrent cardioembolic stroke: possible therapeutic relevance. Cerebrovasc Dis. 1999;9:314–9. doi: 10.1159/000016004. [DOI] [PubMed] [Google Scholar]
- 31.Adams HP, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association / American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular radiology and intervention council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in research interdisciplinary working groups. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 32.Montaner J, Perea-Gainza M, Delgado P, et al. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. 2008;39:2280–7. doi: 10.1161/STROKEAHA.107.505354. [DOI] [PubMed] [Google Scholar]
- 33.Naya T, Yukiiri K, Hosomi N, et al. Brain Natriuretic Peptide as a surrogate marker for cardioembolic stroke with paroxysmal atrial fibrillation. Cerebrovasc Dis. 2008;26:434–40. doi: 10.1159/000155640. [DOI] [PubMed] [Google Scholar]
- 34.Khechinashvii G, Asplund K. Electrocardiographic changes in patients with acute stroke: a systematic review. Cerebrovasc Dis. 2002;14:67–76. doi: 10.1159/000064733. [DOI] [PubMed] [Google Scholar]
- 35.Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidince and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36:1115–9. doi: 10.1161/01.STR.0000166053.83476.4a. [DOI] [PubMed] [Google Scholar]
- 36.Douen AG, Pageau N, Medic S. Serial electrocardiographic assessments significantly improve detection of atrial fibrillation 2.6-fold in patients with acute stroke. Stroke. 2008;39:480–82. doi: 10.1161/STROKEAHA.107.492595. [DOI] [PubMed] [Google Scholar]
- 37.Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left-ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol. 1998;81:822– 7. doi: 10.1016/s0002-9149(98)00003-4. [DOI] [PubMed] [Google Scholar]
- 38.Doufekias E, Segal AZ, Kizer JR. Cardiogenic and aortogenic brain embolism. J Am Coll Cardiol. 2008;51:1049–59. doi: 10.1016/j.jacc.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 39.Morris JG, Duffis EJ, Fisher M. Cardiac workup of ischemic stroke. Can we improve our diagnostic yield? Stroke. 2009;40:2893–8. doi: 10.1161/STROKEAHA.109.551226. [DOI] [PubMed] [Google Scholar]
- 40.Bansil S, Karim H. Detection of atrial fibrillation in patients with acute stroke. J Stroke Cerebrovasc Dis. 2004;13:12–5. doi: 10.1016/j.jstrokecerebrovasdis.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Tagawa M, Takeuchi S, Chinushi M, et al. Evaluating patients with acute ischemic stroke with special reference to newly developed atrial fibrillation in cerebral embolism. Pacing Clin Electrophysiol. 2007;30:1121–8. doi: 10.1111/j.1540-8159.2007.00823.x. [DOI] [PubMed] [Google Scholar]
- 42.Fogh CH, Boysen CA. Abnormalities on ECG and telemetry predict stroke outcome at 3 months. J Neurol Sci. 2005;234:99–103. doi: 10.1016/j.jns.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 43.Schaer BA, Zellwegger MJ, Cron TA, Kiser CA, Osswald S. Value of routine Holter monitoring for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemic events. Stroke. 2004;35:e68–e70. doi: 10.1161/01.STR.0000117568.07678.4B. [DOI] [PubMed] [Google Scholar]
- 44.Schaer B, Sticherling C, Lyrer P, Osswald S. Cardiological work-up in stroke patients- a comprehensive study of test results and therapeutic implications. Eur J Neurol. 2009;16:268–73. doi: 10.1111/j.1468-1331.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 45.Liao J, Khalid Z, Scallan C, Morillo C, O’Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after ischemic stroke. A systematic review. Stroke. 2007;38:2935–40. doi: 10.1161/STROKEAHA.106.478685. [DOI] [PubMed] [Google Scholar]
- 46.Wallmann D, Tuller D, Wustmann K, et al. Frequent atrial premature beats predict paroxysmal atrial fibrillation in stroke patients: an opportunity for a new diagnostic strategy. Stroke. 2007;38:2292–4. doi: 10.1161/STROKEAHA.107.485110. [DOI] [PubMed] [Google Scholar]
- 47.Barthelemy JC, Feasson-Gerard S, Garnier P, et al. Automatic event recorders reveal paroxysmal atrial fibrillation after unexplained strokes or transient ischemic attacks. Ann Noninvasive Electrocardiol. 2003;8:194–9. doi: 10.1046/j.1542-474X.2003.08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004;35:1647–51. doi: 10.1161/01.STR.0000131269.69502.d9. [DOI] [PubMed] [Google Scholar]
- 49.Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology. 2008;71:1696–1701. doi: 10.1212/01.wnl.0000325059.86313.31. [DOI] [PubMed] [Google Scholar]
