Abstract
Background:
The combination of sorafenib (vascular endothelial growth factor receptor 2 inhibitor) and sirolimus (mammalian target of rapamycin inhibitor) might work synergistically.
Methods:
A phase I dose-escalation study with sorafenib twice a day (b.i.d.) and sirolimus once daily (q.d.) was performed to determine the recommended dose of the combination in patients with solid tumours. Secondary objectives were to determine the safety profile and maximum tolerated dose (MTD), and to evaluate the pharmacokinetics (PK) of the combination.
Results:
Dose-limiting toxicities were transaminitis and cutaneous toxicity. The most frequently reported adverse events were elevated transaminases, hypophosphatemia, fatigue, anorexia, diarrhoea, nausea, rash and palmar–plantar erythrodysaesthesia. Sirolimus did not change the PK of sorafenib; in contrast, sorafenib reduced the AUC(0−96) and Cmax of sirolimus. No objective responses were observed; eight patients showed stable disease for a median of 16.3 weeks (range 8–24). The MTD of the combination was sorafenib 200 mg b.i.d. with sirolimus 1 mg q.d.
Conclusion:
The combination of sorafenib and sirolimus showed enhanced toxicity, which could not be explained by the PK of both drugs. The relative low doses at the MTD, in combination with the PK results, do not warrant further development of this combination.
Keywords: phase I, sorafenib, sirolimus, tyrosine kinase inhibitor, mTOR inhibitor
Multiple signalling pathways contribute to tumour growth and development. Single-agent strategies with small molecule signal transduction inhibitors or antibodies against one of these targets have shown clinical activity in several tumour types, although, only modest prolongation of overall survival has been shown. Combining agents that target pathways at multiple sites may enhance anti-tumour activity by biochemical and clinical synergism, reduce drug resistance or be successful with lower doses resulting in less toxicity (Dent et al, 2009). Sorafenib inhibits the receptor tyrosine kinases, vascular endothelial growth factor receptor 2 (VEGFR2), VEGFR3, Fms-related tyrosine kinase 3, c-KIT, platelet-derived growth factor receptor, and threonine kinases B-RAF and C-RAF. Sorafenib inactivates the RAS-RAF-MEK-ERK pathway, with subsequent inhibition of tumour-cell proliferation and angiogenesis. It has been approved for the second-line treatment in metastatic renal-cell cancer and hepatocellular carcinoma. The recommended dose of sorafenib is 400 mg twice daily (b.i.d.) in metastatic renal-cell cancer. Sirolimus is an orally administered mammalian target of rapamycin (mTOR) inhibitor and is the active metabolite of intravenously administered temsirolimus, registered for first-line treatment in poor prognosis metastatic renal-cell cancer. The threonine kinase mTOR is a key element of the intracellular signalling pathways involved in tumour cell proliferation, growth, survival and angiogenesis. Its activation leads to progression from the G1 to S phase of the cell cycle (Abraham and Gibbons, 2007). Mammalian target of rapamycin is activated aberrantly in tumours. This activation is increased by several signalling pathways, including the phosphatidyl-inositol 3-kinase/Akt, epidermal growth factor, Ras/mitogen-activated protein kinase pathways (Meric-Bernstam and Gonzalez-Angulo, 2009). One of the most important downstream proteins affected by mTOR is hypoxia-induced factor 1α. Hypoxia-induced factor 1α is a transcription factor, essential for the expression of genes necessary for cell growth in hypoxic conditions, as in tumours. Transcription of VEGF gene is regulated by hypoxia-induced factor 1α (Forsythe et al, 1996). Mammalian target of rapamycin is an attractive target for anti-cancer therapy. Sirolimus is approved as an immunosuppressive agent indicated for the prophylaxis of organ rejection in renal transplant patients. Currently sirolimus is studied in several types of cancer at doses of 0.5–10 mg orally daily (Stallone et al, 2005; Reardon et al, 2006; Rizell et al, 2008).
