Abstract
Many cellular processes including neuronal activity are sensitive to changes in intracellular and/or extracellular pH— both of which are regulated by acid-base transporter activity. HCO3−-dependent transporters are particularly potent regulators of intracellular pH in neurons and astrocytes, and also contribute to the composition of the cerebrospinal fluid (CSF). The molecular physiology of HCO3− transporters has advanced considerably over the past ~14 years as investigators have cloned and characterized the function and localization of many Na-Coupled Bicarbonate Transporters of the Slc4 family (NCBTs). In this review, we provide an updated overview of the function and localization of NCBTs in the nervous system. Multiple NCBTs are expressed in neurons and astrocytes in various brain regions, as well as in epithelial cells of the choroid plexus. Characteristics of human patients with SLC4 gene mutations/deletions and results from recent studies on mice with Slc4 gene disruptions highlight the functional importance of NCBTs in neuronal activity, somatosensory function, and CSF production. Furthermore, energy-deficient states (e.g., hypoxia and ischemia) lead to altered expression and activity of NCBTs. Thus, recent studies expand our understanding of the role of NCBTs in regulating the pH and ionic composition of the nervous system that can modulate neuronal activity.
Keywords: brain, electrogenic, astrocyte, genetic, neuron, pH
The regulation of intracellular pH (pHi) is critical for proper cell function because many cellular processes are sensitive to changes in pH (Roos and Boron, 1981; Bevensee and Boron, 2007). pH-sensitive processes include fertilization (Johnson and Epel, 1976), cell coupling (O'Beirne et al., 1987; Roos and Boron, 1981), alterations in cell structure (Parton et al., 1991), and the activity of metabolic enzymes such as phosphofructokinase (Trivedi and Danforth, 1966). In the central nervous system (CNS), which is comprised of cells with high surface-to-volume ratios and an extracellular space that is quite tortuous, the movement of acid-base equivalents across plasma membranes (e.g., by passive diffusion and pHi-regulating transporters) not only changes pHi, but also has the additional consequence of changing extracellular pH (pHo) in the opposite direction. Changes in pHi and/or pHo have important functional consequences in the nervous system, particularly because the activity of many voltage-sensitive and ligand-gated ion channels associated with neuronal activity are sensitive to changes in pHi/pHo.
The regulation and homeostasis of pHi is controlled primarily by acid-base transporters localized at the plasma membrane. As described in more detail below, acid-base transporters are characterized as HCO3−-dependent or -independent acid extruders or acid loaders. Na-Coupled Bicarbonate Transporters (NCBTs) in conjunction with Anion Exchangers (AEs) are encoded by Solute carrier 4 (Slc4) genes, and many NCBTs including electrogenic and electroneutral Na/Bicarbonate Cotransporters (NBCs) and Na-Driven Cl-Bicarbonate Exchangers (NDCBEs) are dominant contributors to the pH physiology of cells, especially those in the nervous system. While other Slc genes (e.g., Slc26) also encode bicarbonate transporters, we restrict our review to the Slc4-encoded Na-coupled ones— primarily from vertebrates. This review in conjunction with recent reviews of Na-independent and/or -dependent Slc4 anion exchangers (Alper, 2009; Romero et al., 2009) and Slc26 transporters (Dorwart et al., 2008; Romero et al., 2009) provides a comprehensive overview of bicarbonate transporters.
Our understanding of the physiologic importance of NCBTs —especially in the nervous system— has expanded tremendously over the past decade following the cloning of the first NCBT cDNA encoding the electrogenic NBC from salamander kidney (Romero et al., 1997). Recent data from studies on various NCBT knockout mice have highlighted the importance of cation-coupled bicarbonate transporters in neurologic processes, including neuronal activity and somatosensory function. In the present review on NCBTs, we first provide a general overview of the importance of pH regulation, the energetics of proton movement and acid-base transporter activity, and the molecular physiology of the bicarbonate transporters. Next, we present both historical and contemporary information on the function and localization of NCBTs in the nervous system. Finally, we discuss the latest advances in understanding the role of NCBTs in the functioning nervous system and in energy-deficient neurologic conditions.
1) Overview of pH Regulation and NCBT Activity
A) General importance of pH regulation in the nervous system
Changes in pH can have a powerful influence on brain function, for example by altering neuronal activity and synaptic transmission. Although there are exceptions, an increase in pHo generally stimulates neuronal firing, whereas a decrease has the opposite effect (Chesler and Kaila, 1992; Balestrino and Somjen, 1988; Ransom, 2000). In fact, an increase in pH can trigger epileptiform activity in both humans and rodents (Cohen and Kassirer, 1982; Aram and Lodge, 1987; Woodbury et al., 1984; Jarolimek et al., 1989; Lee et al., 1996; Marshall and Engberg, 1980; Velísek et al., 1994). A decrease in pH has the opposite effect. For example, lowering pHo from 7.4 to 6.7 reversibly reduced the amplitude, increased the interval, and slowed the propagation of low extracellular Mg2+-induced epileptiform discharges in combined entorhinal cortex-hippocampal slices (Velísek et al., 1994). Lowering pHo to 6.2 inhibited the discharges completely. pHo-induced increases in neuronal activity are often associated with increased activity of N-methyl-D-aspartate (NMDA) receptors that display pronounced pH sensitivity in the physiologic range (Tang et al., 1990; Traynelis and Cull-Candy, 1990) with increased open probability at high pH.
Perhaps it is not surprising that changes in pH can alter neuronal firing in light of the myriad of ligand- and voltage-gated ion channels sensitive to changes in pHi and/or pHo (Traynelis, 1998; Tombaugh and Somjen, 1998). Many of these voltage-gated channels generate and propagate action potentials. pH changes presumably alter ion-channel activity by modifying electrostatic interactions between charged amino acids and inducing structural alterations. In isolated rat CA1 hippocampal neurons, a moderate extracellular acidosis (pH 6.4) depresses the peak Na+ current by ~15%, whereas alkalosis has the opposite effect (Tombaugh and Somjen, 1996). K+ channels are more sensitive to changes in pHi than pHo in the physiologic range. In general, a decrease in pHi inhibits K channels (Byerly and Moody, 1986).
Compared to Na+ and K+ channels, Ca2+ channels are typically more sensitive to changes in pH. In neurons, an increase in pHo reversibly enhances both high voltage-activated Ca2+ currents (e.g., from L-, N-, P-, Q-, and R-type Ca2+ channels; Tombaugh and Somjen, 1996) and low voltage-activated Ca2+ currents (e.g., from the T-type Ca2+ channel; Tombaugh and Somjen, 1997). Regarding effects of changes in pHi, the activity of high voltage-activated Ca2+ channels in chick dorsal root ganglion neurons is reversibly enhanced by applying a weak base (which raises pHi) and depressed by applying a weak acid (which lowers pHi) (Mironov and Lux, 1991). Similar results have been obtained on rat CA1 neurons (Tombaugh and Somjen, 1997).
Channels in the nervous system that are activated by changes in pHo can influence neurotransmission. For example, DeVries (2001) has presented evidence that transient acidification at the synaptic cleft can inhibit a presynaptic voltage-gated Ca2+ current in retinal cone photoreceptors due to a pH-mediated shift in the voltage dependence of the Ca2+ channel. Furthermore, the regulation of pHo by horizontal cells may modulate or contribute to the feedback inhibition of these Ca2+ channels in the cone photoreceptors (Vessey et al., 2005). A change in pH is the primary stimulus for activating some channels such as acid-sensing ion channels (ASICs), which are stimulated by a decrease in pHo (Waldmann et al., 1999). The importance of these pH-sensitive channels in neurotransmission is highlighted by studies on ASIC1 knockout mice that have impaired hippocampal long-term potentiation, as well as reduced excitatory postsynaptic potentials and NMDA receptor activation (Wemmie et al., 2002). Neurons cultured from the hippocampus of these ASIC1 knockout mice fail to elicit acid (pH 5)-evoked cation currents. The ASIC1-knockout mice also display defects in performance on the Morris water maze test and eye-blink conditioning— findings that implicate these channels in synaptic plasticity, learning, and memory.
pH changes in the brain can also regulate neurotransmission by modulating the release and/or uptake of neurotransmitters and neuromodulators. In studies performed on isolated rat-brain synaptosomes, the depolarization-induced release of total glutamate was 15% less at pHo 6.0 than 7.4, although the uptake of glutamate was unaltered in the pHo range 6.0–7.4 (Fedorovich et al., 2003). However, such pH effects are dependent on the neurotransmitter/neuromodulator. For instance, in the same study, uptake of the acetylcholine precursor choline displayed a pHo dependence, with uptake being 80% less at pHo 6.0 compared to 7.4. In a different study on superfused rat hypothalamic synaptosomes, both an extracellular and an intraterminal acidification stimulated 3H-dopamine release through a Ca2+-dependent exocytotic process (Cannizzaro et al., 2003).
B) Cellular physiology and energetics of acid-base transporters
For a cell with a resting membrane potential of −60 mV, and bathed in a pH-7.3 solution, the electrochemical gradient favors the passive entry of protons (or conjugate weak acid) into the cell, and the passive exit of weak bases such as bicarbonate out of the cell, thereby leading to a decrease in pHi (towards the equilibrium pH of 6.3). The extent of such passive movement depends on the cell membrane’s permeability to protons or acid-base species through nonspecific pathways (see review by DeCoursey, 2003), or ion channels such as GABA-activated Cl− channels that can conduct HCO3− (Kaila and Voipio, 1987; Chen et al., 1990, 1992; Kaila et al., 1992). Passive H+ movement can also occur through Hv1-type voltage-gated H+ channels (Sasaki et al., 2006; Ramsey et al., 2006) originally described in snail neurons (Thomas and Meech, 1982), although depolarization-induced activation typically drives H+ out of cells. Finally, the transmembrane electrochemical gradient for H+ will favor the retention of metabolically produced H+. Thus, most cells are continually exposed to a chronic acid load that tends to lower pHi. Probably because so many important cellular processes are sensitive to pH (as described above), cells have evolved a system of acid-base transporters to regulate pHi above the equilibrium pH.
Acid-base transporters are classified as acid loaders or acid extruders. Acid loaders transport protons into or base equivalents out of cells, whereas acid extruders transport those ions in the opposite direction. Acid-base transporters are further classified as being HCO3−-independent or -dependent, and for many cells, the HCO3−-dependent transporters are the more powerful of the two classes in regulating pHi. NCBTs usually function as acid extruders by transporting HCO3− into cells and increasing pHi, although they can also function as acid loaders. The direction of transport is determined by the transporter stoichiometry, ion gradients, and membrane potential (for an electrogenic transporter). Every NCBT is Na+-dependent. However, as detailed in the next section, NCBTs represent a diverse family of transporters that are either electroneutral or electrogenic (with more than one Na:HCO3− stoichiometry), chloride-independent or chloride-dependent, and stilbene-sensitive or -insensitive.
C) Molecular physiology of NCBTs
We have made significant advances in understanding the importance of NCBTs in the functional nervous system with the cDNA cloning, molecular characterization, and localization of these proteins. Below, we describe the general characteristic features of the different NCBTs and their splice variants. A more extensive review of the molecular nature of NCBTs and their splice variants is presented in Boron et al. (2009), as well as Romero et al. (2009) who also discuss the contribution of Slc4 transporters to the functioning CNS.
a) NBCe1
The first cDNA encoding a NCBT was identified by Romero et al. (1997) who expression cloned the electrogenic NBC (NBCe1) from the proximal tubule of the salamander kidney— a preparation in which the first NBC was functionally identified (Boron and Boulpaep, 1983). Cloned NBCe1 expressed in Xenopus oocytes elicits a DIDS-sensitive, Na-dependent increase in pHi following a CO2-induced acidification when oocytes are exposed to a CO2/HCO3− solution. The electrogenicity of the transporter is evident by a hyperpolarization when oocytes are exposed to the HCO3− solution, and a depolarization when external Na+ is removed (Romero et al., 1997). NBCe1 was subsequently cloned and identified from other preparations (Table I), including zebrafish recently (Sussman et al., 2009). Three splice variants of NBCe1 (−A, −B, and −C) have been identified, and these variants differ only at their N and/or C termini. A common theme among the NCBTs is alternative splicing that produces variable N and C termini that can influence transporter function and regulation, and perhaps expression (see review by Boron et al., 2009). For example, the N terminus of NBCe1-B and −C (but not −A) contains an autoinhibitory domain that reduces transporter activity (McAlear et al., 2006). NBCe1 variants are also regulated by classic signaling pathways and protein-protein interactions (see Pushkin and Kurtz, 2006; Wu et al., 2009).
Table I.
