Abstract
Photodynamic therapy (PDT) is a viable treatment option for a wide range of applications, including oncology, dermatology, and ophthalmology. Singlet oxygen is believed to play a key role in the efficacy of PDT, and on-line monitoring of singlet oxygen during PDT could provide a methodology to establish and customize the treatment dose clinically. This work is the first report of monitoring singlet oxygen luminescence in vivo in human subjects during PDT, demonstrating the correlation of singlet oxygen levels during PDT with the post-PDT photobiological response.
Keywords: photodynamic therapy, singlet oxygen, luminescence
Photodynamic therapy (PDT) is a viable treatment option for a variety of applications, including oncology, dermatology, and ophthalmology.1 In particular, 5-aminolevulinic acid (ALA)-PDT is widely used to treat a range of dermatologic conditions.2 PDT is based on the interaction of a photosensitizer (PS), light, and oxygen, in which photoactivation of PS generates cytotoxic molecular species. Customized dosimetry could, in principle, impact the efficacy of treatment outcome and of the effective use of resources. Dosimetry in PDT is complex, as the treatment effect is generated by an interaction of multiple components.
A number of dose metrics have been evaluated to monitor the outcome of ALA-PDT in dermatological treatment.3,4 Since singlet oxygen (1O2), which phosphoresces to the ground triplet state, is believed to be a key cytotoxic species in PDT,1 its direct monitoring is of great interest. Until now, in-vivo optical detection of 1O2 luminescence at 1270 nm remained elusive because of its low signal yield. In recent pioneering works, Niedre et al.5 and Yamamoto et al.6 measured PDT-generated 1O2 luminescence in vivo in mice, and demonstrated its correlation with treatment outcome. Recently, we have developed a fiber-based 1O2 monitoring device7 that is compatible with human studies. We present the first clinical trial measuring PDT-generated 1O2 levels in skin of healthy volunteers before and after ALA application, and correlating the 1O2 luminescence signal with the photobiological effects of ALA-PDT, possibly indicating treatment outcome.
A total of 18 healthy subjects (14 males and 4 females) were enrolled with written, informed consent in the clinical study approved by our internal Institutional Review Board to ensure adherence to the Declaration of Helsinki protocols. Power analysis indicated that the sample size of 18 subjects provided 80% power (α = 0.05, β = 0.20) to detect a significant linear relationship between the change in 1O2 luminescence signal and photobiological skin response following PDT using repeated-measures mixed model regression analysis (version 6.0, nQuery Advisor, Statistical Solutions, Saugus, Massachusetts). A newly developed, entirely fiber-based 1O2 dosimeter was used to detect 1O2 luminescence in vivo.7 The device uses a low power, time-resolved diode laser of 635 nm (10-kHz repetition rate, 5-μs pulse duration, less than 1 μJ/pulse) for PS activation. 1O2 signal was measured at the end of each laser pulse to reduce background fluorescence signal. Simultaneously, three optical signal strengths at 1.22, 1.27, and 1.315 μm are recorded using narrow bandpass filters and a photon multiplier tube (PMT) (Hamamatsu H9170-45) with a fast photon counter (model MSA-300, Becker and Hickl, Berlin). The average signal from 1.22 and 1.315 is used to estimate the background signal and then subtracted from the 1O2 signal at 1.27 μm to eliminate back-ground noise. Measurements typically take only a few seconds.
Each subject had two treatment sites on the right upper arm outlined and randomly assigned for ALA-PDT (Levulan Kerastick, DUSA Pharmaceuticals, Wilmington, Massachusetts) with either one-hour (Ishort) or three-hour (Ilong) incubation periods. ALA incubation time was varied to control PS accumulation, keeping in mind that longer incubation times result in increased PpIX levels,8 leading to different 1O2 production and treatment outcomes. 1O2 measurements were done before ALA application (pre-ALA) when no exogenous PS was administered and immediately before the therapeutic light dose (pre-PDT) following PpIX production from ALA application.
