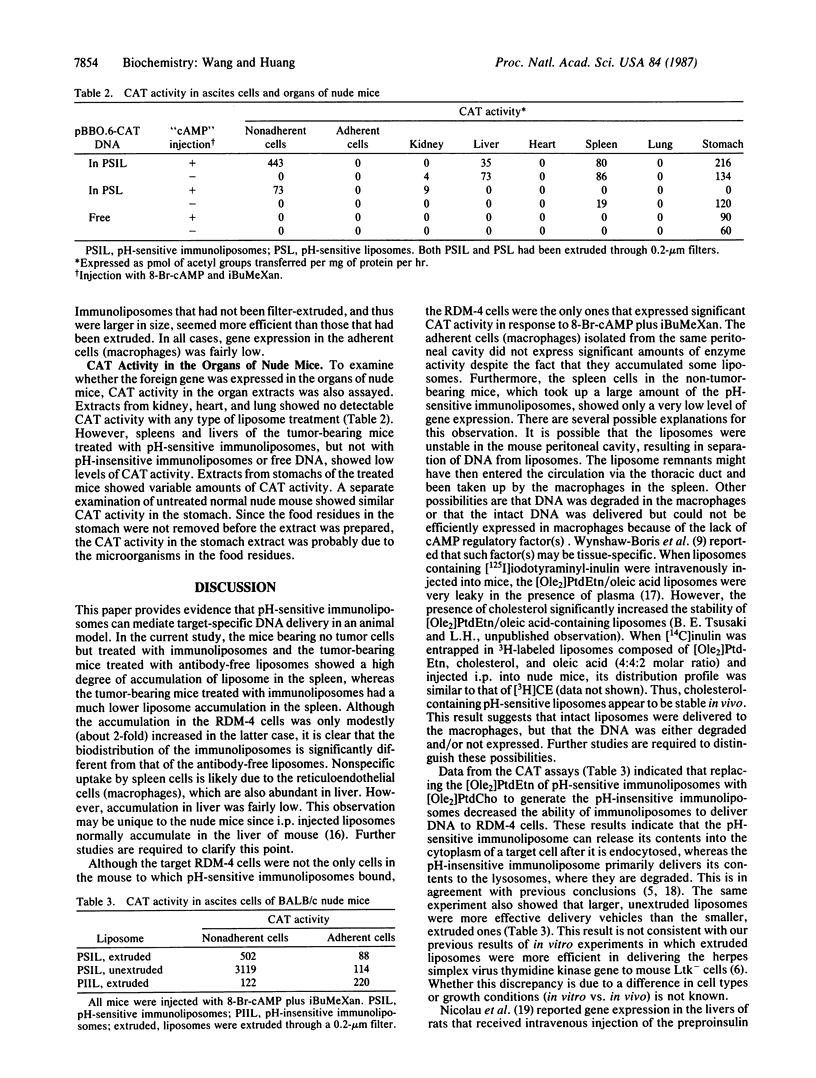
Abstract
A plasmid containing the Escherichia coli chloramphenicol acetyltransferase (CAT) gene under the control of a mammalian cAMP-regulated promoter was entrapped in H-2Kk antibody-coated liposomes composed of dioleoyl phosphatidylethanolamine, cholesterol, and oleic acid (pH-sensitive immunoliposomes). The entrapped or free DNA was injected intraperitoneally into immunodeficient (nude) BALB/c mice bearing ascites tumor generated by H-2Kk-positive RDM-4 lymphoma cells. About 20% of the injected immunoliposomes were taken up by the target RDM-4 cells. Uptake was much less when liposomes without antibody were used. The presence of the targeting antibody on liposomes also significantly decreased the nonspecific uptake of liposomes by the spleen. Significant CAT enzyme activity was detected in RDM-4 cells from mice treated with DNA entrapped in the pH-sensitive immunoliposomes. Furthermore, CAT expression in RDM-4 cells was under the control of cAMP, as only the cells from mice injected with 8-bromo-cAMP and 3-isobutyl-1-methylxanthine showed CAT activity. CAT activity in liver and spleen was much lower (by factors of 12 and 5, respectively) than in the RDM-4 cells, and the activities in these reticuloendothelial organs were not regulated by cAMP. CAT activity in RDM-4 cells from mice injected with DNA entrapped in pH-insensitive immunoliposomes (containing phosphatidylcholine in place of phosphatidylethanolamine) was approximately one-fourth that in RDM-4 cells from mice injected with pH-sensitive immunoliposomes, indicating the superior delivery efficiency of the pH-sensitive liposomes. These results are discussed in terms of the DNA-carrier potential of immunoliposomes in therapy of cancer and genetic diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Cline M. J. Gene therapy. Mol Cell Biochem. 1984;59(1-2):3–10. doi: 10.1007/BF00231302. [DOI] [PubMed] [Google Scholar]
- Connor J., Huang L. Efficient cytoplasmic delivery of a fluorescent dye by pH-sensitive immunoliposomes. J Cell Biol. 1985 Aug;101(2):582–589. doi: 10.1083/jcb.101.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J., Norley N., Huang L. Biodistribution of pH-sensitive immunoliposomes. Biochim Biophys Acta. 1986 Dec 10;884(3):474–481. doi: 10.1016/0304-4165(86)90197-2. [DOI] [PubMed] [Google Scholar]
- Connor J., Yatvin M. B., Huang L. pH-sensitive liposomes: acid-induced liposome fusion. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1715–1718. doi: 10.1073/pnas.81.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapergolas G., Neerunjun E. D., Gregoriadis G. Penetration of target areas in the rat by liposome-associated bleomycin, glucose oxidase and insulin. FEBS Lett. 1976 Apr 1;63(2):235–239. doi: 10.1016/0014-5793(76)80102-0. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Straubinger R. M., Baldwin P. A., Friend D. S., Papahadjopoulos D. Proton-induced fusion of oleic acid-phosphatidylethanolamine liposomes. Biochemistry. 1985 Jun 18;24(13):3091–3098. doi: 10.1021/bi00334a004. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Huang A., Tsao Y. S., Kennel S. J., Huang L. Characterization of antibody covalently coupled to liposomes. Biochim Biophys Acta. 1982 May 27;716(2):140–150. doi: 10.1016/0304-4165(82)90262-8. [DOI] [PubMed] [Google Scholar]
- Kantoff P. W., Kohn D. B., Mitsuya H., Armentano D., Sieberg M., Zwiebel J. A., Eglitis M. A., McLachlin J. R., Wiginton D. A., Hutton J. J. Correction of adenosine deaminase deficiency in cultured human T and B cells by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6563–6567. doi: 10.1073/pnas.83.17.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maxwell I. H., Maxwell F., Glode L. M. Regulated expression of a diphtheria toxin A-chain gene transfected into human cells: possible strategy for inducing cancer cell suicide. Cancer Res. 1986 Sep;46(9):4660–4664. [PubMed] [Google Scholar]
- Nicolau C., Le Pape A., Soriano P., Fargette F., Juhel M. F. In vivo expression of rat insulin after intravenous administration of the liposome-entrapped gene for rat insulin I. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1068–1072. doi: 10.1073/pnas.80.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool G. L., French M. E., Edwards R. A., Huang L., Lumb R. H. Use of radiolabeled hexadecyl cholesteryl ether as a liposome marker. Lipids. 1982 Jun;17(6):448–452. doi: 10.1007/BF02535225. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Setoyama C., de Crombrugghe B. Regulation of a collagen gene promoter by the product of viral mos oncogene. Nature. 1985 Mar 21;314(6008):286–289. doi: 10.1038/314286a0. [DOI] [PubMed] [Google Scholar]
- Soriano P., Dijkstra J., Legrand A., Spanjer H., Londos-Gagliardi D., Roerdink F., Scherphof G., Nicolau C. Targeted and nontargeted liposomes for in vivo transfer to rat liver cells of a plasmid containing the preproinsulin I gene. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7128–7131. doi: 10.1073/pnas.80.23.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Hughes K. W., Huang L. Improved Cytoplasmic Delivery to Plant Protoplasts via pH-Sensitive Liposomes. Plant Physiol. 1986 Sep;82(1):179–184. doi: 10.1104/pp.82.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynshaw-Boris A., Lugo T. G., Short J. M., Fournier R. E., Hanson R. W. Identification of a cAMP regulatory region in the gene for rat cytosolic phosphoenolpyruvate carboxykinase (GTP). Use of chimeric genes transfected into hepatoma cells. J Biol Chem. 1984 Oct 10;259(19):12161–12169. [PubMed] [Google Scholar]