Combined inhibition of the VEGFR- and mTOR- signalling network may work synergistically. During treatment with a VEGFR inhibitor, such as sorafenib, plasma levels of VEGF will increase, which may contribute to resistance (Sosman and Puzanov, 2009). By addition of an mTOR inhibitor, the VEGF production will be downregulated by the effect of mTOR inhibition on hypoxia-induced factor 1α. On the basis of pre-clinical data and different mechanisms of anti-tumour activity of sorafenib and sirolimus, the current phase I trial was performed with this combination. Special attention was paid to the partly overlapping toxicity profiles and the fact that both drugs are metabolized by CYP3A4. The primary objective of this study was to identify the recommended dose of the combination of sorafenib and sirolimus for subsequent phase II studies. The secondary objectives were to (i) analyse the pharmacokinetic (PK) profiles, (ii) determine the safety profile (ii) determine the maximum tolerated dose (MTD) and (iv) evaluate preliminary activity of the combination of sorafenib and sirolimus.
Patients and methods
Patient eligibility
Patients aged ⩾18 years, with histologically or cytologically confirmed advanced solid malignancies refractory to conventional treatment or without any regular therapy option were enrolled. Eligibility criteria included life expectancy ⩾12 weeks, Eastern Cooperative Oncology Group performance status ⩽1, no previous anti-cancer therapy within 4 weeks of study entry, no previous treatment with sorafenib or sirolimus, adequate hematopoietic (absolute neutrophil count ⩾1.5 × 109 l−1; platelets ⩾100 × 109 l−1), hepatic (bilirubin ⩽1.5 × upper limit of normal (ULN), aspartate transaminase/alanine transaminase ⩽2.5 × ULN, in case of liver metastases ⩽5 × ULN,) and renal function (creatinine ⩽2 × ULN). Patients with clinically symptomatic brain tumours or brain metastases and patients with uncontrolled comorbidity were excluded. The local ethics committee approved the study protocol. Written informed consent was obtained from all patients before any study-related procedures.
Study design and dose-escalation schedule
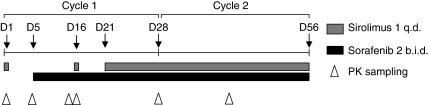
The recommended dose of the combination of sorafenib and sirolimus was determined by dose escalation. Patients were initially treated in cohorts of six per dose level. The number six was chosen to achieve adequate PK profiling of the combination of drugs, especially owing to the described large variances in sirolimus PK parameters (MacDonald et al, 2000). Both drugs were administered orally. Initially planned dose levels (DLs) were as follows: DL 1 (starting DL): sorafenib 200 mg b.i.d. and sirolimus 2 mg q.d., DL 2: sorafenib 400 mg b.i.d. and sirolimus 2 mg q.d., and DL 3: sorafenib 400 mg b.i.d. and sirolimus 4 mg q.d. In case DL 1 would not be feasible, a DL 0 was defined with sorafenib 200 mg b.i.d. and sirolimus 1 mg q.d. An extra intermediate DL (DLim) was amended: DLim: sorafenib 400 mg b.i.d. and sirolimus 1 mg q.d. During the first cycle, a run-in period was used for optimal PK analysis of both single drugs as well as to investigate the influence of sorafenib on sirolimus and vice versa (Figure 1). Therefore, in the first cycle, sirolimus was administered on day 1, once on day 16 and from day 21 as per a daily continuous schedule, while sorafenib was administered from day 5 on a daily continuous schedule. (Figure 1) The duration of one cycle was 28 days. The dose limiting toxicity (DLT) period ended after 28 days of administration of combination of sorafenib and sirolimus, thus 50 days after start of the study treatment. This prolonged DLT period was chosen because of the expectation that the first 7 days were needed to reach a steady concentration of sirolimus.
Figure 1.
Treatment schedule and pharmacokinetic (PK) schedule. The DLT period ended after 28 days of combination of sorafenib and sirolimus administration, thus, 50 days after start of study treatment.
Patients remained in study medication for as long as the investigator felt it was in their best interest and there was no evidence of progressive disease (PD) or unacceptable toxicity.
Definition of MTD and dose-limiting toxicity
Toxicity was evaluated according to the common toxicity criteria for adverse events (AEs) (CTCAEv3.0). The MTD was defined as the highest dose at which none or one out of six patients developed a DLT.
DLT was defined as any of the following events that was determined to be possibly or probably related to sorafenib and/or sirolimus and occurred during the first 50 days of treatment: any non-haematological grade 3–4 toxicity with the exceptions of nausea and vomiting, and fever that could be rapidly controlled with appropriate measures, grade 4 neutropenia lasting for ⩾7 days or febrile neutropenia defined as absolute neutrophil count ⩽1.0 × 109 l−1 and fever ⩾38.5°C; grade 4 thrombocytopenia and grade 4 uncontrolled hypertension. The protocol was amended to define that hypophosphatemia grade 3 or 4 was not a DLT, as this is a well-known toxicity of sorafenib (Escudier et al, 2007).