Molecular characterization and localization of Na-Coupled Bicarbonate Transporters (NCBTs) in tissues
| NCBT | Variants | Alternate names | SLC | Reported tissue distribution1 | References |
|---|---|---|---|---|---|
| NBCe1 | A–C | aNBC2, akNBC3, hhNBC4, hpNBC5, kNBC6, kNBC-1, NBC1, pNBC7, rb1NBC8, rb2NBC9, rkNBC10, zNBCe111 | SLC4A4 | Zebrafish (cloning, B variant)12, zebrafish tissues (mRNA), zebrafish eye, gill (protein) | Sussman et al., 2009 |
| Ambystoma kidney (cloning, A variant), Ambystoma tissues (mRNA) | Romero et al.,1997 | ||||
| gerbil brain (protein) | Kang et al., 2002 | ||||
| murine duodenum (mRNA, protein), murine pancreas (mRNA) | Praetorius et al., 2001 | ||||
| murine colon, other tissues (mRNA) | Bachmann et al., 2003 | ||||
| mouse brain (protein) | Douglas et al., 2003 | ||||
| mouse kidney (protein) | Pushkin et al., 2004 | ||||
| mouse brain (protein) | Rickmann et al., 2007 | ||||
| mouse kidney, small intestine & colon (mRNA) | Gawenis et al., 2007 | ||||
| rat kidney (cloning, A variant), rat tissues (mRNA) | Romero et al.,1998 | ||||
| rat kidney (cloning, A variant), rat tissues (mRNA) | Burnham et al.,1998 | ||||
| rat epididymis (mRNA, protein) | Jensen et al., 1999 | ||||
| rat, Ambystoma, & rabbit kidney (protein) | Schmitt et al., 1999 | ||||
| rat salivary gland (protein) | Roussa et al., 1999 | ||||
| rat pancreas (cloning, B variant), rat pancreas (protein) | Thévenod et al.,1999 | ||||
| rat brain (cloning, B & C variants), rat tissues (protein) | Bevensee et al., 2000 | ||||
| rat brain (cloning, B variant), kidney, brain, & tissues (mRNA) | Giffard et al., 2000 | ||||
| rat brain (mRNA, protein) | Schmitt et al., 2000 | ||||
| rat & Ambystoma kidney (protein) | Maunsbach et al., 2000 | ||||
| rat eye & conjunctiva (protein) | Bok et al., 2001 | ||||
| rat brain (protein) | Douglas et al., 2001 | ||||
| rat kidney (protein) | Kwon et al., 2002 | ||||
| rat kidney (mRNA) | Xu et al., 2003 | ||||
| rat kidney & pancreas (mRNA,protein),human pancreas (protein) | Satoh et al., 2003 | ||||
| rat kidney (mRNA) | Abuladze et al., 2004 | ||||
| rat pancreas (mRNA) | Abuladze et al., 2004 | ||||
| rat kidney (mRNA) | Praetorius et al., 2004b | ||||
| rat brain (protein) | Jung et al., 2007 | ||||
| rat heart (mRNA) | Yamamoto et al., 2007 | ||||
| rat brain (mRNA, protein) | Majumdar et al., 2008 | ||||
| rat pancreas (mRNA, protein) | Soyfoo et al., 2009 | ||||
| bovine cornea (mRNA, protein) | Sun et al., 2000 | ||||
| human kidney (cloning, A variant), human kidney & pancreas (mRNA) | Burnham et al.,1997 | ||||
| human kidney (mRNA) | Abuladze et al.,1998 | ||||
| human pancreas (cloning, B variant), human tissues & mouse pancreas (mRNA) | Abuladze et al., 1998 | ||||
| human heart (cloning, B variant), human pancreas (mRNA) | Choi et al., 1999 | ||||
| human cornea (mRNA) | Usui et al., 1999 | ||||
| human pancreas (mRNA, protein) | Marino et al., 1999 | ||||
| human cornea (mRNA, protein) | Sun and Bonanno, 2003 | ||||
| human tissues (mRNA), human duodenum (protein) | Damkier et al., 2007 | ||||
| NBCe2 | A–F | NBC4 | SLC4A5 | mouse & rat choroid plexus (mRNA) | Praetorius et al., 2004b |
| mouse & rat choroid plexus (protein) | Bouzinova et al., 2005 | ||||
| mouse & rat choroid plexus (protein) | Damkier et al., 2009 | ||||
| rat liver (cloning, E & F variants), rat tissues (mRNA), | Xu et al., 2003 | ||||
| rat liver (cloning, C variant), rat testis (mRNA), rat liver & kidney (mRNA, protein) | Abuladze et al., 2004 | ||||
| rat heart (mRNA) | Yamamoto et al., 2007 | ||||
| human testis (cloning, A variant), human tissues (mRNA) | Pushkin et al., 2000b | ||||
| human heart (cloning, B variant) | Pushkin et al., 2000a,b | ||||
| human testis (cloning, C variant), human tissues (mRNA) | Sassani et al., 2002 | ||||
| human tissues (cloning, C variant) | Virkki et al., 2002 | ||||
| human tissues (mRNA), human kidney (protein) | Damkier et al., 2007 | ||||
| NBCn1 | A–H | NBC3 | SLC4A7 | mouse ear & eye (protein) | Bok et al., 2003 |
| mouse ear (protein) | Lopez et al., 2005 | ||||
| mouse brain (protein) | Chen et al., 2007 | ||||
| mouse tissues (mRNA, protein) | Boedtkjer et al., 2008 | ||||
| mouse choroid plexus (protein) | Damkier et al., 2009 | ||||
| rat smooth muscle (cloning, B–D variants), rat tissues & aorta (mRNA) | Choi et al., 2000 | ||||
| rat kidney (protein) | Vorum et al., 2000 | ||||
| rat aorta & murine duodenum (mRNA), murine duodenum (protein) | Praetorius et al., 2001 | ||||
| rat brain (protein) | Risso-Bradley et al., 2001 | ||||
| rat kidney (protein) | Kwon et al., 2002 | ||||
| rat salivary glands, kidney (mRNA) | Gresz et al., 2002 | ||||
| rat kidney (mRNA, protein) | Odgaard et al., 2004 | ||||
| rat kidney (mRNA, protein) | Praetorius et al., 2004a | ||||
| rat brain (mRNA), rat, mouse choroid plexus (mRNA, protein) | Praetorius et al., 2004b | ||||
| rat brain (cloning, E variant), rat brain (mRNA, protein) | Cooper et al., 2005 | ||||
| rat tissues (mRNA, protein) | Damkier et al., 2006 | ||||
| rat heart (mRNA) | Yamamoto et al., 2007 | ||||
| rat brain (protein) | Cooper et al., 2009 | ||||
| human skeletal muscle (cloning, A variant), human skeletal muscle & heart (mRNA) | Pushkin et al.,1999a | ||||
| human salivary glands & kidney (mRNA), rat & human salivary glands & kidney (protein) | Gresz et al., 2002 | ||||
| human choroid plexus (protein) | Praetorius & Nielsen, 2006 | ||||
| human tissues (mRNA), human kidney & duodenum (protein) | Damkier et al., 2007 | ||||
| NBCn2 | A–D | NCBE, rb1NCBE13, rb2NCBE14 | SLC4A10 | insulin-secreting cell line (cloning, A variant), the cell line, mouse pancreas, & rat tissues (mRNA) | Wang et al., 2000 |
| mouse brain, other tissues (mRNA) | Hübner et al., 2004 | ||||
| mouse brain (mRNA, protein) | Jacobs et al., 2008 | ||||
| mouse brain (protein) | Damkieret al., 2009 | ||||
| mouse brain (protein) | Liu et al., 2010 | ||||
| rat brain (cloning, B & C variants), mouse brain (mRNA & protein), rat tissues (mRNA) | Giffard et al., 2003 | ||||
| rat brain (mRNA), rat & mouse choroid plexus (mRNA, protein) | Praetorius et al., 2004b | ||||
| rat & mouse brain (protein) | Chen et al., 2007 | ||||
| rat & mouse brain (protein) | Chen et al., 2008c | ||||
| human choroid plexus (protein) | Praetorius & Nielsen, 2006 | ||||
| human brain & choroid plexus, other tissues (mRNA) | Damkier et al., 2007 | ||||
| human brain (cloning, A & B variants), human brain (mRNA, A & B variants) | Parker et al., 2008a | ||||
| NDAE | NDAE1 | Drosophila (cloning), Drosophila CNS & other tissues (mRNA) | Romero et al., 2000 | ||
| Drosophila nervous system, sensory organs, & other tissues (protein) | Sciortino et al., 2001 | ||||
| NDCBE | A–D, D' | kNBC-3, NDCBE1 | SLC4A8 | squid giant fiber lobe (cloning, A variant), squid tissues (mRNA) | Virkki et al., 2003 |
| mouse kidney (cloning, A variant), mouse tissues (mRNA) | Wang et al., 2001 | ||||
| rat brain (protein) | Risso-Bradley et al., 2001 | ||||
| rat brain (mRNA) | Praetorius et al., 2004b | ||||
| rat & mouse brain (protein) | Chen et al., 2008a,b | ||||
| human brain (cloning, B variant), human brain & tissues (mRNA) | Grichtchenko et al., 2001 | ||||
| human brain, choroid plexus, & other tissues (mRNA), human brain (protein) | Damkier et al., 2007 | ||||
| human brain (cloning, A, C, D, D' variants), human brain (mRNA, A variant), human tissues (mRNA, C, D, & D') | Parker et al., 2008b |
In many studies, the molecular probes used (e.g., primers in PCR, oligonucleotides with in-situ hybridization, and antibodies with immunochemical techniques) recognized or are expected to recognize multiple variants (often unavoidably).
Ambystoma NBC (aNBC)
Ambystoma kidney NBC (akNBC)
human heart NBC (hhNBC)
human pancreas NBC (hpNBC)
kidney NBC (kNBC)
pancreas NBC (pNBC)
rat brain 1 NBC (rb1NBC)
rat brain 2 NBC (rb2NBC)
rat kidney NBC (rkNBC)
zebrafish NBCe1 (zNBCe1)
The amino sequence is 82% identical to NBCe1-B
rat brain 1 NCBE (rb1NCBE)
rat brain 2 NCBE (rb2NCBE)
The importance of NBCe1 in physiologic functions is highlighted by results from studies on human patients with NBCe1 gene mutations and data from a more recent NBCe1 knockout mouse. At present, investigators have identified human patients with one of either two frameshift mutations, seven missense mutations, or three nonsense mutations in the SLC4A4 gene. All these patients exhibit proximal renal tubule acidosis, and most of them also have ocular abnormalities, including glaucoma and/or cataract formation (Igarashi et al., 1999; Dinour et al., 2004; Horita et al., 2005; Demirci et al., 2006; Suzuki et al., 2010). Although neurological abnormalities are not a consistent finding in all patients, six of them experience migraines (Suzuki et al., 2010), three are mentally retarded (Igarashi et al., 1999; Igarashi et al., 2001; Horita et al., 2005), and two others displayed developmental disorders (Horita et al., 2005; Demirci et al., 2006). These mutations can lead to loss of NBC-mediated HCO3− transport by either decreasing activity of the transporter (Igarashi et al., 1999; Dinour et al., 2004; Horita et al., 2005) or reducing expression at the plasma membrane (Li et al., 2005; Horita et al., 2005; Toye et al., 2006; Suzuki et al., 2010).
Gawenis et al. (2007) developed an NBCe1 null-mutant mouse by disrupting the Slc4a4 gene. The NBCe1 null-mutant mice, which died early before weaning, had severe metabolic acidosis, abnormalities in dentition, growth retardation, splenomegaly, hyponatremia, and impaired HCO3− secretion in the colon. The mice had very thin and transparent skulls due to defects in bone mineralization― an effect likely due to the metabolic acidosis. Although the null-mutant mice did not exhibit any overt neurological defects, further neurologic studies may reveal alterations in learning and memory and susceptibility to seizures. Of course, the compensatory expression of other acid-base transporters in these knockout mice during development may have masked an important role of NBCe1 in the nervous system.
b) NBCe2
From human heart and testis, Ira Kurtz’s group cloned cDNAs encoding two variants of another member of the NBC family —NBCe2 or NBC4— that exhibit sequence similarity to NBCe1 (Pushkin et al., 2000a; Pushkin et al., 2000b). Subsequently, four additional NBCe2 variants were identified. The six variants (NBCe2-A to −F) differ at their C termini and/or last third of the transmembrane domain region. The A through D variants, but not E and F, are reported in the human genome database. At present, only the C variant of the cloned NBCe2s has been functional characterized and determined to be electrogenic (Virkki et al., 2002). Similar to the aforementioned studies on NBCe1, NBCe2-C expressed in oocytes and studied with voltage- and pH-sensitive microelectrodes elicits a DIDS-sensitive, Na-dependent increase in pHi following a CO2-induced acidification. The electrogenicity of the transporter is evident by a hyperpolarization when oocytes are exposed to the HCO3− solution, a depolarization when external Na+ is removed, and Na+- and HCO3−-induced currents under voltage-clamp conditions. These electrogenic signals are blocked by DIDS. NBCe2 at the RNA and protein levels has been detected in rodent and human tissues (Table I).
c) NBCn1
cDNA encoding the transporter (NBCn1-A) was first cloned from human skeletal muscle (Pushkin et al., 1999a), and then three additional variants (NBCn1-B, −C, and − D) were cloned shortly thereafter from rat smooth muscle (Choi et al., 2000). When expressed in oocytes, NBCn1-A (originally named NBC3) stimulated Cl−-independent, DIDS-insensitive 22Na uptake (Pushkin et al., 1999a). In subsequent pHi experiments on NBCn1-expressing oocytes, removing external Na+ in the presence of CO2/HCO3− produced an acidification, while raising external K+ to depolarize the membrane had no effect on pHi. These findings are consistent with NBCn1 being electroneutral.
The electroneutrality of NBCn1 was particularly evident from microelectrode studies on oocytes expressing NBCn1-B cloned from rat smooth muscle (Choi et al., 2000). NBCn1-B-expressing oocytes displayed a Na+- and HCO3−-dependent, yet Cl−- independent pHi recovery following a CO2-induced acid load. This pHi recovery was only modestly inhibited by DIDS. While the transporter is electroneutral based on the absence of a CO2/HCO3−-induced hyperpolarization, a Na+ conductance is associated with the transporter. NBCn1 is ubiquitously expressed (Table I). In additional to the aforementioned four splice variants, four additional ones have been identified. These eight variants (NBCn1-A to −H) have an identical transmembrane domain region, but different splice cassettes at their cytoplasmic N and/or C termini.
Bok et al. (2003) have developed an NBCn1 knockout mouse that is blind and displays defects in hearing. Details of this study are presented in a subsequent section.
d) NBCn2 (NCBE)
cDNA encoding the transporter was first cloned from an insulin- secreting cell line MIN6 cDNA library and called a Na-driven Chloride/Bicarbonate Exchanger (NCBE) (Wang et al., 2000). Based on 22Na+, 36Cl−, and pHi studies with oocytes or HEK293 cells heterologously expressing the transporter, the authors concluded that the transporter exchanges extracellular Na+ and HCO3− for intracellular Cl− and H+. However, the observations reported in the study that removing external Cl− inhibited both 22Na+ uptake and 36Cl− efflux in NCBE-expressing oocytes (which are difficult to Cl− deplete) are opposite to that predicted for a functional Na-driven Cl-HCO3 exchanger. Furthermore, according to a study with voltage- and pH-sensitive microelectrodes, the transporter expressed in oocytes is electroneutral and responsible for a DIDS-sensitive, Na+- and HCO3−-dependent, but Cl−-independent pHi recovery following a CO2-induced acidification (Parker et al., 2008a). Using both Cl−-sensitive microelectrodes to measure surface Cl− transients and 36Cl− to monitor Cl− efflux, the authors determined that Cl− flux across the membrane is not coupled to Na+ and HCO3− transport, but rather, is linked to CO2/HCO3−-stimulated, but Na+-independent, Cl− self exchange (Parker et al., 2008a). Interestingly, the group did observe that NBCn2 (NCBE) is capable of NDCBE-like activity in the absence of extracellular Cl−.
The function of NBCn2 (NCBE) may also be cell-type dependent. Giffard et al. (2003) presented evidence that two NBCn2 (NCBE) splice variants (−B and −C) expressed in NIH 3T3 cells display external Na+, Cl−, and HCO3−-dependent pHi recoveries from acid loads. In a more extensive analysis, Damkier et al. (2010) recently reported that NBCn2 (NCBE) functions as a Na-driven Cl-HCO3 exchanger when expressed in NIH-3T3 fibroblasts. The transporter exhibited a DIDS-sensitive, HCO3−-dependent pHi increase elicited by returning external Na+ following an acid load. In parallel experiments, returning external Na+ stimulated a DIDS-sensitive, HCO3−-dependent 36Cl− efflux, but no HCO3−-dependent 36Cl− influx.
Four splice variants (NBCn2 (NCBE)-A through −D) have been identified with different internal cytoplasmic N terminal splice cassettes and/or different cytoplasmic C termini. NBCn2 (NCBE) displays pronounced expression in brain (Table I), and the −B and −C variants were cloned from rat brain (Giffard et al., 2003). Jacobs et al. (2008) developed an NBCn2 (NCBE) knockout mouse that displays a high seizure threshold and a reduced brain ventricular volume. Details of the study are described in a subsequent section.
e) NDCBE/NDAE
The first cDNA encoding a Na-Driven Anion Exchanger (NDAE) was identified from Drosophila, and the protein expressed in oocytes mediates DIDS-sensitive transport of Na+, Cl−, and H+/HCO3− (Romero et al., 2000). However, NDAE does not have an absolute requirement for HCO3− and exhibits some electrogenicity. Shortly thereafter, a cDNA encoding a related Na-Driven Cl-Bicarbonate Exchanger (NDCBE) was cloned from human brain (Grichtchenko et al., 2001). In functional studies using voltage-, Na+-, and pH-sensitive microelectrodes, as well as a 36Cl− efflux assay, NDCBE expressed in oocytes induced a DIDS-sensitive, Na+- and HCO3−-dependent pHi recovery from a CO2-induced acidification. Na+ and HCO3− efflux from oocytes mediated by NDCBE required extracellular Cl−. Also, CO2/HCO3−-induced stimulation of the transporter elicited a DIDS-sensitive and Na+-dependent increase in 36Cl− efflux, as well as a DIDS-sensitive increase in intracellular [Na+]. Four variants of NDCBE have been identified that differ at the cytoplasmic N and/or C termini. Similar to NBCn2 (NCBE), NDCBE also exhibits pronounced expression in brain (Table I).
On a related note, the cloned cDNA encoding the anion bicarbonate transporter ABTS-1 from C. elegans is ~50% identical to the cDNA encoding NDCBE (Sherman et al., 2005). ABTS-1 heterologously expressed in Xenopus oocytes exhibits Cl-HCO3 exchanger activity.
2) Functional Evidence for NCBTs in the Nervous System
A) Neurons
The Na-driven Cl-HCO3 exchanger was the first pHi-regulating transporter functionally identified and shown to be the major acid extruding mechanism in classic neuronal preparations including the squid giant axon (Boron and De Weer, 1976a; Boron and De Weer, 1976b; Russell and Boron, 1976; Russell and Boron, 1981; Boron and Russell, 1983) and the snail neuron (Thomas, 1976a; Thomas, 1976b; Thomas, 1977). Work on these two preparations was performed simultaneously, but independently, by Walter Boron and Roger Thomas— two pioneers in the field of pHi regulation.
In both preparations, the recovery of pH from an acid load required external HCO3− (Boron and De Weer, 1976a; Thomas, 1977), external Na+ (Thomas, 1977; Boron and Russell, 1983), intracellular Cl− (Russell and Boron, 1976; Thomas, 1977), and intracellular ATP (Russell and Boron, 1976; Boron and Russell, 1983). The transporter was also inhibited by SITS (Russell and Boron, 1976; Boron and Russell, 1983; Thomas, 1977). Based on pHi measurements, as well as 22Na+ and 36Cl − efflux/influx data, the transporter moves 1 Na+ and 2 HCO3− (or equivalent species) in one direction and 1 Cl− in the opposite direction (Boron and Russell, 1983). The acid extrusion rate was pHi dependent and stimulated by a decrease in pHi (Boron and Russell, 1983). In the squid axon, intra-axonal ATP is required for a functional transporter, although not as an energy source (Boron et al, 1988) because the ATP effect can be mimicked by non-hydrolysable ATPγS. The transporter’s KM for ATP is 124 µM (Davis et al, 2008).
The Na-driven Cl-HCO3 exchanger also appears to be the major HCO3− -dependent acid extruder in mammalian neurons. In CA1 neurons acutely isolated from the hippocampus of neonatal rats, applying CO2/HCO3− elicited a pHi recovery from the initial CO2-induced acidification that was Na+-dependent and DIDS-sensitive (Schwiening and Boron, 1994). Cl− dependence of the transporter was evident by progressive decreases in the rate and magnitude of HCO3−-induced pHi increases when the neurons were exposed several times to the CO2/HCO3− solution in the continued absence of external Cl− (Fig. 1, gh vs. de vs. ab). The transporter appears to slow itself down by transporting Cl− out of the cell and lowering intracellular Cl−, which can not be replenished in the absence of external Cl−. This slowing down could also be explained by a Na/HCO3 cotransporter that functions like a Na-driven Cl-HCO3 exchanger in the absence of external Cl−. In additional studies, results from pHi experiments implicated the presence of a Na-driven Cl-HCO3 exchanger in other mammalian neurons (see Bevensee and Boron, 1998).