Following 1O2 measurements, each treatment site was irradiated with a therapeutic light dose of 20 J/cm2 (irradiance 100 mW/cm2) with a separate fiber-based continuous diode laser at 635 nm (HPD 7401, High Power Devices, North Brunswick, New Jersey).
Standardized digital photographs of the treatment sites were taken within 15 min and at 24 h after this treatment and used for the evaluation of phototoxic reactions by four blinded investigators. Each investigator rated the degree of erythema and edema between a scale of 0 (no response) to 10 (most pronounced). Median of the four readings is reported to minimize any potential interobserver variability. The photobiological response to the Ilong treatment sites were more pronounced and more clinically significant compared to Ishort treatment sites (Fig. 1). Consistent with Ref. 9, edema and erythema response peaked at 15 min and 24 h following PDT, respectively. Subsequent analysis was performed using the maximal photobiological skin response: acute edema at 15-min post-PDT and erythema at follow-up 24-h post-PDT. PS-induced 1O2 signal was defined by change in 1O2 luminescence between pre-ALA (time of minimum PpIX concentration) and pre-PDT (time of maximum PpIX concentration) time points.
Fig. 1.
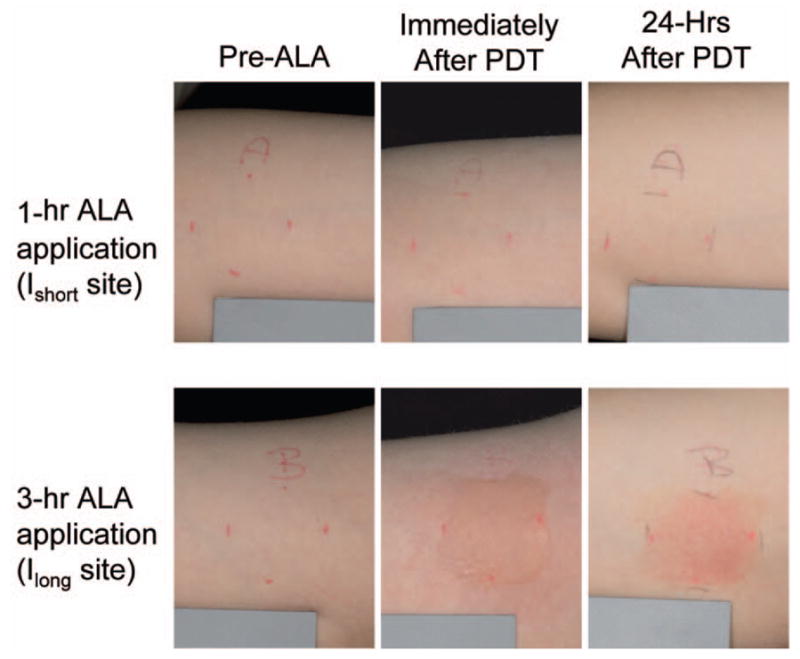
Representative images of treatment sites with 1 h (Ishort) or 3 h (Ilong) of ALA application time. The phototoxic reaction in Ilong sites (edema immediately after PDT and erythema 24 h afterward) is more intense than in Ishort sites.
Several researchers have shown that the degree of erythema and edema following ALA-PDT is predictive of clinical outcome in various dermatologic conditions.4,10 To evaluate the relationship between 1O2 signal and photobiological skin responses (edema and erythema), we applied repeated-measures linear mixed model analysis.11 Using this model, the 1O2 luminescence signal significantly correlated with acute edema in Ilong sites (P=0.01) and 24-h follow-up erythema for Ilong and Ishort combined (P=0.028) (Table 1).
Table 1.