Patient evaluation and follow-up
Toxicity assessment, haematology and clinical biochemistry were performed at baseline and weekly during the first two cycles, and thereafter once every 2 weeks. Full physical examination and ECOG performance status were recorded at baseline and before each new cycle. Concomitant medications were recorded at every visit.
Response was evaluated according to the Response Evaluation Criteria in Solid Tumours (Therasse et al, 2000) at baseline and after every second cycle.
Pharmacokinetic assessments
Sorafenib is metabolized primarily in the liver and undergoes oxidative metabolism, mediated by CYP3A4, as well as glucuronidation by UGT1A9. The elimination half-life is 25–48 h. Sirolimus is a substrate for both CYP3A4 and P-glycoprotein. It is extensively metabolized by O-demethylation and/or hydroxylation. The terminal half-life in stable renal transplant recipients was 62±12 h, however, the effective half-life is shorter and the mean steady-state concentrations are achieved after 5–7 days.
To evaluate the single-agent PK of sirolimus, blood samples were obtained at 15 time points for up to 4 days after the first administration, as follows: at baseline, 20 and 40 min, and at 1, 2, 3, 4, 6, 8, 12, 15, 24, 36, 48, 72 and 96 h after the first administration on day 1. On day 15, the PK of single-agent sorafenib was analysed by collecting blood samples at baseline and 30 min, at 1, 2, 4, 8, 10 and 12 h after administration of sorafenib. To evaluate continuous dosing of sorafenib combined with single-dose sirolimus, blood samples were collected starting on day 16, when patients were already treated with sorafenib since day 5 and single-dose sirolimus was added. Samples were obtained at same time points after day 1 for sirolimus, and at baseline after 30 min and at 1, 2, 4, 8, 12 and 24 h for sorafenib. Fully validated LC/MS/MS assay methods were applied to quantify blood concentrations of sirolimus and plasma concentrations of sorafenib. Lower limits of quantization were 0.26 μg l−1 for sirolimus and 0.01 mg l−1 for sorafenib.
On days 1 and 15 of the second cycle, samples were collected to determine steady-state concentrations of sorafenib in plasma and of sirolimus in blood after combined continuous dosing at time points as follows: baseline, after 20, 30 and 40 min, and after 1, 2, 3, 4, 6, 8, 10 and 12 h after administration. In this way, we collected data of both drugs on PK of single-agent sirolimus, single-agent sorafenib, effect of single-dose sirolimus on steady-state level of sorafenib and effect of combination therapy on steady-state plasma or blood levels. Peak plasma or blood concentrations (Cmax), overall drug exposure (area under the plasma concentration vs time curve; AUC) and terminal plasma half-life (t1/2) were calculated using non-compartmental methods according to the Bayer guideline ‘Harmonization of Data Evaluation in Pharmacokinetics – A Task Force Report – ’ (1992 R-Report No. R-5747 and 2000 Amendment A to Report No. R-5747). A paired t-test on log-transformed values was used to calculate changes in AUC, Cmax and t1/2. A P-value ⩽0.05 was considered significant.
Results
General trial conduct
Between July 2007 and May 2009, 20 patients were screened for inclusion in the trial. A total of 19 patients started treatment as one patient had a screening failure because of the appearance of clinically symptomatic brain metastases. Patient characteristics are summarised in Table 1. Three patients had early PD within the evaluation period of the first 50 days and were replaced as per protocol. One patient was not evaluated for DLT owing to incorrect medication intake by the patient herself. One patient (7%) received one cycle, nine patients (60%) received two cycles, three patients (20%) received four cycles and two patients (13%) received six cycles. No relation between number of cycles received and DLs was apparent.