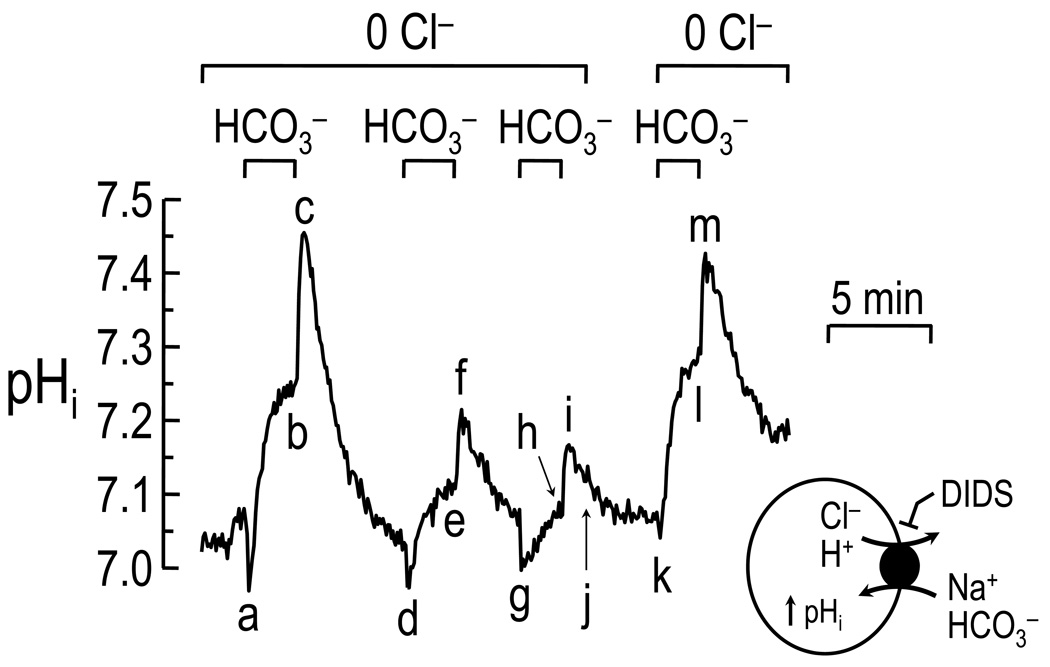
Fig. 1.
Intracellular Cl− dependence of the Na-coupled HCO3− transporter in a single CA1 neuron acutely isolated from the hippocampus of a neonatal rat. pHi was measured using the pH-sensitive dye BCECF. At the beginning of the experiment, the neuron was bathed in a Cl−-free, HEPES-buffered solution. Switching to a Cl−-free solution containing CO2/HCO3− elicited an initial decrease in pHi (point a) due to CO2 influx, followed by a rapid increase in pHi (ab) due to a DIDS-sensitive, Na+- and HCO3−-dependent transporter. Returning to the HEPES-buffered solution caused a large increase in pHi (bc) due to CO2 efflux, followed by a slower decrease in pHi due to the activity of acid-loading mechanisms. Exposing the neuron to the Cl−-free, HCO3− solution two additional times (def and ghi) elicited progressively slower and less pronounced pHi recoveries from the initial CO2-induced acidifications (de and gh), presumably due to a progressive decrease in intracellular Cl−. Returning the neuron to a Cl−-containing solution briefly (jk) presumably refills intracellular Cl−, thereby allowing the return of the robust HCO3−-induced pHi increase (kl), even in the absence of external Cl−. Modified from Schwiening and Boron (1994) with kind permission from John Wiley & Sons Ltd.
According to early studies, electroneutral NCBTs are the dominant acid extruding bicarbonate transporters in neurons (see below). However, more recent data support the function and expression of an electrogenic NBC in cultured mammalian neurons. For example, in pHi experiments on cultured rat hippocampal neurons, removing external Na+ elicited a pHi decrease that was partially due to a HCO3−-dependent, Cl−-independent process sensitive to niflumic acid (Williams et al., unpublished data). In whole-cell and perforated patch-clamp experiments on the same preparation, applying a CO2/HCO3− solution elicited a Na+-dependent outward current, whereas removing external Na+ in the presence of CO2/HCO3− induced an inward current in a population of the neurons (Majumdar et al., unpublished data). Further studies, particularly with NCBT inhibitors, are required to identify and characterize this presumed electrogenic NBC in the hippocampal neurons.
As discussed in the localization section below, using immunohistochemical techniques, Cooper et al. (2005) reported the expression of NBCn1 in the synapses and somatodendrites in embryonic rat hippocampal neurons. They proposed that the possible role of NBCn1 is to regulate pHi at the synapse and therefore modulate neuronal activity.
B) Glia
Boron and Boulpaep (1983) working on perfused proximal tubules from the salamander kidney functionally identified an electrogenic NBC as the first cation-coupled HCO3− transporter. The basolateral transporter was responsible for a SITS-sensitive decrease in both pHi and intracellular Na+ activity (aNa+i), as well as a depolarization of the basolateral membrane upon lowering basolateral pH or HCO3−. Removing Cl− had no effect on these changes in pHi or basolateral membrane potential. The electrogenicity of the transporter was particularly evident from the following now-recognized hallmark observation: lowering/removing external Na+ elicited a simultaneous depolarization and decrease in pHi. This transporter in basolateral membrane vesicles from rabbit kidney cortex has a 1:3 Na+:HCO3− stoichiometry based on a thermodynamic analysis (Soleimani et al., 1987).
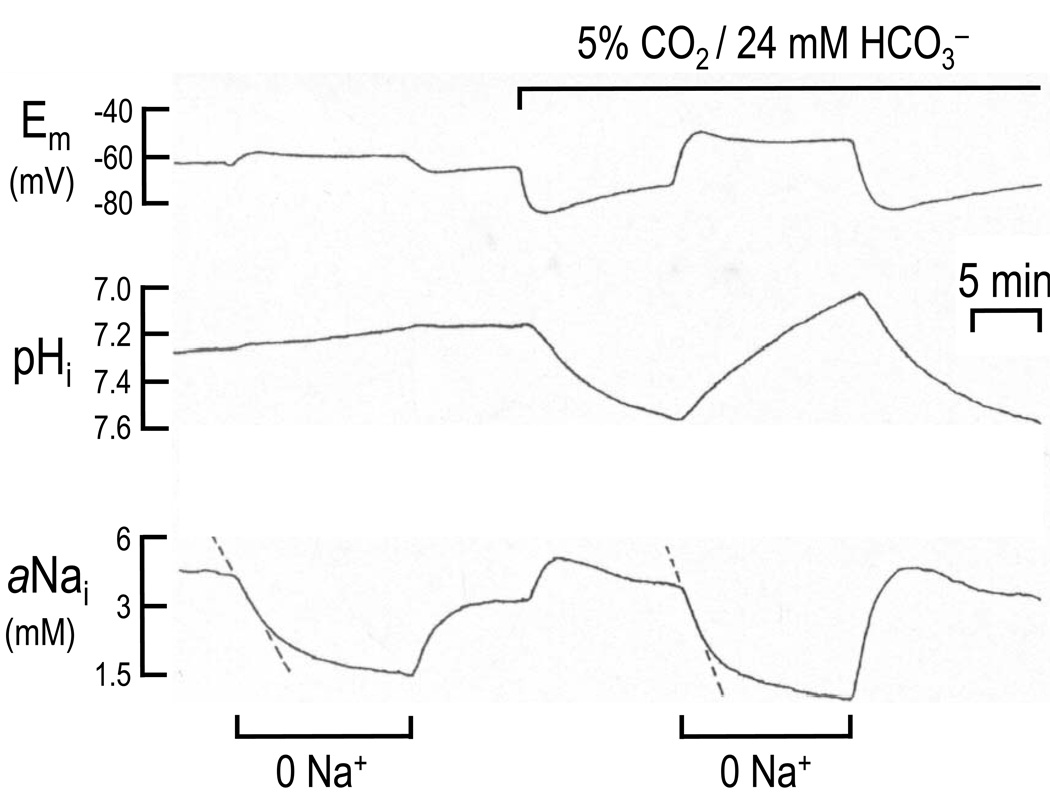
In the CNS, an electrogenic NBC was first characterized by Deitmer and Schlue working on the giant neuropile glial cells of the leech (Deitmer and Schlue, 1987; Deitmer and Schlue, 1989; Deitmer, 1992). The transporter was responsible for a CO2/HCO3−-induced alkalization and hyperpolarization that was DIDS-sensitive and Na+-dependent (Deitmer and Schlue, 1989). The HCO3−-induced alkalization was not affected by either removing extracellular Cl − or depleting the cells of intracellular Cl−. The HCO3− solution also elicited an expected increase in intracellular Na+. The electrogenic nature of the transporter was also evident from the following simultaneous, HCO3−-dependent changes elicited by removing external Na+ (Fig. 2): a pronounced depolarization (top trace), a decrease in pHi (middle trace), and a faster decrease in aNa+i than seen in the nominal absence of HCO3− (bottom trace) (Deitmer, 1992). In leech glial cells, the transporter appears to have a stoichiometry of 1:2 Na:HCO3− (Deitmer and Schlue, 1989; Deitmer, 1991).
Fig. 2.
Electrogenic Na/Bicarbonate Cotransporter (NBC) activity in a giant neuropile glial cell from leech. Membrane potential (Em), pHi, and aNai were measured using ion-sensitive and voltage electrodes. For the glial cell bathed in a HEPES-buffered solution, removing external Na+ had little effect on Em and pHi, and elicited a decrease in aNai. However, after the glial cell was switched to a CO2/HCO3− solution, removing external Na+ caused a depolarization, a decrease in pHi, and a faster decrease in aNai than observed in HEPES. These three responses are consistent with an electrogenic NBC moving Na+, HCO3−, and net-negative charge out of the cell when exposed to the Na+-free solution. Modified from Deitmer (1992), Fig. 3, © Springer-Verlag, 1992 with kind permission of Springer Science+Business Media.
Using similar approaches, the transporter was subsequently identified and/or studied in other glial cell preparations including connective glial cells from leech (Szatkowski and Schlue, 1992), astrocytes from the Necturus optic nerve (Astion and Orkand, 1988), as well as mammalian astrocytes cultured from rat forebrain (Boyarsky et al., 1993), hippocampus (Bevensee et al., 1997a; Bevensee et al., 1997b; Pappas and Ransom, 1994), cerebellum (Brune et al., 1994) and cortex (Shrode and Putnam, 1994). In at least rat hippocampal astrocytes, the mammalian electrogenic NBC appears to have a 1:2 Na+:HCO3− stoichiometry (Bevensee et al., 1997a; Bevensee et al., 1997b).
Electrogenic NBC activity has also been functionally characterized in mammalian brain slices. In gliotic hippocampal slices, electrogenic NBC activity contributes to a depolarization-induced alkalization and an extracellular acid shift (Grichtchenko and Chesler, 1994a; Grichtchenko and Chesler, 1994b). Applying Ba2+ or raising extracellular K+ produces a Na+-dependent intracellular alkalization and an extracellular acidification that are both enhanced in the presence of CO2/HCO3−. In contrast to results from in vitro studies on cultured astrocytes, the in situ NBC is not sensitive to stilbene derivatives.
Groups have reported evidence for Na-driven Cl-HCO3 exchanger activity in astrocytes, including those cultured from rat brain (Mellergård et al., 1993; Shrode and Putnam, 1994). For example, in rat astrocyte and C6 glioma cell cultures, exposing cells to a CO2/HCO3− solution elicited a pHi recovery following the initial CO2-induced acidification. This HCO3−-induced pHi recovery was dependent on external Na+, blocked by DIDS, and inhibited by incubating the cells for 2 h in the absence of external Cl− (Shrode and Putnam, 1994).
There is evidence for a Na+- and HCO3−-dependent, and perhaps Cl−- independent transporter in cultured oligodendrocytes from embryonic mouse spinal cord (Kettenmann and Schlue, 1988) and adult rat cerebellum (Boussouf et al., 1997). However, the electrogenicity/electroneutrality of an oligodendrocyte NBC has yet to be determined. Support for a functional electrogenic NBC in oligodendrocytes comes from immunolocalization data in which an NBCe1-A/B antibody labels dendrites of oligodendrocytes cultured from mouse and rat (Ro and Carson, 2004).
C) Epithelial cells of choroid plexus
As reviewed in more detail by McAlear and Bevensee (2003), according to early studies on pH-regulating transporters in the choroid epithelium, a Na-H exchanger and an anion-exchanger (AE) on the basolateral membrane in conjunction with a possible NBC or HCO3−-conducting anion channel on the apical membrane contribute to both the ionic composition and pH of the cerebrospinal fluid (CSF). According to more recent functional data, multiple Na-coupled HCO3− transporters appear to be involved.
Using the pH-sensitive dye BCECF, Bouzinova et al. (2005) measured pHi of epithelial cells from the rat choroid plexus to characterize HCO3− transporters involved in both the pHi recovery from an acid load and the pHi increase elicited by applying CO2/HCO3− at resting pHi in the nominal absence of the physiologic buffer. In both assays, the HCO3−-induced pHi increase was Na+ dependent, but only the pHi increase elicited by applying CO2/HCO3− at resting pHi was inhibited by DIDS. Cl−-free media had no effect on the pHi recoveries. In light of additional immunologic evidence presented that the cells express NBCe2, as well as previous studies demonstrating the expression of NBCn1 and NBCn2 (NCBE) (see below), the authors conclude that rat choroid plexus epithelial cells have both a DIDS-insensitive NCBT (probably NBCn1) and a DIDS-sensitive NCBT (probably either NBCe2 and/or NBCn2 (NCBE)). More recently, Chen et al. (2008b) have localized NDCBE to the basolateral membrane of the choroid plexus of fetal (but not adult) rats.
In a recent functional study, Millar and Brown (2008) measured small DIDS-sensitive, Na+-dependent, HCO3−-induced outward currents from I–V plots obtained from mouse choroid plexus epithelial cells. From the reversal potentials of these I–V plots, the authors concluded that the NBC —which may be NBCe2 according to the previous discussion— has a 1:3 Na+:HCO3− stoichiometry. With the membrane potential of the choroid plexus epithelial cell estimated to be −35 mV to −60 mV, this NBC would be expected to mediate HCO3− secretion into the CSF given the Na+ and HCO3− gradients across the apical membrane.
3) Localization of NCBTs in the Nervous System
A) Electrogenic Na/HCO3 cotransporter, NBCe1
NBCe1 was the first NCBT localized in the CNS—not surprising because it was the first NCBT cloned and subsequently used to generate molecular probes including PCR primers, cRNA probes, and antibodies. Schmitt et al. (2000) and Giffard et al. (2000) performed the early NBCe1 localization studies on rat brain using antibodies and polynucleotide probes— nearly all of which did not distinguish among the NBCe1 splice variants. Both groups found wide-spread expression of NBCe1 throughout the brain in a pattern consistent with glial-cell expression. According to double-labeling studies using an antibody to the glial fibrillary acidic protein (GFAP) and an in situ hybridization probe to NBCe1, the transporter was evident in astrocytes and Bergmann glia in the cerebellum (Giffard et al., 2000). Schmitt et al. (2000) performed in situ hybridization co-localization studies with probes to the astrocytic glutamate transporter 1 (GLT1) and NBCe1. The authors observed NBCe1 mRNA expression in the astrocytes of the cortex, dentate gyrus, and brainstem. In double-labeling studies using antibodies to GFAP and NBCe1, the authors found expression of NBCe1 in astrocytes in hippocampus and cerebellum. NBCe1 mRNA was also evident in neurons of the piriform and entorrhinal cortex, cerebellum, olfactory bulb, dentate gyrus and striatum. Using an antibody to the microtubule associated protein 2 (MAP2), a neuronal marker, Schmitt et al. also found NBCe1 expression in Purkinje neurons of the cerebellum, as well as neurons in the dentate gyrus, pyramidal and molecular cell layers, and stratum oriens of the hippocampus.
NBCe1 has also been identified in human brain. Using human NBCe1 primers that did not discriminate among the NBCe1 splice variants, Damkier et al. (2007) found NBCe1 mRNA in human cerebellum, cerebrum, hippocampus, and choroid plexus.
