Abstract
Background
Sunitinib is widely used as first-line treatment for metastatic clear cell renal cancer (MCRC). No reports are known of treatment after sunitinib failure. As irinotecan, cisplatin, and mitomycin-C (IPM) chemotherapy has been reported to influence MCRC after progression on cytokine therapy, we report on the outcome of 11 patients treated with IPM after sunitinib failure.
Patients and methods
Eleven patients with progression of disease on sunitinib therapy were treated with 4, monthly cycles of monthly IPM.
Results
Nine out of 11 patients progressed during IPM therapy. The median time to progression was 1.4 months (95% CI: 0.7–2.1 months), while the overall survival was 4.2 months (95% CI: 0.9–2.3). Overall 10 patients have died of progressive renal cancer. One patient had a radiological response to therapy and remains progression free 11 months after treatment. Four of the 10 patients required a dose reduction for grade 3 or 4 toxicities.
Conclusions
IPM alone does not appear to benefit patients with MCRC who previously progressed during sunitinib therapy. The median progression-free survival and overall survival for these patients is short.
Keywords: renal cancer, chemotherapy, sunitinib
Introduction
Multitargeted tyrosine kinase inhibitors (TKIs) have revolutionised the treatment of metastatic clear cell renal cancer (Motzer et al 2006; Escuider et al 2007). Sunitinib has superseded cytokine therapy as first line treatment for this disease (Motzer et al 2007).
Patient results after treatment with cytokine therapy have been reported (Ryan et al 2002; Shamash et al 2003; Stadler et al 2003; Motzer et al 2004; Porta et al 2004), but currently there are no data on treatment and outcome after progression on sunitinib therapy.
Single agent chemotherapy has little effect in metastatic clear cell renal cancer (Kish et al 1994; De Mulder et al 1996). However, the role of combination chemotherapy in cytokine refractory disease was already under investigatation before the introduction of TKIs (Ryan et al 2002; Stadler et al 2003; Porta et al 2004; Patard et al 2008). Our group published a phase II study using irinotecan cisplatin and mitomycin (IPM) in cytokine refractory disease (Shamash et al 2003). Results were comparable to historical controls treated with chemotherapy, with a progression-free survival of 4.8 months and overall survival (OS) of 9.2 months.
We have continued to use IPM in patients with refractory renal cell cancer and in this short report we describe our experience (as part of a prospective observational study) using this regimen after progression on sunitinib therapy.
Methods
Since the publication of the IPM regimen (Shamash et al 2003) the policy of our unit has been to offer this treatment to patients with sunitinib refractory metastatic renal cancer. Patients with histologically confirmed metastatic clear cell renal cancer, who were progressing on (RECIST criteria) (n = 10) or intolerant to sunitinib (n = 1), were included, after giving informed consent according to standard guidelines. CT scanning of the chest, abdomen, and pelvis was performed every 2 complete cycles of treatment.
The IPM regimen
Treatment was administered on an outpatient basis as follows: intravenous (iv) irinotecan 70 mg/m2, cisplatin 40 mg/m2, mitomycin-C 6 mg/m2 was administered on day 1, and irinotecan 70 mg/m2, cisplatin 40 mg/m2 was administered on day 15 of a 28-day cycle. Anti-emetic granisetron 1 mg iv and dexamethasone 8 mg iv were co-administered routinely with each cycle. Blood cell counts and chemistry panels were repeated with every treatment. Treatment was delayed by one week in the event of bone marrow suppression (total leukocyte count <3 × 109 cells/L, neutrophil count <1.5 × 109 cells/L, or platelet count <100 × 109 cells/L). This regimen has been standard second line therapy for metastatic renal tumors at our unit since the publication of our work in 2003 (Shamash et al 2003).
Statistics
Survival was calculated according to the method of Kaplan and Meier.
Results (Table 1)
Table 1.
Patient characteristics and outcome
| Risk at time of sunitinib therapy (Motzer et al 2004) | Initial response to sunitinib therapy | TTP during sunitinib therapy | Risk at time of IPM (Motzer et al 2004) | Response to IPM after 2 cycles | Grade 3 or 4 toxicity on IPM | TTP on IPM (months) | Overall survival from time of starting IPM (months) | Overall survival from time of diagnosis of metastatic renal cancer |
|---|---|---|---|---|---|---|---|---|
| int | PR | 14.3 | int | PD | – | 1,4 | 4.2 | 31 |
| Poor | PD | 2.8 | poor | PD | – | 0.5 | 0.5 | 6.1 |
| Int | PD | 4.8 | poor | PD | Dia | 0.9 | 2.1 | 9.2 |
| Int | SD | 8.3 | poor | PD | – | 1.8 | 3.0 | 27.4 |
| Good | PR | 12.2 | poor | PD | Dia, lethergy | 1.4 | 1.6 | 33.0 |
| Poor | SD | 7.2 | int | PD | Lethargy | 2.8 | 6.2 | 16.9 |
| int | PR | 6.2 | poor | PR | – | 11.0PF | 11.0A | 34.3A |
| Poor | SD | 2.1 | poor | SD | Neut. Sepsis. Lethargy | 2.3T | 7.8 | 18.1 |
| int | SD | 10.5 | poor | PD | – | 0.9 | 5 | 20.5 |
| poor | SD | 2.7 | poor | PD | – | 1.8 | 5.3 | 24.4 |
| Int | PR | 11.3 | int | PD | – | 1.4 | 2.1 | 15.6 |
Notes: Alive
Progression free
Stopped due to toxicity.