Table 1. Patient characteristics (n=19).
| Age, median (range) | 49 (28–64) |
| Male/female | 11/8 |
| Performance score | |
| 0 | 14 (74%) |
| 1 | 5 (26%) |
| Tumour type | |
| Sarcoma | 8 (42%) |
| Colorectal cancer | 3 (16%) |
| Melanoma | 2 (11%) |
| Non-small cell lung cancer | 2 (11%) |
| Hepatocellular carcinoma | 2 (11%) |
| Thyroid cancer | 1 (5%) |
| Breast cancer | 1 (5%) |
| Previous treatment | 19 (100%) |
| Systemic treatment | |
| Chemotherapy | 15 (79%) |
| Targeted therapy | 3 (16%) |
| Hormonal treatment | 2 (11%) |
| Surgery | 17 (89%) |
| Radiotherapy | 9 (47%) |
Dose-limiting toxicities and MTD
On DL 1 (sorafenib 200 mg b.i.d., sirolimus 2 mg q.d.) three out of six patients experienced a DLT. Two patients developed a grade 3 elevation of aspartate transaminase, and one patient a grade 3 elevation of alanine transaminase starting around day 28, that is 7 days after starting the combination treatment. In one patient this was accompanied by grade 3 fatigue, grade 3 anorexia and grade 3 weight loss. In another patient experiencing a DLT, grade 3 anorexia was also present. In all three patients, the transaminases values returned to baseline levels after discontinuation of sorafenib and sirolimus.
As this dose level was not tolerated, we decreased to DL 0 (sorafenib 200 mg b.i.d., sirolimus 1 mg q.d.). One out of six patients had a DLT due to cardiac ischaemia. This 49-year-old female patient with controlled hypertension and a chondrosarcoma presented on day 48 of the study, with chest pain due to cardiac ischaemia. A coronary angiography showed a small occlusion of the left coronary artery due to arterial thrombosis, and apical ballooning. The patient discontinued the study medication and recovered completely.
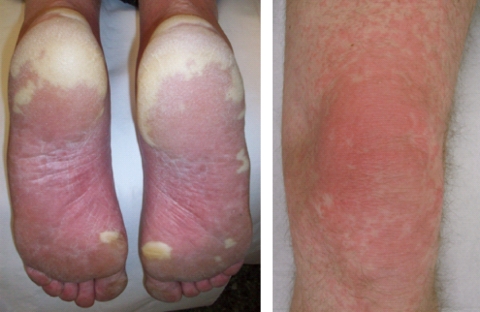
As DL 0 was tolerated, we amended the protocol to escalate to an DLim (sorafenib 400 mg b.i.d., sirolimus 1 mg q.d.), as the registered regular dosing of sorafenib is 400 mg b.i.d. On this DLim, three out of four patients experienced a DLT. All three patients experienced a grade 3 palmar–plantar erythrodysaesthesia (PPE, also called ‘hand–foot syndrome’ Figure 2), in one patient accompanied with grade 3 acneiform dermatitis (Figure 2). One of the patients also had a grade 3 fatigue. In all three patients, the PPE recovered completely after discontinuation of the study drugs. Consequently, the MTD was established as sorafenib 200 mg b.i.d. and sirolimus 1 mg q.d.
Figure 2.
Left panel: Plantar palmar erythrodysaesthesia (PPE, also called ‘hand foot syndrome’). Right panel: Acneiform dermatitis.
Overall safety and tolerability
Adverse events and serious AEs.
All patients experienced several AEs. Table 2 summarizes AEs occurring with a frequency >30% or grade 3 or worse. The most frequently reported AEs were elevated aspartate transaminase and alanine transaminase (95% and 63%), anaemia (89%), hypophosphatemia (84%), anorexia (80%), cough (79%), fatigue (79%), PPE (69%), nausea (68%) and diarrhoea (68%).
Table 2. Haematological and non-haematological adverse events occurring in >30% of patients or reaching grade 3–4 severity. (n=19).