As mentioned earlier, NBCe1 has three splice variants. Recently, Rickmann et al. (2007) reported on the expression of NBCe1-A and B in mouse brain using immunohistochemistry and immunoelectron microscopy. The authors found expression of NBCe1-A throughout brain, particularly in neuronal populations in the cerebellum, hippocampus, cerebral cortex, and olfactory bulb. In contrast, they reported the expression of NBCe1-B in astroglia and Bergmann glia. The results from studies by Rickmann et al. were different from earlier cloning/localization results in three ways. First, the cDNA encoding the A variant of NBCe1 has not been identified in a homology cloning study of NBCe1 from rodent brain (Bevensee et al., 2000). Second, Giffard et al. (2000) reported preliminary data that an in situ hybridization probe specific to the A variant did not appreciably label cells in rat brain. Finally, the antibody used by Rickmann et al. to localize NBCe1-B is also expected to recognize the C variant.
Using mRNA and protein localization techniques, Majumdar et al. (2008) examined the presence and expression profiles of the three NBCe1 variants in rat brain. According to in situ hybridization data, NBCe1-B and likely −C (rather than −A) are the predominant variants expressed in rat brain, with high levels in the cerebellum and hippocampus. Using immunolabeling techniques and antibodies to neuronal and astrocytic markers, the authors found i) diffuse labeling throughout the brain with antibodies to the A/B variant and C variant, and ii) evidence that NBCe1-B is localized intracellularly in neurons (e.g., in the hippocampus and cortex), whereas NBCe1-C is expressed at the plasma membrane in glia surrounding neurons and possibly neurons themselves (e.g., in pyramidal and granule layers of the hippocampus, the Purkinje layer of the cerebellum, and the cortex.)
NBCe1 mRNA and protein levels are developmentally regulated. In the aforementioned in situ hybridization study on rat brain, Giffard et al. (2000) first detected NBC mRNA at embryonic day-17 (E17) in the spinal cord. In rat forebrain, NBC mRNA was first evident at postnatal day-0 (P0). The authors found that NBC mRNA persisted into adulthood and the level was highest at P15. Using immunoblotting techniques with a nonspecific NBCe1 antibody, Douglas et al. (2001) found that NBCe1 expression increases gradually from E16 to P105 in the cerebral cortex, cerebellum, and brain-stem diencephalon, but to a much lesser degree in the cortex.
Sussman et al. (2009) recently cloned by homology the cDNA encoding NBCe1 from zebrafish (zNBCe1), which is most similar to mammalian NBCe1-B. By 72 h post fertilization, zNBCe1 transcript was detected in brain, ependymal cells lining the brain ventricles, and the inner nuclear layer of the retina. In the adult zebrafish, zNBCe1 protein was found in the retina, particularly the ganglion cell layer and the photoreceptor layer.
B) Electrogenic Na/HCO3 cotransporter, NBCe2
NBCe2 mRNA in brain has been localized to rat and mouse choroid plexus epithelial cells (Praetorius et al., 2004b). In immunofluorescence studies with an antibody to the N-terminal 22 residues of NBCe2, Bouzinova et al. (2005) identified expression in the apical membrane of mouse and rat choroid plexus. Such apical expression was reinforced by results from electron microscopy studies with immunogold labeling of the apical microvillar projections from the mouse choroid plexus. Interesting, in a study by Praetorius and Nielsen (2006), the authors did not detect NBCe2 in human choroid plexus. The specific variant of NBCe2 expressed in rodent choroid plexus is not known.
NBCe2 is also present in human brain. Using RT-PCR and human NBCe2 primers that are expected to amplify all splice variants of NBCe2 cDNA, Damkier et al. (2007) found mRNA expression in human cerebellum, cerebrum, hippocampus, and choroid plexus.
C) Electroneutral Na/HCO3 cotransporter, NBCn1
In a preliminary study, Risso Bradley et al. (2001) used immunohistochemistry and found NBCn1 expression in nerve fibers of the hippocampal slice. In a more recent study, Cooper et al. (2005) used RT-PCR techniques and found NBCn1-B in hippocampal neurons cultured from embryonic rats and NBCn1-E in neurons cultured from adult rat hippocampal slices. According to single-cell RT-PCR data with primers to NBCn1, glutamic acid decarboxylase 65 (GAD65), and GAD67, the authors found that NBCn1-B was present in both excitatory (GAD-negative) and inhibitory (GAD-positive) embryonic neurons. In immunocytochemistry studies, the authors found expression of NBCn1 at the plasma membrane of both the soma and dendrites of embryonic neurons. According to double-labeling antibody studies, NBCn1 partially colocalized with post synaptic density protein-95 (PSD-95) at excitatory synapses. More recently, the authors have generated new NBCn1 antibodies and identified NBCn1 expression in neurons in many regions of rat brain, including hippocampal pyramidal neurons, dendate gyrus granular neurons, posterior cortical neurons, and cerebellar Purkinje neurons, as well as in the basolateral membrane of the choroid plexus epithelia (Park et al., 2010). NBCn1 is expressed at the soma and dendrites of CA3 neurons, and partially colocalizes with PSD-95 in the dendrites.
Boedtkjer et al. (2008) used an antibody-independent technique to perform a more systemic analysis of NBCn1 localization in mouse brain. This immunoreactive-independent technique involves generating a transgenic mouse with the LacZ gene under the control of the NBCn1 promoter. A colorometric product of β-galactosidase activity can be used as a marker for NBCn1 transcriptional activity. In brain, β-galactosidase staining was seen in several regions including the cortex, hippocampus, cerebellum, and epithelial cells of the choroid plexus. Staining was evident in all the layers of the hippocampus including the pyramidal cells layers and the dentate gyrus. Staining was also evident in the dentate nucleus and cortical Purkinje cells of the cerebellum. NBCn1 mRNA has also been detected in the cerebrum and cerebellum of rat, and the choroid plexus of both mouse and rat choroid plexus (Praetorius et al., 2004b). Based on immunohistochemistry, the authors reported NBCn1 expression in the basolateral membranes of the epithelial cells of both rat and mouse choroid plexus.
NBCn1 is also present in human brain. Using RT-PCR and human NBCn1 primers that are expected to amplify all splice variants of NBCn1 cDNA, Damkier et al. (2007) found mRNA expression in human cerebellum, cerebrum, hippocampus, and choroid plexus.
Chen et al. subsequently reported the developmental expression of NBCn1 in cortex, hippocampus, subcortex, and cerebellum of postnatal day 16 (P16), P30, P104, and P118 mice. NBCn1-B expression was highest in cortex and hippocampus of P16 mice (Chen et al., 2007). Postnatal changes in NBCn1-B expression were brain region dependent. For example, NBCn1-B expression with age gradually increased in the hippocampus, decreased in the cortex, and remained the same in the subcortex and cerebellum. Changes in NBCn1 expression may be required for proper development of the various brain regions, and may highlight developmental changes in the pH physiology of the regions.
D) Electroneutral Na/HCO3 cotransporter, NBCn2 (NCBE)
In addition to NBCe2 as described above, NBCn2 (NCBE) is also present in the epithelial cells of the choroid plexus (Praetorius et al., 2004b). In RT-PCR studies, Praetorius et al. detected NBCn2 (NCBE) mRNA in the cerebrum and cerebellum of rat, and the choroid plexus of both mouse and rat. In immunochemical studies on the choroid plexus, NBCn2 (NCBE)-B protein was evident on the basolateral membrane of the epithelial cells.
Giffard et al. (2003) cloned two NBCn2 (NCBE) variants from rat brain: rb1NCBE (NBCn2 (NCBE)-B) and rb2NCBE (NBCn2 (NCBE)-C). Subsequently, they reported developmental and regional mRNA expression patterns of NBCn2 (NCBE) in rat and mouse brain. NBCn2 (NCBE) mRNA in rat brain was evident from Northern blot analysis. According to in situ hybridization data, NBCn2 (NCBE)-B mRNA expression was widespread in the brain and spinal cord of rats as early as E19, and such expression persisted until adulthood. At higher magnifications, NBCn2 (NCBE)-B mRNA was detected in the Purkinje cells of the cerebellum and CA3 principal neurons of the hippocampus. According to results from RT-PCR studies, mRNA expression of the C variant was greater than that of the B variant in mouse cultured astrocytes, whereas both variant mRNAs expressed similarly in mouse cultured neurons. The authors report that the C variant compared to B variant mRNA was greater in cortex, striatum, and hippocampus of mouse brain, while the reverse was true for these regions from rat brain. To examine the cellular expression profiles of the two variants in mouse brain, the authors transfected cultured mouse astrocytes with retroviral vectors encoded FLAG-tagged NBCn2 (NCBE) constructs and subsequently performed immunocytochemistry with anti-FLAG antibodies. Although both variants were expressed at the plasma membrane, the B variant was more localized to intracellular vesicles, whereas the C variant was more localized to the actin cytoskeleton.
NBCn2 (NCBE) is also present in human brain. Using RT-PCR and human NBCn2 (NCBE) primers that are expected to recognize all splice variants of NBCn2 (NCBE) cDNA, Damkier et al. (2007) found higher NBCn2 (NCBE) mRNA levels in human cerebrum, hippocampus, and choroid plexus than in the cerebellum.
Using immunoblotting techniques and a polyclonal antibody that recognizes the N-terminal 135 amino acids of NBCn2 (NCBE), Chen et al. (2008c) found abundant expression of NBCn2 (NCBE) in both rat and mouse hippocampus, cerebellum, cerebral cortex, and subcortex to a lesser extent. Interestingly, NBCn2 (NCBE) expression was evident in neurons cultured from rat hippocampus or freshly dissociated from mouse hippocampus, but not dissociated astrocytes from mouse. NBCn2 (NCBE) was localized to both the plasma membrane of the soma and the processes of cultured rat hippocampal neurons. In agreement with the aforementioned results by Praetorius et al. (2004b), where the authors detected NBCn2 (NCBE) expression in the basolateral membranes of choroid plexus, Chen et al. found basolateral expression of NBCn2 (NCBE) in the choroid plexus from E18 and adult rats.
Similar to the results by Chen et al. (2008c), Jacobs et al. (2008) used a different polyclonal antibody and found NBCn2 (NCBE) protein in lysates from mouse brain, as well as mixed neuron/glia cultures, but not pure glia cultures. In immunohistochemical studies, the authors observed broad NBCn2 (NCBE) expression in cortex, cerebellum, and olfactory bulb. Based on results from colocalization immunohistochemical studies with antibodies to cell-specific proteins, NBCn2 (NCBE) expression was evident in the CA3 layer of the hippocampus, some GAD-positive interneurons in the neocortex, and parvalbumin-positive Purkinje neurons in the cerebellum (but not interneurons in the molecular layer). Based on immunochemical results with three antibodies, all four NBCn2 (NCBE) variants (−A through −D) appear to be expressed in mouse CNS. The A variant appears to be the dominant one throughout mouse brain, whereas the D variant is mainly expressed in the subcortex and medulla (Liu et al., 2010).
In a developmental study using in situ hybridization techniques, Hübner et al. (2004) first detected NBCn2 (NCBE) mRNA as early as E12.5 in mouse brain regions, including cerebellum and cortex. mRNA levels increased from E15.5 to E18.5 in hippocampus, and from E12.5 to E18.5 in cortex. The transcript expression followed a neuronal pattern and was not detected in large fiber tracts. mRNA was found in the neuronal cell layer of the retina, the retinal pigment epithelium, as well as in certain regions of the central auditory pathway including the geniculate nucleus and auditory brain stem nuclei. NBCn2 (NCBE) mRNA was also detected in the choroid plexus as expected from the aforementioned studies.
Chen et al. (2007) used immunoblotting to examine and compare expression levels of NBCn2 (NCBE) in the cortex, cerebellum, hippocampus, and subcortex of mouse brain with development from P16 to P118. In general, expression was highest in the cortex and hippocampus at the different age groups, and expression in any given region did not change with age. This developmental expression profile is different than with NBCn1, which displays increased levels with age (Chen et al., 2007).
E) Electroneutral Na-driven Cl-HCO3 exchanger, NDCBE/NDAE
According to in-situ and immunohistochemical studies, NDAE is present in the central and peripheral nervous systems, as well as dorsal and Bolwig’s sensory organs in the developing Drosophila (Romero, et al., 2000; Sciortino et al., 2001).
Regarding NDCBE, Risso Bradley et al. (2001) in a preliminary immunohistochemistry study reported NDCBE expression in the soma and dendrites of rat cerebellar Purkinje neurons. In a subsequent RNA study on rat brain, Praetorius et al. (2004b) used RT-PCR techniques and found NDCBE mRNA in cerebrum and cerebellum, but not in the choroid plexus.
In a more extensive protein study, Chen et al. (2008b) recently used immunochemical approaches and an antibody to the N-terminus of human NDCBE to localize protein expression in mouse and rat brain. In immunoblotting studies, the authors assessed NDCBE expression in membrane proteins from the cortex, subcortex, cerebellum, and hippocampus from mouse brain. NDCBE expression was higher in the cortex, subcortex, and cerebellum compared to the hippocampus. According to results from immunohistochemistry studies, NDCBE is widely distributed in mouse and /or rat brain, with particular expression in cortex, hippocampus, cerebellum, substantia nigra, brainstem, and olfactory bulb. In further studies with cell-specific antibodies, the authors found that NDCBE is expressed more so in neurons than astrocytes in the hippocampus and cerebellum of mouse, and neuronal expression is evident in both the soma and processes. Similar findings were obtained with neurons and astrocytes cultured from rat/mouse hippocampus. NDCBE protein was detected in the basolateral membrane of choroid plexus epithelial cells from E18, but not adult rat brains (Chen et al., 2008b).
NDCBE is also expressed in human brain. By RT-PCR, Damkier et al. (2007) found NDCBE mRNA expression in human cerebrum, hippocampus, and choroid plexus. In addition, in immunohistochemistry studies using an antibody that is expected to recognize both NDCBE-A and −B, the authors found expression in pyramidal cells of human hippocampus.
3) Role of NCBTs in Nervous System Function
A) General neuronal excitability
a) Neuron-glia interactions
As discussed above, a decrease in pHo typically inhibits neuronal firing, whereas an increase in pHo typically stimulates activity. Repetitive neuronal firing or depolarization as seen in spreading depression or epilepsy is often associated with a decrease in pHi. As mentioned earlier, a predominant acid-extrusion mechanism in astrocytes is an electrogenic NBC. Because an electrogenic NBC when active alters both pHi and the pHo by moving HCO3− across the plasma membrane of cells, this transporter links neuronal activity with changes in pH. According to a classic model proposed by Chesler (1990) and Ransom (1992), an electrogenic NBC in astrocytes may actually modulate neuronal excitability through such changes in pH. When a neuron fires an action potential, there is an increase in extracellular K+ (K+o) that depolarizes neighboring astrocytes. This depolarization leads to stimulation of an electrogenic NBC activity in astrocytes and transport of Na+, HCO3− and net-negative charge into the cells. The ensuing decrease in pHo tends to dampen further neuronal activity by inhibiting many pH-sensitive voltage- and ligand-gated channels. This negative-feedback model is predicted to be neuroprotective under pathophysiological conditions associated with repetitive and excessive neuronal firing such as with spreading depression and epilepsy.
The direction of HCO3− transport will be influenced by the transporter’s stoichiometry, as well as the membrane potential (Vm) of the NCBT-expressing brain cell if the transporter is electrogenic. For an electrogenic NBC transporting net-negative charge and expressed in a typical cell with a negative Vm, the chemical gradient favors NBC-mediated HCO3− influx. However, the electrical driving force favors NBC-mediated HCO3 efflux, which will be enhanced by an NBC that moves more net-negative charges per transport cycle (i.e., has a Na :HCO3 stoichiometry of 1:3 vs. 1:2), and by a more negative membrane potential. For an electrogenic NBC with a 1:2 Na+:HCO3− stoichiometry, the electrochemical gradient would likely favor the transport of HCO3− into a neuron or astrocyte with either a typical resting Vm or a depolarized Vm (e.g., during an action potential), thereby decreasing pHo and inhibiting neuronal activity. However, for an electrogenic NBC with a 1:3 Na+:HCO3− stoichiometry, the electrochemical gradient would likely favor the transport of HCO3− out of a neuron or astrocyte at a typical resting Vm, thereby increasing pHo and promoting neuronal activity.