Effect of 1O2 signal on photobiological responses based on a linear mixed model regression analysis of the data. Slope of the linear correlation between 1O2 signal and photobiological response in either Ishort sites, Ilong sites, or the combined data are reported. Edema is measured at post-PDT; erythema is measured at follow-up. The slope test shows the comparison of slopes estimated from Ishort sites and Ilong sites using an F-test. ST is statistically significant. CI=confidence interval.
| 1O2 signal | Edema+ |
Erythema× |
||
|---|---|---|---|---|
| Slope (95% CI) | P value | Slope (95% CI) | P value | |
| Slope test† | 0.009 (ST) | 0.527 | ||
| Ishort sites only | −0.002 (−0.015, 0.011) | 0.747 | ||
| Ilong sites only | 0.035 (0.011, 0.059) | 0.010 (ST) | ||
| Combined sites | 0.017 (0.002, 0.031) | 0.028 (ST) | ||
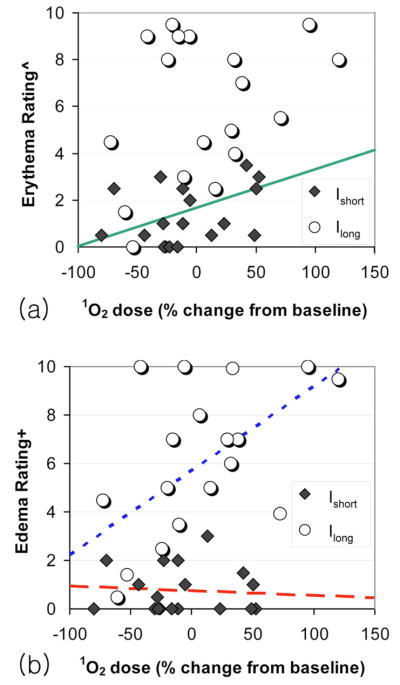
Equality of slopes for Ishort and Ilong sites was assessed with an F-test to decide whether a common slope parameter could be modeled to describe this correlation.12 The relationship between erythema and 1O2 signal showed no significant difference in slopes between Ishort and Ilong sites (P=0.527). As a result, correlation between follow-up erythema and 1O2 signal was investigated with a common slope fitted to the combined dataset for the Ishort and Ilong sites (Fig. 2). The interpretation is that the percent change in 1O2 signal from baseline is positively correlated for both sites in a similar way, and thus can be described using a common slope.
Fig. 2.
Scatter plots illustrating the relationship between 1O2 luminescence signal versus (a) acute edema rating and (b) erythema rating 24 h after PDT. Regression-based fits to the Ishort, Ilong, and combined data are shown by the red dashed line, the blue dotted line, and the solid green line, respectively. (Color online only.)
On the other hand, the correlation between acute edema and 1O2 signal was modeled separately for Ishort and Ilong sites because they had unequal slopes (P=0.009) (Fig. 2). In fact, greater edema was significantly correlated with greater percent change from baseline in 1O2 signal but only for the Ilong sites (P=0.01), not Ishort sites (P=0.747) (Table 1). Interestingly, only Ilong sites showed a significant correlation between 1O2 signal and acute edema (Fig. 2). It is possible that the minor acute edema at the Ishort sites (median score of 0.25 out of 10) may not have been clearly discernable by the blinded investigators in a 2-D photograph, and thus may not have correlated significantly with the quantitative 1O2 signal.
Unexpectedly, a decrease in 1O2 signal after the incubation period was observed in some treatment sites. This might be related to modification of skin optical properties by ALA and its carrier.13 A carrier-only control treatment site could potentially provide additional information when incorporated in future studies.
This is the first study showing the feasibility of monitoring PS-generated 1O2 signal in vivo in human subjects. 1O2 signal measured immediately prior to PDT light irradiation correlated significantly with the photobiological response to ALA-PDT in normal skin. As a result, monitoring 1O2 production in the skin is predictive of the clinical ALA-PDT outcome and is a helpful tool for customizing the clinical ALA-PDT treatment.
Acknowledgments
This work was supported by NIH SBIR Phase II grant R44 CA096243 and in part by NIH grant P01 CA84203. We also thank Oleg Akilov, Mehron Puoris’haag, Fernanda Sakamoto, and Kana Watanabe for evaluating the phototoxic response from the clinical photographs.