|
DLT period (first 50 days)
|
All cycles
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adverse event | Grade 1 (n) | Grade 2 (n) | Grade 3 (n) | Grade 4 (i) | All (%) | Grade 1 (n) | Grade 2 (n) | Grade 3 (n) | Grade 4 (n) | All (%) |
| Haematological adverse events | ||||||||||
| Anaemia | 9 | 4 | 0 | 0 | 68 | 9 | 8 | 0 | 0 | 89 |
| Neutropenia | 0 | 2 | 0 | 0 | 11 | 4 | 2 | 0 | 0 | 32 |
| Non-haematological adverse events | ||||||||||
| Anorexia | 7 | 0 | 2 | 0 | 47 | 7 | 6 | 2 | 0 | 79 |
| Nausea | 7 | 3 | 0 | 0 | 53 | 8 | 5 | 0 | 0 | 68 |
| Vomiting | 2 | 1 | 1 | 0 | 21 | 7 | 1 | 1 | 0 | 47 |
| Dysphagia | 4 | 0 | 0 | 0 | 21 | 6 | 0 | 1 | 0 | 37 |
| Diarrhoea | 5 | 4 | 0 | 0 | 47 | 7 | 5 | 1 | 0 | 68 |
| Constipation | 3 | 0 | 0 | 0 | 16 | 6 | 0 | 0 | 0 | 32 |
| Weight loss | 5 | 0 | 1 | 0 | 32 | 7 | 1 | 1 | 0 | 47 |
| Fatigue | 2 | 6 | 2 | 0 | 53 | 2 | 8 | 5 | 0 | 79 |
| Acne | 4 | 1 | 1 | 0 | 32 | 2 | 1 | 1 | 0 | 32 |
| Dry skin | 6 | 1 | 0 | 0 | 37 | 8 | 1 | 0 | 0 | 47 |
| Rash | 4 | 2 | 0 | 0 | 32 | 6 | 4 | 0 | 0 | 53 |
| PPE | 6 | 2 | 3 | 0 | 58 | 6 | 2 | 5 | 0 | 68 |
| Alopecia | 3 | 1 | 0 | 0 | 21 | 9 | 1 | 0 | 0 | 53 |
| Hoarseness | 5 | 1 | 0 | 0 | 32 | 6 | 1 | 0 | 0 | 37 |
| Cough | 11 | 1 | 0 | 0 | 63 | 14 | 1 | 0 | 0 | 79 |
| Dyspnoea | 7 | 0 | 0 | 0 | 37 | 8 | 0 | 0 | 0 | 42 |
| Abdominal pain | 5 | 4 | 0 | 0 | 47 | 6 | 6 | 0 | 0 | 63 |
| Thoracic pain | 1 | 0 | 0 | 0 | 5 | 7 | 1 | 0 | 0 | 42 |
| Dizziness | 5 | 0 | 0 | 0 | 26 | 6 | 0 | 0 | 0 | 32 |
| Hypertension | 1 | 1 | 4 | 0 | 32 | 1 | 1 | 5 | 0 | 37 |
| Cardiac ischaemia | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 1 | 5 |
| Sensory neuropathy | 6 | 0 | 0 | 0 | 32 | 7 | 1 | 0 | 0 | 42 |
| Elevated AST | 10 | 2 | 3 | 0 | 79 | 12 | 3 | 3 | 0 | 95 |
| Elevated ALT | 9 | 1 | 1 | 0 | 58 | 10 | 1 | 1 | 0 | 63 |
| Elevated bilirubin | 2 | 3 | 0 | 0 | 26 | 3 | 3 | 0 | 0 | 32 |
| Hypoalbumin | 3 | 5 | 0 | 0 | 42 | 4 | 6 | 1 | 0 | 58 |
| Hypophosphatemia | 0 | 11 | 4 | 0 | 79 | 0 | 12 | 4 | 0 | 84 |
| Hypokalemia | 7 | 0 | 0 | 0 | 37 | 9 | 0 | 0 | 0 | 47 |
| Hypercholesterolaemia | 7 | 1 | 0 | 0 | 42 | 7 | 2 | 0 | 0 | 47 |
| Hypertryglyceremia | 4 | 2 | 0 | 0 | 32 | 6 | 2 | 0 | 1 | 47 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase.
As shown in Table 3, the frequency of anorexia, fatigue, diarrhoea and PPE was high in the first 50 days on study treatment, and increased significantly during prolonged study participation. Most patients had a combination of several grade 1–3 toxicities. Therefore, the combination therapy was intolerable for most patients.