In addition to regulating pH and modulating neuronal activity, an electrogenic NBC may also contribute to other changes in the extracellular space (ECS) associated with neuronal activity. As mentioned by Østby et al. (2009), neuronal stimulation causes a ~30% shrinkage of the ECS in brain. To examine the impact of specific pathways involved in such shrinkage, the authors generated five models that incorporated the following traditional ion/water pathways: both the efflux of K+ out of and the influx of Na+ into firing neurons, the influx of Na+, K+, and Cl− via channels into astrocytes, the influx of K+ and the efflux of Na+ via the Na-pump into astrocytes, and the osmotic movement of water into/out of astrocytes. In addition, the authors also evaluated the impact of two additional transporter-mediated pathways: i) the influx of Na+ and HCO3− via the NBC into astrocytes, and ii) the influx of Na+, K+, and Cl− via the Na/K/Cl cotransporter 1 (NKCC1) into astrocytes. Although shrinkage could be reasonably well modeled in the absence of these additional transporter-mediated pathways, their presence provided better, more physiologically relevant results in agreement with the literature. Thus, an astrocytic NBC may act in concert with NKCC1 and additional ion/water channels to mediate neuronal activity-evoked decreases in the volume of the ECS.
b) NBCn2 (NCBE) knockout mouse— elevated seizure threshold
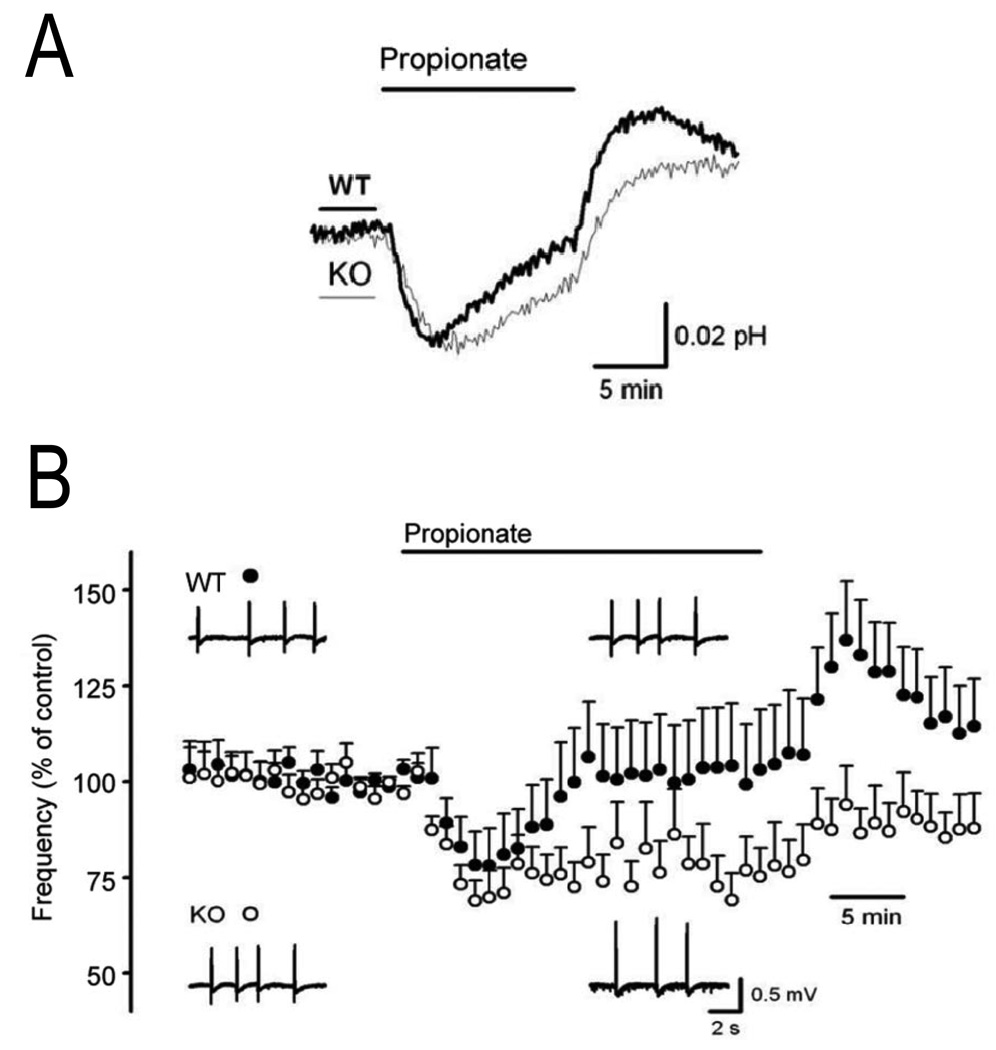
Recent knockout studies have highlighted the functional importance of NCBTs in the nervous system. Jacobs et al. (2008) developed an NBCn2 (NCBE) knockout mouse by targeted disruption of exon 12 of Slc4a10 gene. The knockout mice have a high seizure threshold and exhibit delayed onset of proconvulsive- and hyperthermia-induced seizures. A larger number of knockout vs. wild-type mice survive lethal doses of proconvulsants. In measuring pHi with fluorescence imaging and the pH-sensitive dye BCECF, the authors found that CA3 pyramidal neurons from the knockout mice displayed slower pHi recoveries from propionate-induced acid loads than neurons from the wild-type mice (Fig. 3A). The slower pHi recoveries in the knockout mice correlated with propionate-induced decreases in the frequency of 4-aminopyridine (4-AP)-elicited interictal events in the CA3 region (Fig. 3B). Furthermore, in the continued presence of propionate, the frequency of interictal-like events in this region recovered to control levels in slices from the wild-type mice, but not the knockout mice. The lack of frequency recovery correlated with the incomplete pHi recovery in neurons from the knockout mice. Thus, NBCn2 (NCBE)-mediated pHi regulation appears to influence excitability of at least the CA3 region.
Fig. 3.
Reduced pHi regulation and neuronal excitability in the CA3 stratum pyramidale layer of hippocampal slices from NBCn2 (NCBE) Slc4a10 knockout mice. (A) pHi in the CA3 layer of slices from wild-type (WT) and NBCn2 (NCBE) knockout (KO) mice was measured by fluorescence imaging with the pH-sensitive dye BCECF. Exposing slices to a bath solution containing 20 mM propionate elicited an initial decrease in mean pHi due to the influx of propionic acid, followed by an increase in pHi due to acid extruders such as NBCn2 (NCBE). Both the rate and magnitude of the mean pHi increase was less in slices from KO mice (grey trace, n=12) vs. WT mice (black trace, n=10). Removing bath propionate elicited a pHi increase due to the efflux of propionic acid, followed by a pHi decrease due to acid loading mechanisms. (B) 4-aminopyridine (4-AP)-elicited interictal-like events in the CA3 layer of slices from WT and NBCn2 (NCBE) KO mice were measured using field potential electrodes. The slower propionate-induced pHi recoveries in the KO slices shown in panel A correlated with a propionate-induced decrease in the relative mean (+SEM) frequency of interictal events in the KO slices (open circles, n=27) compared to the WT slices (filled circles, n=20). The frequency of the events recovered to control levels in WT slices, but not KO slices during the propionate exposure. Modified from Jacobs et al. (2008). © 2007 by The National Academy of Sciences of the USA.
Interestingly, in contrast to results from the knockout mice studies by Jacobs et al. (2008) described above, disruption of the SLC4A10 gene in a human patient is associated with partial complex epilepsy, in addition to loss of cognitive function, and moderate mental retardation (Gurnett et al., 2008). However, this disruption resulted from a chromosomal translocation with multiple unidentified genes being affected. Therefore the role of NBCn2 (NCBE) in epilepsy and seizure disorders has yet to be fully elucidated.
c) Gerbil seizure model— elevated NBCe1 expression
The expression of NCBTs in the nervous system may also be regulated by neuronal activity. Kang et al. (2002) compared NBCe1 expression in the hippocampus of gerbils that were separated into seizure-resistant and seizure-sensitive groups depending on the degree of motor arrest induced by stroking the back of the neck. In seizure-sensitive animals, NBCe1 expression by immunohistochemistry increased in the CA1–3 regions of the hippocampus at the 30-min time point following seizure induction. At the 3-h time point, expression increased further in the CA2–3 regions and also increased in the dentate gyrus (granule cells). At the 6-h time point, NBCe1 expression in all hippocampal regions decreased to pre-seizure levels.
The functional impact of a change in the activity/expression of an NCBT such as NBCn2 (NCBE) or NBCe1 on seizure activity is anticipated to be complex because of changes in pHi, pHo, the concentrations of transported ions, and intracellular/extracellular volumes. Further studies are required to elucidate the mechanisms by which altered function/expression of NCBTs influence neuronal activity.
B) Somatosensory function
a) NBCn1 knockout mouse— visual impairments
NBCn1, which is expressed in both the eye and ear, is necessary for somatosensory function (Bok et al., 2003; Lopez et al., 2005). Mice with a disruption of the Slc4a7 gene that encodes for NBCn1 (Slc4a7−/−) are blind and have hearing defects (Bok et al., 2003). We will initially focus on the eye, and then discuss the ear in the next paragraph. Based on results from confocal immunofluorescence studies on wild-type mice (Slc4a7+/+), NBCn1 is expressed in the outer plexiform layer of retina, and more specifically in photoreceptor synaptic terminals. In knockout compared to wild-type mice, there was a progressive deterioration of the photoreceptor region of the retina beginning at two months of age and leading to complete loss at 11 months. At four months of age, the morphology of the fundi region of knockout mice had markedly deteriorated as demonstrated by attenuation of blood vessels and diffuse granularity. The authors propose that eye defects in the NBCn1-knockout mice may result from interrupted NBC-mediated buffering of H+ that would lower pHi and inhibit Ca2+ efflux via the Ca2+-pump and/or Ca2+ influx via pH-sensitive L-type voltage-gated Ca2+ channels at the photoreceptor synaptic terminal.
b) NBCn1 knockout mouse— auditory impairments
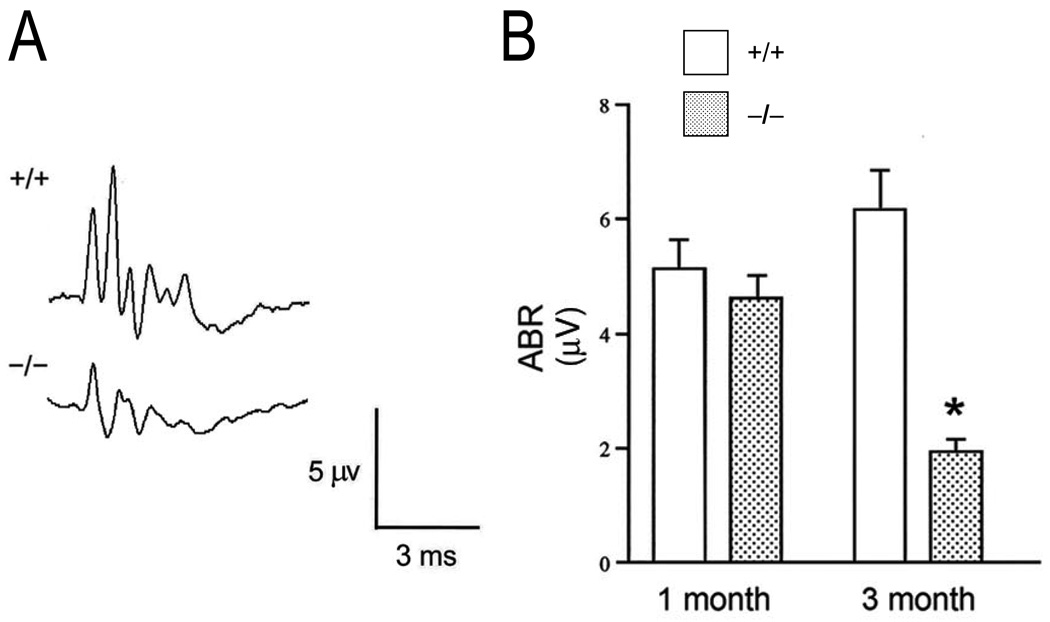
In the same study introduced above, Bok et al. (2003) also found hearing defects in the NBCn1 knockout mice. Based on results from cochlear immunohistochemical studies on wild-type mice, NBCn1 is expressed in fibrocytes of the spiral ligament. The authors found that one-month old knockout mice displayed degenerating inner and outer hair cells of cochlea (which progressed with age), and abnormal morphology of the stria vascularis and spiral ligament. The Reissner’s membrane was collapsed— a finding consistent with a role for NBCn1 in endolymph formation. In knockout mice (−/−) compared to wild-type (+/+) mice at three months of age, auditory brainstem responses (ABR) elicited by acoustic clicks to the ear were blunted (Fig. 4A); the mean ABR amplitude from the combined stimulus intensities was ~70% less at three months (Fig. 4B). Thus, NBCn1 plays an important role in the development and function of the auditory system.
Fig. 4.
Auditory impairment in NBCn1 (Slc4a7) knockout mice. (A) Auditory function was assessed by examining auditory brain responses (ABRs) in response to various click stimuli. Click stimuli of 60 dB sound-pressure level elicited an ABR waveform that was blunted in three-month-old Slc4a7−/− vs. Slc4a7+/+ mice. (B) Summary data from panel-A type responses with means (+SEMs) computed from ABR waves I, II, and III, and from all stimulus intensities for Slc4a7+/+ (open bars) and Slc4a7−/ mice (closed bars). n = 5 for each bar. *P < 0.04. Modified from Bok et al. (2003). Reprinted by permission from Macmillan Publishers Ltd: Nature Genetics (Bok et al., (2003)), © Nature Publishing Group (2003).
According to Bok et al. (2003), the NBCn1 knockout mouse serves as a model for Usher syndrome, which is a human disorder associated with similar hearing and vision disabilities. SLC4A7 appears responsible for type 2B of Usher syndrome (Pushkin et al., 1999b), which has been mapped in a consanguineous Tunisian family with vision and hearing defects (Hmani-Aifa et al., 2002; Hmani et al., 1999).
More recently, Lopez et al. (2005) performed a detailed characterization of the time course of cochlear hair cell degeneration and loss from postnatal P2 to P90 NBCn1 knockout mice. Based on results from light and transmission electron microscopy studies, the authors observed degeneration of the hair cells as early as P21. The degeneration progressed with age until complete degeneration at P90. There was atrophy in the organ of Corti beginning at P21. At P90, both the organ of Corti and the myelin that surrounded spiral ganglia neurons were degenerated. In addition, there was formation of vacuoles in the cytoplasm of the spiral ganglia neurons. Apoptotic cells were detected in the cochlea as early as P8.
C) Cerebrospinal Fluid Secretion
a) NBCn2 (NCBE) knockout mouse
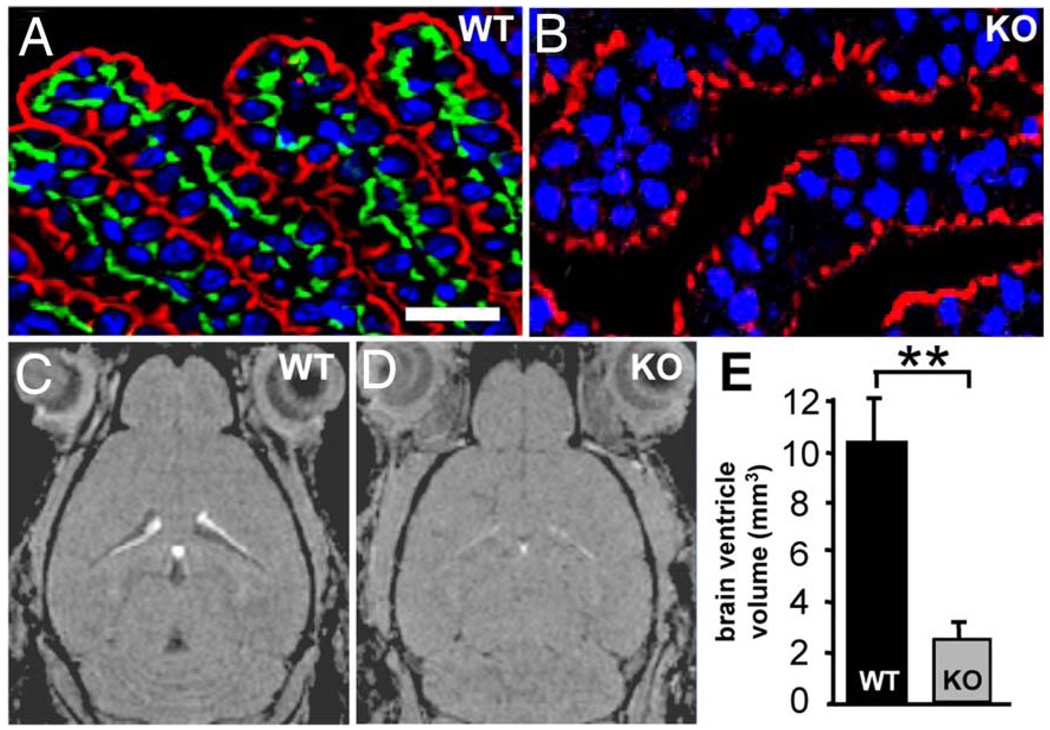
In the same study described above on NBCn2 (NCBE) knockout mice, Jacobs et al. (2008) examined the impact of NBCn2 (NCBE) loss on choroid plexus function and ventricle volume. NBCn2 (NCBE) expression was evident on the basolateral membrane of the choroid plexus epithelium from wild-type mice (Fig. 5A, green), but not knockout mice (Fig. 5B). Using magnetic resonance imaging, the authors observed a ~75% reduction in brain ventricular volume in knockout vs. wild-type mice (Figs. 5C–E). The decreased volume was not the result of edema or increased intracranial pressure, but was instead due to enlarged lateral intercellular spaces and reduced apical microvilli in the choroid plexus according to data from histological and electron microscopy studies (Fig. 5). To examine NBCn2 (NCBE)-mediated CSF production, the authors performed fluorescence imaging experiments with the pH-sensitive dye BCECF to characterize HCO3−-dependent pHi regulation in the epithelial cells of choroid plexus from the knockout mice. Na+- and HCO3−-dependent pHi recoveries from NH4+ prepulse-induced acid loads were reduced in the knockout vs. wild-type mice. The authors propose that NBCn2 (NCBE) promotes CSF secretion by contributing to net vectorial movement of Na+ and HCO3− across the polarized choroid plexus epithelium. NBCn2 (NCBE) transports Na+ and HCO3− from blood across the basolateral membrane of the choroid plexus epithelium. Another NBC and the Na+ pump then transport the intracellular Na+ and/or HCO3− across the apical membrane into the CSF. H2O follows the solute movement through aquaporin-1 H2O channels. Decreased HCO3−-dependent CSF production is likely responsible for the reduced brain ventricular volume in the NBCn2 (NCBE) knockout mice.