Abbreviations: TTP, time to progression; Int, intermediate; Neut, neutropenic; Dia, diarrhea.
The median age of the 11 patients with clear cell renal tumors was 53.5 (range: 42–69). The median OS from time of diagnosis of metastatic renal cancer was 20.5 months (range: 6.1–19.7).
Treatment prior to IPM chemotherapy
Cytokine therapy
The Memorial Sloan Kettering risk factors for these patients at initial diagnosis were: poor risk disease (n = 1), intermediate risk disease (n = 8), and favourable risk disease (n = 2). All but 1 patient had had a nephrectomy and all had received immune therapy as first line therapy followed by sunitinib therapy as second line therapy. None of the patients responded to immune therapy (interferon-alpha n = 9, interferon, 5FU, and il-2 n = 2) and the median time to progression on cytokine therapy was 4 months (range: 2–17 months).
Sunitinib therapy
All 11 patients went on to receive sunitinib therapy. Two patients had progression of disease, 5 had stable disease, and 4 had a partial response to treatment after 2 cycles of therapy. The median number of cycles administered was 4 and the median time on therapy was 7.2 months (range: 2.1–11.3 weeks). The prognostic factors for these pre-treated patients are shown in Table 1.
Response to IPM chemotherapy post sunitinib therapy
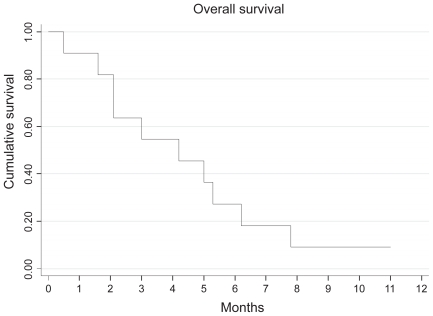
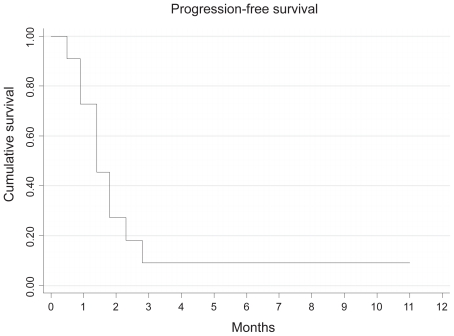
Only 2 patients received all 4 cycles of IPM chemotherapy as planned. All but 2 patients progressed either clinically or radiologically by the end of the second cycle of chemotherapy. One patient had stable disease but did not complete treatment (due to grade 3 toxicity), while the other had a partial response to treatment. This patient is alive and continues in remission 11 months after chemotherapy. The median time to progression from time of starting chemotherapy was 1.4 months (95% CI: 0.9–2.3 months), while the OS was 4.2 months (95% CI:1.6–6.2 months). Ten patients have died of progressive renal cancer (Figures 1 and 2).
Figure 1.
Kaplan Meier curve showing overall survival associated with IPM chemotherapy after progression on sunitinib therapy.
Figure 2.
Kaplan Meier curve showing progression-free survival with IPM chemotherapy.
Toxicity associated with IPM chemotherapy
Four of the 11 patients required a dose reduction for grade 3 or grade 4 toxicity, which included diarrhea (n = 2) and neutropenic sepsis (n = 1) and lethargy (n = 2). One of these patients stopped the IPM after 3 cycles because of toxicity.
Discussion
Sunitinib is now widely used as first line therapy in metastatic clear cell renal cancer. There are no published data on the management of patients with progression of disease on sunitinib therapy. Importantly, it is not clear whether patients should stop sunitinib therapy at progression (Motzer et al 2006, 2007). The use of sequential TKIs, such as a switch from sunitinib to sorafenib or axitinib on progression is common, but not yet of proven benefit (Patard et al 2008). These sequential regimens are undergoing intense prospective evaluation and the results are eagerly awaited.
It is widely accepted that renal cancer is largely resistent to chemotherapy in the majority of patients. The data presented here describes 11 patients who switched to IPM chemotherapy at progression (n = 10) or intolerance (n = 1) after sunitinib. The median time to progression (TTP) and median OS were 1.4 and 4.2 months, respectively. These results appear shorter when compared with IPM after cytokine therapy (TTP 4.8 months and OS 9.2 months) (Shamash et al 2003). Although the numbers are small, the data suggest that IPM chemotherapy in this setting does not appear to be of benefit. One patient did respond to therapy, but the significance of this is not clear (Dreicer 2006). Moreover this regimen was not particularly well tolerated in this setting with more than a third of the patients developing grade 3 or 4 toxicity. This finding, in conjunction with the poor response data, prompted us to halt further use of this regimen in this setting.