References
- 1.Hasan T, Ortel B, Solban N, Pogue B. Photodynamic therapy of cancer. In: Kufe, Bast, Hait, Hong, Pollock, Weichselbaum, Holland, Frei, editors. Cancer Medicine. 7. B.C. Decker, Inc; Hamilton, Ontario: 2006. pp. 537–548. [Google Scholar]
- 2.Babilas P, Landthaler M, Szeimies RM. Photodynamic therapy in dermatology. Eur J Dermatol. 2006;16(4):340–348. [PubMed] [Google Scholar]
- 3.Ericson MB, Sandberg C, Stenquist B, Gudmundson F, Karlsson M, Ros AM, Rosen A, Larko O, Wennberg AM, Rosdahl I. Photodynamic therapy of actinic keratosis at varying fluence rates: assessment of photobleaching, pain and primary clinical outcome. Br J Dermatol. 2004;151(6):1204–1212. doi: 10.1111/j.1365-2133.2004.06211.x. [DOI] [PubMed] [Google Scholar]
- 4.Jeffes EW, McCullough JL, Weinstein GD, Fergin PE, Nelson JS, Shull TF, Simpson KR, Bukaty LM, Hoffman WL, Fong NL. Photodynamic therapy of actinic keratosis with topical 5-aminolevulinic acid. A pilot dose-ranging study. Arch Dermatol. 1997;133(6):727–732. [PubMed] [Google Scholar]
- 5.Niedre MJ, Yu CS, Patterson MS, Wilson BC. Singlet oxygen luminescence as an in vivo photodynamic therapy dose metric: validation in normal mouse skin with topical amino-levulinic acid. Br J Cancer. 2005;92(2):298–304. doi: 10.1038/sj.bjc.6602331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto J, Yamamoto S, Hirano T, Li S, Koide M, Kohno E, Okada M, Inenaga C, Tokuyama T, Yokota N, Terakawa S, Namba H. Monitoring of singlet oxygen is useful for predicting the photodynamic effects in the treatment for experimental glioma. Clin Cancer Res. 2006;12(23):7132–7139. doi: 10.1158/1078-0432.CCR-06-0786. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Zhu L, Minhaj AM, Hinds MF, Vu DH, Rosen DI, Davis SJ, Hasan T. Pulsed diode laser-based monitor for singlet molecular oxygen. J Biomed Opt. 2008;13(3):034010. doi: 10.1117/1.2927465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juzeniene A, Juzenas P, Ma LW, Iani V, Moan J. Topical application of 5-aminolaevulinic acid, methyl 5-aminolaevulinate and hexyl 5-aminolaevulinate on normal human skin. Br J Dermatol. 2006;155(4):791–799. doi: 10.1111/j.1365-2133.2006.07484.x. [DOI] [PubMed] [Google Scholar]
- 9.Brooke RC, Sinha A, Sidhu MK, Watson RE, Church MK, Friedmann PS, Clough GF, Rhodes LE. Histamine is released following aminolevulinic acid-photodynamic therapy of human skin and mediates an aminolevulinic acid dose-related immediate inflammatory response. J Invest Dermatol. 2006;126(10):2296–2301. doi: 10.1038/sj.jid.5700449. [DOI] [PubMed] [Google Scholar]
- 10.Tosca AD, Balas CJ, Stefanidou MP, Katsantonis JC, Georgiou SK, Tzardi MN. Photodynamic treatment of skin malignancies with aminolevulinic acid. Emphasis on anatomical observations and in vivo erythema visual assessment. Dermatol Surg. 1996;22(11):929–934. [PubMed] [Google Scholar]
- 11.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Linear, Logistic, Survival, and Repeated Measures Models. Springer; Berlin: 2005. Regression Methods in Biostatistics. [Google Scholar]
- 12.Diggle PJ, Liang K, Zeger SL. Analysis of Longitudinal Data. Oxford University Press; Oxford, UK: 1994. [Google Scholar]
- 13.Welzel J, Reinhardt C, Lankenau E, Winter C, Wolff HH. Changes in function and morphology of normal human skin: evaluation using optical coherence tomography. Br J Dermatol. 2004;150(2):220–225. doi: 10.1111/j.1365-2133.2004.05810.x. [DOI] [PubMed] [Google Scholar]