Fig. 5.
Reduced brain ventricular volume in NBCn2 (NCBE) Slc4a10 knockout mice. (A, B) According to results from immunohistochemical studies, NBCn2 (NCBE) is expressed on the basolateral membrane of the choroid plexus epithelium from wild-type but not knockout mice (green), but not on the apical membrane labeled with an antibody to the Na-pump (red). (C–E) Based on magnetic resonance imaging, the mean (+SEM) brain ventricular volume is ~75% less in knockout mice (n=4) vs. wild-type mice (n=3). **P < 0.04. Modified from Jacobs et al. (2008). © 2007 by The National Academy of Sciences of the USA.
On a related note, Sussman et al. (2009) observed that morpholino knockdown of NBCe1 in zebrafish leads to hydrocephalus, as well as ocular abnormalities such as retinal distention and small eyes. As proposed by the authors, NBCe1 may be required for fluid absorption from CSF to the brain parenchyma, or proper ion homeostasis of cells in the ventricular lining.
There is evidence that NCBT knockout mice may express compensatory acid-base transporters to regulate pHi. For example, compensatory pHi-regulating mechanisms appear in the choroid plexus epithelial cells of the NBCn2 (NCBE) knockout mice. According to results from immunofluorescence studies, Damkier et al. (2009) found that the Na-H exchanger 1 (NHE1), which is usually localized to the apical membrane in the choroid plexus, is expressed in the basolateral membrane in the choroid plexus epithelial cells from the knockout mice. In functional studies on the choroid plexus epithelium from knockout mice, there was an increase in the activity of an apical EIPA-insensitive NHE responsible for Na+-dependent pHi recoveries from NH4+-induced acid loads. The apical NHE was sensitive to EIPA in the wild-type mice. According to the authors, NBCn2 (NCBE) knockout increases the expression of an EIPA-sensitive NHE (NHE1) from the apical to basolateral membrane, and also increases the activity of an EIPA-insensitive NHE on the apical membrane.
D) Genetic links to human neurologic disorders
As described above, many of the human patients with frameshift, missense, or nonsense mutations in the SLC4A4 gene have ocular abnormalities, including glaucoma and/or cataract formation (Igarashi et al., 1999; Demirci et al., 2006; Dinour et al., 2004). In addition, three of them are mentally retarded and another two display developmental disabilities. Findings from additional genetic studies have yielded associations between neurologic disorders and other more recently identified NCBT genes. For example, in a genetic linkage study, Kok et al. (2003) identified two families in which a form of hereditary sensory neuropathy type I (HSN I) with gastroesophageal reflux (GER) and cough is linked to chromosome 3p22-p24. Although, 28 genes are mapped to this interval, one of two positional candidate genes identified based on expression in peripheral nerve and spinal cord is SLC4A7 (encodes NBCn1).
NBCn1 also appears associated with a propensity to substance addictions. In examining allelic variations in the SLC4A7 gene, Ishiguro et al. (2007) found a strong association between single nucleotide polymorphism (SNP) markers of the gene and substance abusers. The authors proposed that allelic variations of SLC4A7 may increase addiction vulnerability by influencing drug and/or neurotransmitter pharmacodynamics and pH-dependent transport across cell membranes.
Finally, NCBTs have been linked to autism. In performing a high-resolution genomic microarray analysis on 264 families with or without autistic individuals, Sebat et al. (2007) identified a pair of monozygotic autistic twins with deleted exon 1 of SLC4A10 (encoding NBCn2 (NCBE)), as well as a patient with Asperger syndrome linked to a genomic deletion that included the SLC4A11 gene (encoding a unique Na-dependent borate transporter in the SLC4 family).
4) Role of NCBTs in Energy-deficient Neurologic Conditions
A) Ischemia
Jung et al. (2007) examined the expression profiles of four sodium transporters, including NBCe1, in the ischemic penumbra in a rat model of focal cerebral ischemia induced by occlusion of the left middle cerebral artery. By immunoblotting procedures, the authors reported increased NBCe1 expression in ischemic penumbra tissues 3 and 6 h post surgery in the ischemic model vs. sham-operated controls. Although the reason for this ischemia-induced increase in NBCe1 expression is not known, the accompanying increase in electrogenic NBC activity may protect against associated decreases in pHi. Furthermore, ischemia-induced increases in extracellular K+ (Leis et al., 2005) may be involved. According to the model of Jung et al., an ischemia-induced increase in extracellular K+ will depolarize brain cells (e.g., astrocytes) and stimulate electrogenic NBC activity. The increased cellular influx of HCO3− will buffer ischemia-induced decreases in pHi. On the other hand, the accompanying Na+ influx is expected to promote cellular edema.
One of the hallmarks of ischemia is both intracellular and extracellular acidification. Furthermore, McKee et al. (2005) have demonstrated a decrease in Mg2+ concentration in both the CSF and serum early on in ischemic stroke. Cooper et al. (2009) recently examined the expression of NBCn1-B and its contribution to the cytotoxicity of rat hippocampal neurons under ischemic-like conditions with low pHo and the absence of extracellular Mg2+. Using immunoblot procedures on cultured rat hippocampal neurons, the authors reported increased expression of NBCn1 in cultured neurons under the ischemic-like conditions. Regarding the effects of low pHo, the authors found an increase in NBCn1-B protein expression by immunofluorescence in the cell body, plasma membrane, and dendrites of neurons cultured at pHo 6.5, but not 7.4 for 1–3 h. According to results from subsequent cytotoxicity studies, glutamate cytotoxicity was decreased in neurons from NBCn1 siRNA knockdown animals after incubation in Mg2+-free media for 2 and 6 h, but not when the cells were incubated at a low pHo of 6.3 for 6 h. The authors speculated that 0 Mg2+ stimulates NMDA receptor activity, which in turn induces cytotoxicity. The cytotoxicity appears to be promoted by NBCn1-mediated acid extrusion. More recently, the group has reported that chronic metabolic acidosis in mice increases NBCn1 expression in CA3 hippocampal, posterior cortical, and cerebellar granular neurons (Park et al., 2010).
Hypoxia
Hypoxia influences both the function and expression of NCBTs. In pHi experiments with BCECF on astrocytes cultured from the rat hippocampus, Bevensee and Boron (2008) observed that acute hypoxia (3% O2) stimulated total acid extrusion during pHi recoveries from NH4+-induced acid loads in the nominal absence of CO2/HCO3−, but actually inhibited SITS-sensitive acid extrusion during pHi recoveries in the presence of CO2/HCO3−. The data are consistent with acute hypoxia stimulating the activity of the Na-H exchanger activity, but inhibiting the activity of the SITS-sensitive electrogenic Na/HCO3− cotransporter in the astrocytes. Hypoxia also stimulated an acid-loading mechanism responsible for the pHi recovery from an alkali load in the astrocytes. Thus, acute hypoxia stimulates a HCO3−-independent acid extruder and loader, but inhibits a HCO3−-dependent acid extruder in the astrocytes.
To examine hypoxia-induced changes in the expression of NDCBE in mouse brain, Chen et al. (2008a) performed immunoblotting of membrane proteins from different regions of the brain from adult and neonatal mice subjected to chronic continuous hypoxia (11% O2) for 14 or 28 d. At both the 14- and 28-d time points, chronic hypoxia decreased NDCBE-B expression by 20–50% in cortex, subcortex, hippocampus, and cerebellum of adults. In the neonates, the NDCBE-B protein expression was decreased by ~25% in both the hippocampus after 14 d and the subcortex after 28 d hypoxic treatments. The authors proposed that the general reduced effect of chronic hypoxia on NDCBE expression in neonates vs. adults might be due to a smaller compensated respiratory alkalosis and/or reduced hypoxia sensitivity of the neonates.
Chen et al. (2007) performed similar studies on NBCn1-B and NBCn2 (NCBE). At both the 14- and 28-d time points, chronic hypoxia decreased the expression of NBCn1-B and NBCn2 (NCBE) by 15–50% in cortex, subcortex, hippocampus, and cerebellum of both neonates and adults. The authors proposed that the hypoxia-induced decrease in protein expression of NBCn1-B and NBCn2 (NCBE) may result in decreased energy consumption in the brain by reducing either protein synthesis, the requirement for Na+-pump mediated Na+ extrusion, and/or neuronal activity caused by associated changes in pHi/pHo.
Unlike Chen et al. (2007) who examined the effect of chronic continuous hypoxia on expression of NCBTs in mouse brain, Douglas et al. (2003) examined the effect of chronic intermittent hypoxia on the expression of NBCe1 in different regions of the postnatal mouse CNS. The authors performed immunoblotting on membrane fractions from cerebellum, cortex, hippocampus, and brainstem-diencephalon using three different NBCe1 antibodies. One antibody recognized all three NBCe1 variants, one recognized the C terminus of the NBCe1-A and −B variants, and the final one recognized the different C terminus of the C variant. Using the antibody that recognized all three variants, Douglas et al. observed a 33% decrease in NBCe1 expression in the cerebellum of hypoxia-exposed mouse compared to controls. Using the antibody to the C-terminus of NBCe1-A/B, the authors reported a decrease in the expression of NBCe1-A/B in the hippocampus of hypoxic mice. However, with the antibody to the C-terminus of the C variant, NBCe1-C did not appear to show any appreciable change in expression in brain regions from hypoxic and normoxic mice. Therefore, the effects of chronic intermittent hypoxia on NBCe1 expression are both transporter variant and brain-region dependent.
5) Conclusions and Future Directions
We have provided a detailed overview of the function and localization of NCBTs in the nervous system and their relevance to neurologic function. Although the primary role of NCBTs in the nervous system is likely to regulate pHi/pHo tightly, some NCBTs such as NBCe2 also contribute to the production and ionic composition of CSF, thereby controlling brain ventricular volume. The following three noteworthy observations have arisen from molecular studies of NCBTs in the nervous system. First, individual NCBTs or associated variants are expressed in different regions and cell types in the brain. Differential expression of NCBTs may contribute to heterologous pHi regulation of brain cells, and results from studies designed to characterize the functional properties of specific NCBTs in both endogenous cells and heterologous expression systems will provide further insight into such heterologous pHi regulation.
The second noteworthy observation is that more than one NCBT can be expressed in a single type of brain cell. Such apparent redundancy reinforces the hypothesis that cells make use of multiple NCBTs with different regulatory properties to modulate pHi/pHo under various conditions and stimuli. For instance, many NCBTs such as NBCn1, NBCe1-C, and NBCn2 (NCBE)-C and −D contain PDZ-binding motifs that are likely targets for regulatory proteins containing PDZ domains. NBCn1 at least partially colocalizes with post synaptic density protein-95 (PSD-95) in neurons (Cooper et al., 2005; Park et al., 2010). In addition, the IP3-receptor binding protein released with IP3 (IRBIT) binds to the N terminus of NBCe1-B (but not −A) to increase transporter activity (Shirakabe et al., 2006). Further studies are required to identify neurologically relevant protein partners and classic signaling pathways that may regulate specific NCBTs in the brain under both physiologic and pathophysiologic conditions.
Finally, results from animal models of disease and NCBT knockout mice have provided considerable insight into the importance of NCBTs in somatosensory function and CSF production, as well as during pathologic states including epilepsy and ischemia/hypoxia. However, future studies are required to determine if altered expression of NCBTs in disease states is a causative effect or adaptive response. Furthermore, generating and characterizing additional mice with more specific NCBT variant knockdowns will be required to understand the importance of individual NCBT variants, as well as to expand our knowledge of the complexity of the bicarbonate transporter family in the brain.
ACKNOWLEDGEMENTS
We are very grateful to Dr. Mark D. Parker (Department of Physiology and Biophysics, Case Western Reserve University) who critiqued our manuscript and provided many valuable comments and suggestions. This work was supported by NIH/NINDS NS046653 (MOB).