A number of prognostic factors have been identified for patients who have progressed on cytokine therapy (Motzer et al 2004). Eight of the 11 patients presented here were in this poor prognosis group. The median survival for these patients is predicted to be only 5.4 months, which may help explain, in part, why the results are poor. Indeed future investigation in this area should perhaps focus on patients with better prognostic factors.
There are 3 other possible explanations for these poor results. Firstly IPM was used as third line rather than second line therapy (after cytokine failure and sunitinib failure). Secondly, patients who progress on sunitinib may have more advanced and aggressive disease at the time of progression, compared with those who fail on cytokine therapy. This issue is unresolved in previously published studies (Motzer et al 2006), although such results might be expected to indicate higher response rates with chemotherapy than those seen in this work. Finally, it is possible that stopping sunitinib is associated with acceleration of disease, as seen with imatinib in gastro-intestinal stromal tumors (GIST) (Blay et al 2007).
The results of the IPM regimen, in the cytokine refractory setting, are comparable to other combination regimes used in this setting (gemicitabine and 5FU: TTP 6.6 months, OS 11 months (Rini et al 2000); gemicitabine and oxaliplatin TTP 2.5 months, mean OS 9.5 months (Porta et al 2004). The addition of cisplatin to gemicitabine and 5FU did not enhance these results any further (George et al 2002). Therefore one would not necessarily expect the results with these other chemotherapy regimes to be dramatically different, although the in vitro data on this topic is limited. Nevertheless the response and survival data presented here is poor and, in our opinion, does not warrant further investigation in this setting.
In view of the poor survival rates of these patients, this may not be the ideal setting to investigate new treatment in isolation in renal cancer. Other studies may wish to recruit patients with better prognostic factors or add treatments to sunitinib rather than stop it. It does not appear that IPM chemotherapy benefits patients with metastatic clear cell renal cancer who previously received sunitinib therapy.
Footnotes
Disclosures
None of the authors have any conflicts of interest to disclose.
References
- Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25:1107–13. doi: 10.1200/JCO.2006.09.0183. [DOI] [PubMed] [Google Scholar]
- De Mulder PH, Weissbach L, Jakse G, et al. Gemcitabine: a phase II study in patients with advanced renal cancer. Cancer Chemother Pharmacol. 1996;37:491–5. doi: 10.1007/s002800050417. [DOI] [PubMed] [Google Scholar]
- Dreicer R. Tyrosine kinase inhibitors compared with cytokine therapy for metastatic renal cell carcinoma: overview of recent clinical trials differentiating clinical response and adverse effects. Clin Genitourin Cancer. 2006;5(Suppl 1):S19–23. doi: 10.3816/cgc.2006.s.003. [DOI] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, et al. TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- George CM, Vogelzang NJ, Rini BI, et al. A phase II trial of weekly intravenous gemcitabine and cisplatin with continuous infusion fluorouracil in patients with metastatic renal cell carcinoma. Ann Oncol. 2002;13:116–20. doi: 10.1093/annonc/mdf008. [DOI] [PubMed] [Google Scholar]
- Kish JA, Wolf M, Crawford ED, et al. Evaluation of low dose continuous infusion 5-fluorouracil in patients with advanced and recurrent renal cell carcinoma. A Southwest Oncology Group Study. Cancer. 1994;74:916–9. doi: 10.1002/1097-0142(19940801)74:3<916::aid-cncr2820740319>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–63. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- Patard JJ, Pouessel D, Bensalah K, et al. Targeted therapy in renal cell carcinoma. World J Urol. 2008;26:135–40. doi: 10.1007/s00345-008-0237-4. [DOI] [PubMed] [Google Scholar]
- Porta C, Zimatore M, Imarisio I, et al. Gemcitabine and oxaliplatin in the treatment of patients with immunotherapy-resistant advanced renal cell carcinoma: final results of a single-institution Phase II study. Cancer. 2004;100:2132–8. doi: 10.1002/cncr.20226. [DOI] [PubMed] [Google Scholar]
- Rini BI, Vogelzang NJ, Dumas MC, et al. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol. 2000;18:2419–26. doi: 10.1200/JCO.2000.18.12.2419. [DOI] [PubMed] [Google Scholar]
- Ryan CW, Vogelzang NJ, Stadler WM. A phase II trial of intravenous gemcitabine and 5-fluorouracil with subcutaneous interleukin-2 and interferon-alpha in patients with metastatic renal cell carcinoma. Cancer. 2002;94:2602–9. doi: 10.1002/cncr.10528. [DOI] [PubMed] [Google Scholar]
- Shamash J, Steele JP, Wilson P, et al. IPM chemotherapy in cytokine refractory renal cell cancer. Br J Cancer. 2003;88:1516–21. doi: 10.1038/sj.bjc.6600934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler WM, Huo D, George C, et al. Prognostic factors for survival with gemcitabine plus 5-fluorouracil based regimens for metastatic renal cancer. J Urol. 2003;170:1141–5. doi: 10.1097/01.ju.0000086829.74971.4a. [DOI] [PubMed] [Google Scholar]