- 50.Israel CW, Grönefeld G, Ehrlich JR, Li Y, Hohnloser SH. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device. Implications for patient care. J Am Coll Cardiol. 2004;43:47–52. doi: 10.1016/j.jacc.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 51.Varma N, Stambler B, Chun S. Detection of atrial fibrillation by implanted devices with wireless data transmission capability. PACE. 2005;28:S133–S136. doi: 10.1111/j.1540-8159.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 52.Wiesel J, Wiesel DJ, Messineo FC. Home monitoring with a modified automatic sphygmomanometer to detect recurrent atrial fibrillation. J Stroke Cerebrovasc Dis. 2007;16:8–13. doi: 10.1016/j.jstrokecerebrovasdis.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Suissa L, Bertora D, Lachaud S, Mahagne H. score for the Targeting of atrial fibrillation (STAF). A new approach to the detection of atrial fibrillation in the secondary prevention of ischemic stroke. Stroke. 2009;40:2866–8. doi: 10.1161/STROKEAHA.109.552679. [DOI] [PubMed] [Google Scholar]
- 54.Agmon Y, Khanderia BK, gentile F, Sward JB. Clinical and echocardiographic characteristics of patients with left atrial thrombus and sinus rhythm. Circulation. 2002;105:27–31. doi: 10.1161/hc0102.101776. [DOI] [PubMed] [Google Scholar]
- 55.Witt BJ, Ballman KV, Brown RD, Jr, Meverden RA, Jacobsn SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med. 2006;119:354:e1–9. doi: 10.1016/j.amjmed.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 56.Lapeyre AC, Steele PM, Kazmier FV, Chesebro JH, Vlietstra RE, Fuster V. Systemic embolisation in chronic left ventricular aneurysm: incidence and the role of anticoagulation. J Am Coll Cardiol. 1985;6:534–8. doi: 10.1016/s0735-1097(85)80109-1. [DOI] [PubMed] [Google Scholar]
- 57.Pullicino PM, Halperin JL, Thompson JL. Stroke in patients with heart failure and reduced left ventricular ejection fraction. Neurology. 2000;54:288–94. doi: 10.1212/wnl.54.2.288. [DOI] [PubMed] [Google Scholar]
- 58.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Eng J Med. 2001;345:1318–30. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 59.Reisner SA, Brenner B, Haim N, Edoute Y, Markiewicz W. Echocardiography in non bacterial thrombotic endocarditis: from autopsy to clinical entity. J Am Soc Echocardiogr. 2000;13:876–81. doi: 10.1067/mje.2000.106070. [DOI] [PubMed] [Google Scholar]
- 60.Salem DN, Stein PD, Al-Ahmad A, et al. Antithrombotic therapy in valvular heart disease-native and prosthetic. Chest. 2004;126:457S–82S. doi: 10.1378/chest.126.3_suppl.457S. [DOI] [PubMed] [Google Scholar]
- 61.Amarenco P, Cohen A, Tzourio C, et al. Atheroesclerotic disease of the aortic arch and the risk of ischemic stroke. N Eng J Med. 1994;22:1474–79. doi: 10.1056/NEJM199412013312202. [DOI] [PubMed] [Google Scholar]
- 62.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for the management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 63.Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol. 1999;22:391–6. doi: 10.1002/clc.4960220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ha JW, Shin MS, Kang S, et al. Enhanced detection of right-to-left shunt through patent foramen ovale by trnasthoracic echocardiography using harmonic imaging. Am J Cardiol. 2001;87:669–71. doi: 10.1016/s0002-9149(00)01455-7. [DOI] [PubMed] [Google Scholar]
- 65.Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr. 2006;7:147–54. doi: 10.1016/j.euje.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Peterson GE, Brickner ME, Reimold SC. Transesophageal echocardiography: clinical indications and applications. Circulation. 2003;107:2398–2402. doi: 10.1161/01.CIR.0000071540.97144.89. [DOI] [PubMed] [Google Scholar]
- 67.Christin F, Bouffard Y, Rossi R, Delafosse B. Paradoxical symptomatic air embolism after saline contrast transesophageal echocardiography. Echocardiography. 2007;24:867–9. doi: 10.1111/j.1540-8175.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 68.de Bruijn S, Agema W, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiopgraphy in management of patients of any age with transient ischemic attack or stroke. Stroke. 2006;37:2531–4. doi: 10.1161/01.STR.0000241064.46659.69. [DOI] [PubMed] [Google Scholar]
- 69.Strandberg M, Martila RJ, Helenius H, Hartiala J. Transoesophageal echocardiography in selecting patients for anticoagulation after ischaemic stroke or transient ischemic attack. J Neurol Neurosurg Psychiatry. 2002;73:29–33. doi: 10.1136/jnnp.73.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke. 2006;37:859–64. doi: 10.1161/01.STR.0000202592.87021.b7. [DOI] [PubMed] [Google Scholar]