LIST OF ABBREVIATIONS
- AEs
anion exchangers
- ASICs
acid-sensing ion channels
- BT
bicarbonate transporter
- CNS
central nervous system
- CSF
cerebrospinal fluid
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- GFAP
glial fibrillary acidic protein
- aNa+i
intracellular Na+ activity
- MAP2
microtubule associated protein 2
- NCBT
Na-coupled bicarbonate cotransporter
- NBC
Na/bicarbonate cotransporter
- NBCe1 (or 2)
electrogenic Na/bicarbonate cotransporter 1 (or 2)
- NBCn1 (or 2)
electroneutral Na/bicarbonate cotransporter 1 (or 2)
- NDAE
Na-driven anion exchanger
- NDCBE
Na-driven Cl-bicarbonate exchanger
- NCBE
Na-driven Cl-bicarbonate exchanger
- NHE
sodium hydrogen exchanger
- PDZ
post synaptic density-Drosophila disc large tumor suppressor-zonula occludens-1
- pHi
intracellular pH
- pHo
extracellular pH
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PSD-95
post synaptic density protein-95
- Slc4
solute carrier 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- Abuladze N, Pushkin A, Tatishchev S, Newman D, Sassani P, Kurtz I. Expression and localization of rat NBC4c in liver and renal uroepithelium. Am J Physiol Cell Physiol. 2004;287:C781–C789. doi: 10.1152/ajpcell.00590.2003. [DOI] [PubMed] [Google Scholar]
- Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Physiol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aram JA, Lodge D. Epileptiform activity induced by alkalosis in rat neocortical slices: block by antagonists of N-methyl-D-aspartate. Neurosci Lett. 1987;83:345–350. doi: 10.1016/0304-3940(87)90112-1. [DOI] [PubMed] [Google Scholar]
- Astion ML, Orkand RK. Electrogenic Na+/HCO3− cotransport in neuroglia. Glia. 1988;1:355–357. doi: 10.1002/glia.440010508. [DOI] [PubMed] [Google Scholar]
- Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3− cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol. 2003;284:G37–G45. doi: 10.1152/ajpgi.00209.2002. [DOI] [PubMed] [Google Scholar]
- Balestrino M, Somjen GG. Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol. 1988;396:247–266. doi: 10.1113/jphysiol.1988.sp016961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Boron WF. Control of intracellular pH. In: Alpern RJ, Hebert SC, editors. The Kidney: Physiology and Pathophysiology. New York: Elsevier, Inc.; 2008. pp. 1429–1480. [Google Scholar]
- Bevensee MO, Apkon M, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3 cotransport. J Gen Physiol. 1997a;110:467–483. doi: 10.1085/jgp.110.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Boron WF. pH regulation in mammalian neurons. In: Kaila K, Ransom BR, editors. pH and Brain Function. New York: Wiley-Liss, Inc.; 1998. pp. 211–231. [Google Scholar]
- Bevensee MO, Boron WF. Effects of acute hypoxia on intracellular-pH regulation in astrocytes cultured from rat hippocampus. Brain Res. 2008;1193:143–152. doi: 10.1016/j.brainres.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na+-HCO3− cotransporter (NBC) with a novel COOH terminus, cloned from rat brain. Am J Physiol Cell Physiol. 2000;278:C1200–C1211. doi: 10.1152/ajpcell.2000.278.6.C1200. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Weed RA, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role of HCO3−. J Gen Physiol. 1997b;110:453–465. doi: 10.1085/jgp.110.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtkjer E, Praetorius J, Füchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+-HCO3− cotransporter NBCn1 (slc4a7) in mice. Am J Physiol Cell Physiol. 2008;294:C591–C603. doi: 10.1152/ajpcell.00281.2007. [DOI] [PubMed] [Google Scholar]
- Bok D, Schibler MJ, Pushkin A, Sassani P, Abuladze N, Naser Z, Kurtz I. Immunolocalization of electrogenic sodium-bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. Am J Physiol Renal Physiol. 2001;281:F920–F935. doi: 10.1152/ajprenal.2001.281.5.F920. [DOI] [PubMed] [Google Scholar]
- Bok D, Galbraith G, Lopez I, Woodruff M, Nusinowitz S, BeltrandelRio H, Huang W, Zhao S, Geske R, Montgomery C, Van SI, Friddle C, Platt K, Sparks MJ, Pushkin A, Abuladze N, Ishiyama A, Dukkipati R, Liu W, Kurtz I. Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nat Genet. 2003;34:313–319. doi: 10.1038/ng1176. [DOI] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral. J Gen Physiol. 1983;81:53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol. 2009;212:1697–1706. doi: 10.1242/jeb.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Active proton transport stimulated by CO2/HCO3− , blocked by cyanide. Nature. 1976a;259:240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976b;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Hogan E, Russell JM. pH-sensitive activation of the intracellular-pH regulation system in squid axons by ATP-γ-S. Nature. 1988;332:262–265. doi: 10.1038/332262a0. [DOI] [PubMed] [Google Scholar]
- Boron WF, Russell JM. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983;81:373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussouf A, Lambert RC, Gaillard S. Voltage-dependent Na+-HCO3− cotransporter and Na+/H+ exchanger are involved in intracellular pH regulation of cultured mature rat cerebellar oligodendrocytes. Glia. 1997;19:74–84. doi: 10.1002/(sici)1098-1136(199701)19:1<74::aid-glia8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Bouzinova EV, Praetorius J, Virkki LV, Nielsen S, Boron WF, Aalkjaer C. Na+-dependent HCO3− uptake into the rat choroid plexus epithelium is partially DIDS sensitive. Am J Physiol Cell Physiol. 2005;289:C1448–C1456. doi: 10.1152/ajpcell.00313.2005. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ransom B, Schlue WR, Davis MB, Boron WF. Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia. 1993;8:241–248. doi: 10.1002/glia.440080404. [DOI] [PubMed] [Google Scholar]
- Brune T, Fetzer S, Backus KH, Deitmer JW. Evidence for electrogenic sodium-bicarbonate cotransport in cultured rat cerebellar astrocytes. Pflügers Arch. 1994;429:64–71. doi: 10.1007/BF02584031. [DOI] [PubMed] [Google Scholar]
- Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3− cotransporter. J Biol Chem. 1997;272:19111–19114. doi: 10.1074/jbc.272.31.19111. [DOI] [PubMed] [Google Scholar]
- Burnham CE, Flagella M, Wang Z, Amlal H, Shull GE, Soleimani M. Cloning, renal distribution, and regulation of the rat Na+- HCO3− cotransporter. Am J Physiol. 1998;274:F1119–F1126. doi: 10.1152/ajprenal.1998.274.6.F1119. [DOI] [PubMed] [Google Scholar]
- Byerly L, Moody WJ. Membrane currents of internally perfused neurones of the snail, Lymnaea stagnalis, at low intracellular pH. J Physiol. 1986;376:477–491. doi: 10.1113/jphysiol.1986.sp016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro C, Monastero R, Vacca M, Martire M. [3H]-DA release evoked by low pH medium and internal H+ accumulation in rat hypothalamic synaptosomes: involvement of calcium ions. Neurochem Int. 2003;43:9–17. doi: 10.1016/s0197-0186(02)00211-5. [DOI] [PubMed] [Google Scholar]
- Chen JC, Chesler M. A bicarbonate-dependent increase in extracellular pH mediated by GABAA receptors in turtle cerebellum. Neurosci Lett. 1990;116:130–135. doi: 10.1016/0304-3940(90)90398-s. [DOI] [PubMed] [Google Scholar]
- Chen JC, Chesler M. Extracellular alkaline shifts in rat hipocampal slice are mediated by NMDA and non-NMDA receptors. J Neurophysiol. 1992;68:342–344. doi: 10.1152/jn.1992.68.1.342. [DOI] [PubMed] [Google Scholar]
- Chen LM, Choi I, Haddad GG, Boron WF. Chronic continuous hypoxia decreases the expression of SLC4A7 (NBCn1) and SLC4A10 (NCBE) in mouse brain. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2412–R2420. doi: 10.1152/ajpregu.00497.2007. [DOI] [PubMed] [Google Scholar]
- Chen LM, Haddad GG, Boron WF. Effects of chronic continuous hypoxia on the expression of SLC4A8 (NDCBE) in neonatal versus adult mouse brain. Brain Res. 2008a;1238:85–92. doi: 10.1016/j.brainres.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Kelly ML, Parker MD, Bouyer P, Gill HS, Felie JM, Davis BA, Boron WF. Expression and localization of Na-driven Cl-HCO3− exchanger (SLC4A8) in rodent CNS. Neuroscience. 2008b;153:162–174. doi: 10.1016/j.neuroscience.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Kelly ML, Rojas JD, Parker MD, Gill HS, Davis BA, Boron WF. Use of a new polyclonal antibody to study the distribution and glycosylation of the sodium-coupled bicarbonate transporter NCBE in rodent brain. Neuroscience. 2008c;151:374–385. doi: 10.1016/j.neuroscience.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC) Am J Physiol. 1999;276:C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Cohen JJ, Kassirer JP. Acid-Base. Boston: Little Brown; 1982. [Google Scholar]
- Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem. 2005;280:17823–17830. doi: 10.1074/jbc.M408646200. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Yang HS, He P, Kim E, Rajbhandari I, Yun CC, Choi I. Sodium/bicarbonate cotransporter NBCn1/slc4a7 increases cytotoxicity in magnesium depletion in primary cultures of hippocampal neurons. Eur J Neurosci. 2009;29:437–446. doi: 10.1111/j.1460-9568.2008.06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkier HH, Nielsen S, Praetorius J. An anti-NH2-terminal antibody localizes NBCn1 to heart endothelia and skeletal and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;290:H172–H180. doi: 10.1152/ajpheart.00713.2005. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2136–R2146. doi: 10.1152/ajpregu.00356.2007. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Prasad V, Hübner CA, Praetorius J. Nhe1 is a luminal Na+/H+ exchanger in mouse choroid plexus and is targeted to the basolateral membrane in Ncbe/Nbcn2-null mice. Am J Physiol Cell Physiol. 2009;296:C1291–C1300. doi: 10.1152/ajpcell.00062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkier HH, Aalkjaer C, Praetorius J. Na+-dependent HCO3− import by the slc4a10 gene product involves Cl− export. J Biol Chem. 2010;285:26998–27007. doi: 10.1074/jbc.M110.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Hogan EM, Russell JM, Boron WF. ATP dependence of Na+-driven Cl-HCO3 exchange in squid axons. J Membr Biol. 2008;222:107–113. doi: 10.1007/s00232-008-9100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- Deitmer JW. Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. J Gen Physiol. 1991;98:637–655. doi: 10.1085/jgp.98.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW. Bicarbonate-dependent changes of intracellular sodium and pH in identified leech glial cells. Pflügers Arch. 1992;420:584–589. doi: 10.1007/BF00374637. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Schlue WR. The regulation of intracellular pH by identified glial cells and neurones in the central nervous system of the leech. J Physiol. 1987;388:261–283. doi: 10.1113/jphysiol.1987.sp016614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Schlue WR. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: a novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1) Mol Vis. 2006;12:324–330. [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem. 2004;279:52238–52246. doi: 10.1074/jbc.M406591200. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology. 2008;23:104–114. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Schmitt BM, Xia Y, Bevensee MO, Biemesderfer D, Boron WF, Haddad GG. Sodium-hydrogen exchangers and sodium-bicarbonate co-transporters: ontogeny of protein expression in the rat brain. Neuroscience. 2001;102:217–228. doi: 10.1016/s0306-4522(00)00473-5. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Xue J, Chen JY, Haddad CG, Alper SL, Haddad GG. Chronic intermittent hypoxia decreases the expression of Na/H exchangers and HCO3− dependent transporters in mouse CNS. J Appl Physiol. 2003;95:292–299. doi: 10.1152/japplphysiol.01089.2002. [DOI] [PubMed] [Google Scholar]
- Fedorovich SV, Kaler GV, Konev SV. Effect of low pH on glutamate uptake and release in isolated presynaptic endings from rat brain. Neurochem Res. 2003;28:715–721. doi: 10.1023/a:1022809716834. [DOI] [PubMed] [Google Scholar]
- Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/ HCO3− cotransporter. J Biol Chem. 2007;282:9042–9052. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Papadopoulos MC, van Hooft JA, Xu L, Giuffrida R, Monyer H. The electrogenic sodium bicarbonate cotransporter: developmental expression in rat brain and possible role in acid vulnerability. J Neurosci. 2000;20:1001–1008. doi: 10.1523/JNEUROSCI.20-03-01001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H. Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE): developmental expression and addition of a PDZ motif. Eur J Neurosci. 2003;18:2935–2945. doi: 10.1046/j.1460-9568.2003.03053.x. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Chesler M. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience. 1994a;62:1071–1078. doi: 10.1016/0306-4522(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Chesler M. Depolarization-induced acid secretion in gliotic hippocampal slices. Neuroscience. 1994b;62:1057–1070. doi: 10.1016/0306-4522(94)90343-3. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem. 2001;276:8358–8363. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- Gresz V, Kwon TH, Vorum H, Zelles T, Kurtz I, Steward MC, Aalkjaer C, Nielsen S. Immunolocalization of electroneutral Na+-HCO cotransporters in human and rat salivary glands. Am J Physiol Gastrointest Liver Physiol. 2002;283:G473–G480. doi: 10.1152/ajpgi.00421.2001. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, Veile R, Zempel J, Blackburn L, Lovett M, Bowcock A. Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental retardation. Arch Neurol. 2008;65:550–553. doi: 10.1001/archneur.65.4.550. [DOI] [PubMed] [Google Scholar]
- Hmani M, Ghorbel A, Boulila-Elgaied A, Ben Zina Z, Kammoun W, Drira M, Chaabouni M, Petit C, Ayadi H. A novel locus for Usher syndrome type II, USH2B, maps to chromosome 3 at p23–24.2. Eur J Hum Genet. 1999;7:363–367. doi: 10.1038/sj.ejhg.5200307. [DOI] [PubMed] [Google Scholar]
- Hmani-Aifa M, Ben Arab S, Kharrat K, Orten DJ, Boulila-Elgaied A, Drira M, Hachicha S, Kimberling WJ, Ayadi H. Distinctive audiometric features between USH2A and USH2B subtypes of Usher syndrome. J Med Genet. 2002;39:281–283. doi: 10.1136/jmg.39.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al-Gazali L, Shimadzu M, Seki G, Fujita T. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol. 2005;16:2270–2278. doi: 10.1681/ASN.2004080667. [DOI] [PubMed] [Google Scholar]
- Hübner CA, Hentschke M, Jacobs S, Hermans-Borgmeyer I. Expression of the sodium-driven chloride bicarbonate exchanger NCBE during prenatal mouse development. Gene Expr Patterns. 2004;5:219–223. doi: 10.1016/j.modgep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, Takeshima Y, Takumi T, Takahashi T, Yoshikawa N, Nakamura H, Endou H. Novel nonsense mutation in the Na+/ HCO3− cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol. 2001;12:713–718. doi: 10.1681/ASN.V124713. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Walther D, Arinami T, Uhl GR. Variation in a bicarbonate co-transporter gene family member SLC4A7 is associated with propensity to addictions: a study using fine-mapping and three samples. Addiction. 2007;102:1320–1325. doi: 10.1111/j.1360-0443.2007.01877.x. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Ruusuvuori E, Sipila ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynela J, Praetorius J, Voipio J, Hübner CA. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci U S A. 2008;105:311–316. doi: 10.1073/pnas.0705487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolimek W, Misgeld U, Lux HD. Activity dependent alkaline and acid transients in guinea pig hippocampal slices. Brain Res. 1989;505:225–232. doi: 10.1016/0006-8993(89)91447-9. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Schmitt BM, Berger UV, Nsumu NN, Boron WF, Hediger MA, Brown D, Breton S. Localization of sodium bicarbonate cotransporter (NBC) protein and messenger ribonucleic acid in rat epididymis. Biol Reprod. 1999;60:573–579. doi: 10.1095/biolreprod60.3.573. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976;262:661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Jung YW, Choi IJ, Kwon TH. Altered expression of sodium transporters in ischemic penumbra after focal cerebral ischemia in rats. Neurosci Res. 2007;59:152–159. doi: 10.1016/j.neures.2007.06.1470. [DOI] [PubMed] [Google Scholar]
- Kaila K, Paalasmaa P, Taira T, Voipio J. pH transients due to monosynaptic activation of GABAA receptors in rat hippocampal slices. Neuro Report. 1992;2:105–108. doi: 10.1097/00001756-199201000-00028. [DOI] [PubMed] [Google Scholar]
- Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987;330:163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kang TC, An SJ, Park SK, Hwang IK, Suh JG, Oh YS, Bae JC, Won MH. Alterations in Na+/H+ exchanger and Na+/ HCO3− cotransporter immunoreactivities within the gerbil hippocampus following seizure. Brain Res Mol Brain Res. 2002;109:226–232. doi: 10.1016/s0169-328x(02)00559-4. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Schlue WR. Intracellular pH regulation in cultured mouse oligodendrocytes. J Physiol. 1988;406:147–162. doi: 10.1113/jphysiol.1988.sp017373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok C, Kennerson ML, Spring PJ, Ing AJ, Pollard JD, Nicholson GA. A locus for hereditary sensory neuropathy with cough and gastroesophageal reflux on chromosome 3p22-p24. Am J Hum Genet. 2003;73:632–637. doi: 10.1086/377591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon TH, Fulton C, Wang W, Kurtz I, Frokiaer J, Aalkjaer C, Nielsen S. Chronic metabolic acidosis upregulates rat kidney Na-HCO cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol. 2002;282:F341–F351. doi: 10.1152/ajprenal.00104.2001. [DOI] [PubMed] [Google Scholar]
- Lee J, Taira T, Pihlaja P, Ransom BR, Kaila K. Effects of CO2 on excitatory transmission apparently caused by changes in intracellular pH in the rat hippocampal slice. Brain Res. 1996;706:210–216. doi: 10.1016/0006-8993(95)01214-1. [DOI] [PubMed] [Google Scholar]
- Lee A, Rayfield A, Hryciw DH, Ma TA, Wang D, Pow D, Broer S, Yun C, Poronnik P. Na+-H+ exchanger regulatory factor 1 is a PDZ scaffold for the astroglial glutamate transporter GLAST. Glia. 2007;55:119–129. doi: 10.1002/glia.20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis JA, Bekar LK, Walz W. Potassium homeostasis in the ischemic brain. Glia. 2005;50:407–416. doi: 10.1002/glia.20145. [DOI] [PubMed] [Google Scholar]
- Li HC, Szigligeti P, Worrell RT, Matthews JB, Conforti L, Soleimani M. Missense mutations in Na+:HCO3− cotransporter NBC1 show abnormal trafficking in polarized kidney cells: a basis of proximal renal tubular acidosis. Am J Physiol Renal Physiol. 2005;289:F61–F71. doi: 10.1152/ajprenal.00032.2005. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xu K, Chen LM, Sun X, Parker MD, Kelly ML, LaManna JC, Boron WF. Distribution of NBCn2 (SLC4A10) splice variants in mouse brain. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.06.005. doi: 10.1016/j.neuroscience.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez IA, Acuna D, Galbraith G, Bok D, Ishiyama A, Liu W, Kurtz I. Time course of auditory impairment in mice lacking the electroneutral sodium bicarbonate cotransporter NBC3 (slc4a7) Brain Res Dev Brain Res. 2005;160:63–77. doi: 10.1016/j.devbrainres.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Majumdar D, Maunsbach AB, Shacka JJ, Williams JB, Berger UV, Schultz KP, Harkins LE, Boron WF, Roth KA, Bevensee MO. Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuroscience. 2008;155:818–832. doi: 10.1016/j.neuroscience.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na+-HCO3− cotransporter in human pancreas. Am J Physiol. 1999;277:G487–G494. doi: 10.1152/ajpgi.1999.277.2.G487. [DOI] [PubMed] [Google Scholar]
- Marshall KC, Engberg I. The effects of hydrogen ion on spinal neurons. Can J Physiol Pharmacol. 1980;58:650–655. doi: 10.1139/y80-107. [DOI] [PubMed] [Google Scholar]
- Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, Schmitt BM, Boron WF, Aalkjaer C. Immunoelectron microscopic localization of the electrogenic Na/HCO3 cotransporter in rat and ambystoma kidney. J Am Soc Nephrol. 2000;11:2179–2189. doi: 10.1681/ASN.V11122179. [DOI] [PubMed] [Google Scholar]
- McAlear SD, Bevensee MO. pH regulation in non-neuronal brain cells and interstitial fluid. In: Hertz L, editor. Non-neuronal Cells of the Nervous System: Function and Dysfunction. Amsterdam: Elsevier Science; 2003. pp. 707–745. [Google Scholar]
- McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol. 2006;127:639–658. doi: 10.1085/jgp.200609520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee JA, Brewer RP, Macy GE, Borel CO, Reynolds JD, Warner DS. Magnesium neuroprotection is limited in humans with acute brain injury. Neurocrit Care. 2005;2:342–351. doi: 10.1385/NCC:2:3:342. [DOI] [PubMed] [Google Scholar]
- Mellergård P, Ouyang YB, Siesjö BK. Intracellular pH regulation in cultured rat astrocytes in CO2/HCO3−-containing media. Exp Brain Res. 1993;95:371–380. doi: 10.1007/BF00227129. [DOI] [PubMed] [Google Scholar]
- Millar ID, Brown PD. NBCe2 exhibits a 3 HCO3−:1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochem Biophys Res Commun. 2008;373:550–554. doi: 10.1016/j.bbrc.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Lux HD. Cytoplasmic alkalinization increases high-threshold calcium current in chick dorsal root ganglion neurones. Pflügers Arch. 1991;419:138–143. doi: 10.1007/BF00372999. [DOI] [PubMed] [Google Scholar]
- O'Beirne M, Bulloch AG, MacVicar BA. Dye and electrotonic coupling between cultured hippocampal neurons. Neurosci Lett. 1987;78:265–270. doi: 10.1016/0304-3940(87)90371-5. [DOI] [PubMed] [Google Scholar]
- Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjaer C, Leipziger J. Basolateral Na+-dependent HCO3− transporter NBCn1-mediated HCO3− influx in rat medullary thick ascending limb. J Physiol. 2004;555:205–218. doi: 10.1113/jphysiol.2003.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østby I, Øyehaug L, Einevoll GT, Nagelhus EA, Plahte E, Zeuthen T, Lloyd CM, Ottersen OP, Omholt SW. Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLoS Comput Biol. 2009;5:e1000272. doi: 10.1371/journal.pcbi.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas CA, Ransom BR. Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J Neurophysiol. 1994;72:2816–2826. doi: 10.1152/jn.1994.72.6.2816. [DOI] [PubMed] [Google Scholar]
- Park HJ, Rajbhandari I, Yang HS, Lee S, Cocuranu D, Cooper DS, Klein JD, Sands JM, Choi I. Neuronal expression of sodium/bicarbonate cotransporter NBCn1 (SLC4A7) and its response to chronic metabolic acidosis. Am J Physiol Cell Physiol. 2010;298:C1018–C1028. doi: 10.1152/ajpcell.00492.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl- self-exchange activity. J Biol Chem. 2008a;283:12777–12788. doi: 10.1074/jbc.M707829200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MD, Bouyer P, Daly CM, Boron WF. Cloning and characterization of novel human SLC4A8 gene products encoding Na+-driven Cl−/HCO3− exchanger variants NDCBE-A, −C, and −D. Physiol Genomics. 2008b;34:265–276. doi: 10.1152/physiolgenomics.90259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Dotti CG, Bacallao R, Kurtz I, Simons K, Prydz K. pH-induced microtubule-dependent redistribution of late endosomes in neuronal and epithelial cells. J Cell Biol. 1991;113:261–274. doi: 10.1083/jcb.113.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet M, Kuwajima M, Yun CC, Smith Y, Hall RA. Astrocytic and neuronal localization of the scaffold protein Na+/H+ exchanger regulatory factor 2 (NHERF-2) in mouse brain. J Comp Neurol. 2006;494:752–762. doi: 10.1002/cne.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius J, Hager H, Nielsen S, Aalkjaer C, Friis UG, Ainsworth MA, Johansen T. Molecular and functional evidence for electrogenic and electroneutral Na+-HCO3− cotransporters in murine duodenum. Am J Physiol Gastrointest Liver Physiol. 2001;280:G332–G343. doi: 10.1152/ajpgi.2001.280.3.G332. [DOI] [PubMed] [Google Scholar]
- Praetorius J, Kim YH, Bouzinova EV, Frische S, Rojek A, Aalkjaer C, Nielsen S. NBCn1 is a basolateral Na+- HCO3− cotransporter in rat kidney inner medullary collecting ducts. Am J Physiol Renal Physiol. 2004a;286:F903–F912. doi: 10.1152/ajprenal.00437.2002. [DOI] [PubMed] [Google Scholar]
- Praetorius J, Nejsum LN, Nielsen S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol. 2004b;286:C601–C610. doi: 10.1152/ajpcell.00240.2003. [DOI] [PubMed] [Google Scholar]
- Praetorius J, Nielsen S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol Cell Physiol. 2006;291:C59–C67. doi: 10.1152/ajpcell.00433.2005. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Kurtz I. SLC4 base (HCO3−, CO32−) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol. 2006;290:F580–F599. doi: 10.1152/ajprenal.00252.2005. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999a;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Mapping of the human NBC3 (SLC4A7) gene to chromosome 3p22. Genomics. 1999b;58:321–322. [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochim Biophys Acta. 2000a;1493:215–218. doi: 10.1016/s0167-4781(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Two C-terminal variants of NBC4, a new member of the sodium bicarbonate cotransporter family: cloning, characterization, and localization. IUBMB Life. 2000b;50:13–19. doi: 10.1080/15216540050176539. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Gross E, Newman D, Tatishchev S, Lee I, Fedotoff O, Bondar G, Azimov R, Ngyuen M, Kurtz I. Molecular mechanism of kNBC1-carbonic anhydrase II interaction in proximal tubule cells. J Physiol. 2004;559:55–65. doi: 10.1113/jphysiol.2004.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom BR. Glial modulation of neural excitability mediated by extracellular pH: A hypothesis. Prog Brain Res. 1992;94:37–46. doi: 10.1016/s0079-6123(08)61737-9. [DOI] [PubMed] [Google Scholar]
- Ransom BR. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis revisited. Prog Brain Res. 2000;125:217–228. doi: 10.1016/S0079-6123(00)25012-7. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Orlowski B, Heupel K, Roussa E. Distinct expression and subcellular localization patterns of Na+/HCO3− cotransporter (SLC 4A4) variants NBCe1-A and NBCe1-B in mouse brain. Neuroscience. 2007;146:1220–1231. doi: 10.1016/j.neuroscience.2007.02.061. [DOI] [PubMed] [Google Scholar]
- Risso S, Richerson GB, Rojas JD, Bouyer P, Boron WF. Distribution of Na+/HCO3− cotransporters in rodent brain with subtype-specific antibodies. Abstr. Soc. Neurosci. 2001;27 Program #527.11. [Google Scholar]
- Ro HA, Carson JH. pH microdomains in oligodendrocytes. J Biol Chem. 2004;279:37115–37123. doi: 10.1074/jbc.M403099200. [DOI] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol. 1998;274:F425–F432. doi: 10.1152/ajprenal.1998.274.2.F425. [DOI] [PubMed] [Google Scholar]
- Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+-driven anion exchanger (NDAE1) J Biol Chem. 2000;275:24552–24559. doi: 10.1074/jbc.M003476200. [DOI] [PubMed] [Google Scholar]
- Romero MF, Chang M-H, Mount DB. From cloning to structure, function, and regulation of chloride-dependent and independent bicarbonate transporters. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and pathology of chloride transporters and channels in the nervous system: From molecules to diseases. Amsterdam: Elsevier Academic Press; 2009. pp. 43–79. [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Roussa E, Romero MF, Schmitt BM, Boron WF, Alper SL, Thévenod F. Immunolocalization of anion exchanger AE2 and Na+-HCO3− cotransporter in rat parotid and submandibular glands. Am J Physiol. 1999;277:G1288–G1296. doi: 10.1152/ajpgi.1999.277.6.G1288. [DOI] [PubMed] [Google Scholar]
- Russell JM, Boron WF. Role of chloride transport in regulation of intracellular pH. Nature. 1976;264:73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Russell JM, Boron WF. Intracellular pH regulation in squid giant axons. Kroc Found Ser. 1981;15:221–237. [PubMed] [Google Scholar]
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I. Functional characterization of NBC4: a new electrogenic sodium-bicarbonate cotransporter. Am J Physiol Cell Physiol. 2002;282:C408–C416. doi: 10.1152/ajpcell.00409.2001. [DOI] [PubMed] [Google Scholar]
- Satoh H, Moriyama N, Hara C, Yamada H, Horita S, Kunimi M, Tsukamoto K, Iso O, Inatomi J, Kawakami H, Kudo A, Endou H, Igarashi T, Goto A, Fujita T, Seki G. Localization of Na+-HCO3− cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol. 2003;284:C729–C737. doi: 10.1152/ajpcell.00166.2002. [DOI] [PubMed] [Google Scholar]
- Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+-HCO3− cotransporter in mammalian and amphibian kidney. Am J Physiol. 1999;276:F27–F38. doi: 10.1152/ajprenal.1999.276.1.F27. [DOI] [PubMed] [Google Scholar]
- Schmitt BM, Berger UV, Douglas RM, Bevensee MO, Hediger MA, Haddad GG, Boron WF. Na/HCO3 cotransporters in rat brain: expression in glia, neurons, and choroid plexus. J Neurosci. 2000;20:6839–6848. doi: 10.1523/JNEUROSCI.20-18-06839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na+-dependent Cl−-HCO3− exchange. J Physiol. 1994;475:59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino CM, Shrode LD, Fletcher BR, Harte PJ, Romero MF. Localization of endogenous and recombinant Na+-driven anion exchanger protein NDAE1 from Drosophila melanogaster. Am J Physiol Cell Physiol. 2001;281:C449–C463. doi: 10.1152/ajpcell.2001.281.2.C449. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee Y-H, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimäki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King M-C, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman T, Chernova MN, Clark JS, Jiang L, Alper SL, Nehrke K. The abts and sulp families of anion transporters from Caenorhabditis elegans. Am J Physiol Cell Physiol. 2005;289:C341–C351. doi: 10.1152/ajpcell.00071.2005. [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1) Proc Natl Acad Sci USA. 2006;103:9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrode LD, Putnam RW. Intracellular pH regulation in primary rat astrocytes and C6 glioma cells. Glia. 1994;12:196–210. doi: 10.1002/glia.440120305. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Grassi SM, Aronson PS. Stoichiometry of Na+-HCO3− cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987;79:1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyfoo MS, Bulur N, Virreira M, Louchami K, Lybaert P, Crutzen R, Perret J, Delporte C, Roussa E, Thévenod F, Best L, Yates AP, Malaisse WJ, Sener A, Beauwens R. Expression of the electrogenic Na+-HCO3−-cotransporters NBCe1-A and NBCe1-B in rat pancreatic islet cells. Endocrine. 2009;35:449–458. doi: 10.1007/s12020-009-9175-1. [DOI] [PubMed] [Google Scholar]
- Sun XC, Bonanno JA. Identification and cloning of the Na/HCO3− cotransporter (NBC) in human corneal endothelium. Exp Eye Res. 2003;77:287–295. doi: 10.1016/s0014-4835(03)00150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XC, Bonanno JA, Jelamskii S, Xie Q. Expression and localization of Na+-HCO3− cotransporter in bovine corneal endothelium. Am J Physiol Cell Physiol. 2000;279:C1648–C1655. doi: 10.1152/ajpcell.2000.279.5.C1648. [DOI] [PubMed] [Google Scholar]
- Sussman CR, Zhao J, Plata C, Lu J, Daly C, Angle N, Dipiero J, Drummond IA, Liang JO, Boron WF, Romero MF, Chang MH. Cloning, localization, and functional expression of the electrogenic Na+ bicarbonate cotransporter (NBCe1) from zebrafish. Am J Physiol Cell Physiol. 2009;297:C865–C875. doi: 10.1152/ajpcell.00679.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Raint F, De Jonghe P, Li Y, Sekine T, Igarashi T, Fujimoto I, Mikoshiba K, Shimadzu M, Shiohara M, Braverman N, Al-Gazali L, Fujita T, Seki G. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1008705107. www.pnas.org/cgi/doi/10.1073/pnas.1008705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski M, Schlue WR. Mechanisms of pH recovery from intracellular acid loads in the leech connective glial cell. Glia. 1992;5:193–200. doi: 10.1002/glia.440050305. [DOI] [PubMed] [Google Scholar]
- Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proc Natl Acad Sci U S A. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenod F, Roussa E, Schmitt BM, Romero MF. Cloning and immunolocalization of a rat pancreatic Na+ bicarbonate cotransporter. Biochem Biophys Res Commun. 1999;264:291–298. doi: 10.1006/bbrc.1999.1484. [DOI] [PubMed] [Google Scholar]
- Thomas RC. Ionic mechanism of the H+ pump in a snail neurone. Nature. 1976a;262:54–55. doi: 10.1038/262054a0. [DOI] [PubMed] [Google Scholar]
- Thomas RC. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976b;255:715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977;273:317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982;299:826–828. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. pH modulation of voltage-gated ion channels. In: Kaila K, Ransom BR, editors. pH and Brain Function. New York: Wiley-Liss, Inc.; 1998. pp. 395–416. [Google Scholar]
- Tombaugh GC, Somjen GG. Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ currents in isolated rat CA1 neurons. J Physiol. 1996;493(Pt 3):719–732. doi: 10.1113/jphysiol.1996.sp021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. Differential sensitivity to intracellular pH among high- and low-threshold Ca2+ currents in isolated rat CA1 neurons. J Neurophysiol. 1997;77:639–653. doi: 10.1152/jn.1997.77.2.639. [DOI] [PubMed] [Google Scholar]
- Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00094.2006. [DOI] [PubMed] [Google Scholar]
- Traynelis SF. pH modulation of ligand-gated ion channels. In: Kaila K, Ransom BR, editors. pH and Brain Function. New York: Wiley-Liss, Inc.; 1998. pp. 417–446. [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966;241:4110–4112. [PubMed] [Google Scholar]
- Usui T, Seki G, Amano S, Oshika T, Miyata K, Kunimi M, Taniguchi S, Uwatoko S, Fujita T, Araie M. Functional and molecular evidence for Na+-HCO3− cotransporter in human corneal endothelial cells. Pflügers Arch. 1999;438:458–462. doi: 10.1007/s004249900081. [DOI] [PubMed] [Google Scholar]
- Velísek L, Dreier JP, Stanton PK, Heinemann U, Moshé SL. Lowering of extracellular pH suppresses low-Mg2+-induces seizures in combined entorhinal cortex-hippocampal slices. Exp Brain Res. 1994;101:44–52. doi: 10.1007/BF00243215. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO cotransporter (NBCe2) Am J Physiol Cell Physiol. 2002;282:C1278–C1289. doi: 10.1152/ajpcell.00589.2001. [DOI] [PubMed] [Google Scholar]
- Virkki LV, Choi I, Davis BA, Boron WF. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol. 2003;285:C771–C780. doi: 10.1152/ajpcell.00439.2002. [DOI] [PubMed] [Google Scholar]
- Vorum H, Kwon TH, Fulton C, Simonsen B, Choi I, Boron W, Maunsbach AB, Nielsen S, Aalkjaer C. Immunolocalization of electroneutral Na-HCO3− cotransporter in rat kidney. Am J Physiol Renal Physiol. 2000;279:F901–F909. doi: 10.1152/ajprenal.2000.279.5.F901. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H+-gated cation channels. Ann N Y Acad Sci. 1999;868:67–76. doi: 10.1111/j.1749-6632.1999.tb11274.x. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl−/HCO3− exchanger. Cloning, tissue distribution, and functional characterization. J Biol Chem. 2000;275:35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Conforti L, Petrovic S, Amlal H, Burnham CE, Soleimani M. Mouse Na+: HCO3− cotransporter isoform NBC-3 (kNBC-3): cloning, expression, and renal distribution. Kidney Int. 2001;59:1405–1414. doi: 10.1046/j.1523-1755.2001.0590041405.x. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Woodbury DM, Engstrom FL, White HS, Chen CF, Kemp JW, Chow SY. Ionic and acid-base regulation of neurons and glia during seizures. Ann Neurol. 1984;16 Suppl:S135–S144. doi: 10.1002/ana.410160720. [DOI] [PubMed] [Google Scholar]
- Wu J, McNicholas CM, Bevensee MO. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates the electrogenic Na/HCO3 cotransporter NBCe1-A expressed in Xenopus oocytes. Proc Natl Acad Sci U S A. 2009;106:14150–14155. doi: 10.1073/pnas.0906303106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang Z, Barone S, Petrovic M, Amlal H, Conforti L, Petrovic S, Soleimani M. Expression of the Na+-HCO3− cotransporter NBC4 in rat kidney and characterization of a novel NBC4 variant. Am J Physiol Renal Physiol. 2003;284:F41–F50. doi: 10.1152/ajprenal.00055.2002. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shirayama T, Sakatani T, Takahashi T, Tanaka H, Takamatsu T, Spitzer KW, Matsubara H. Enhanced activity of ventricular Na+- HCO3− cotransport in pressure overload hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1254–H1264. doi: 10.1152/ajpheart.00964.2006. [DOI] [PubMed] [Google Scholar]