Table 3. Pharmacokinetics of sorafenib, expressed as geometric means with associated 95% CI.
|
AUC(0–12 h) mg h l−1 (95% CI)
|
Cmax mg l−1 (95%CI)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cycle 1
|
Cycle 2
|
Cycle 1
|
Cycle 2
|
||||||||
| DL | Dose | Day 15 | Day 16 | Ratio d15/d16 | Day 1 | Day 15 | Day 15 | Day 16 | Ratio d15/d16 | Day 1 | Day 15 |
| 1 | Sor 200 mg b.i.d., sir 2 mg q.d | 47.1 (33–69) (n=6) | 47.9 (31–79) (n=6) | 1.02 (0.81–1.26) | 39.3 (21–74) (n=4) | 66.9 (66.3–67.5) (n=2) | 6.04 (4.1–8.9) (n=6) | 6.00 (4.09–8.8) (n=6) | 1.007 (0.76–1.31) | 5.15 (2.9–9.2) (n=4) | 7.27 (6.5–8.1) (n=2) |
| 0 | Sor 200 mg b.i.d., sir 1 mg q.d. | 63.8 (45–89) (n=6) | 64.9 (55–77) (n=6) | 1.02 (0.82–1.25) | 58.4 (50–68) (n=6) | 56.7 (42–76) (n=6) | 7.59 (5.0–11.5) (n=6) | 7.98 (6.4–10.0) (n=6) | 1.05 (0.82–1.35) | 7.12 (5.7–8.8) (n=6) | 7.32 (4.9–10.9) (n=6) |
| IM | Sor 400 mg b.i.d., sir 1 mg q.d. | 102 (59–178) (n=4) | 86.6 (48–155) (n=4) | 0.85 (0.72–0.99) | 74.9 (36–157) (n=4) | 48.4 (47–50) (n=2) | 13.0 (7–23) (n=4) | 11.9 (7–20) (n=4) | 0.92 (0.7–1.2) | 6.64 (5.0–18.7) (n=4) | 5.55 (5.2–5.9) (n=2) |
Abbreviations: CI=confidence interval; IM=intermediate; sir=sirolimus; sor=sorafenib.
Cycle 1, day 15: continuous dosing sor, day 16: effect of single-dose sir on continuous dosing sor; cycle 2, days 1 and 15: effect of continuous dosing sir on continuous dosing sor.
Liver test abnormalities.
Abnormalities in hepatic function tests were frequently observed, especially on DL 1. As early as 1 week after the combined dosing, elevation of aspartate transaminase and alanine transaminase were observed with peak values between days 28–42 (2–4 weeks of combination therapy). Two of the three patients with grade 3 elevations of transaminases suffered from hepatocellular carcinoma and had baseline transaminase values between 1.0–2.5 × ULN. The third patient had colorectal cancer without liver involvement and with a normal hepatic function at baseline. In these three patients, hepatic function restored to baseline after cessation of therapy within weeks. Furthermore, recovery of hepatic function was also observed in patients with less enhanced elevations of transaminases, who did not interrupt the combination treatment, generally between 2–6 weeks after measurement of peak liver function abnormalities.
Cutaneous toxicity.
PPE, maculopapular rash, acne, pruritis and dry skin were the frequently reported AEs with combined sorafenib and sirolimus. In general, skin toxicity was mild. However, on the DLim, severe PPE and rash occurred, generally starting within 10 days after initiation of the combination therapy. The patients experienced a painful red skin, scaling with yellowish demarcations (Figure 2). Emollients did provide some relief, although short drug interruptions of 3–5 days were needed for full clinical recovery. In two patients, sorafenib was reinitiated at a lower dose of 200 mg b.i.d., but one of them had a recurrence, after which we permanently discontinued the sorafenib in this patient.
Pharmacokinetics
The effect of sirolimus on sorafenib PK.
On average, concomitant administration of single oral doses of 1 mg or 2 mg of sirolimus did not influence AUC(0−12 h) (P=0.67) and Cmax (P=0.96) of sorafenib following multiple dosing with 200 mg b.i.d. of sorafenib. In the DLim, when 1 mg sirolimus was administered together with 400 mg b.i.d. of sorafenib, minor non-significant mean decreases in both parameters by 15% (P=0.14) and 8% (P=0.57) were observed. (Table 3)
The effect of sorafenib on sirolimus PK.
On DL 1, pre-treatment with multiple oral doses of 200 mg b.i.d. sorafenib resulted in a mean decrease of Cmax of sirolimus by 55% (P=0.006), while the AUC(0−96 h) of sirolimus non-significantly decreased by 37% (P=0.125) and the mean terminal half-life of sirolimus remained unchanged (P=0.66). Similarly, on the DLim, the Cmax decreased on average by 55% (P=0.027) and the AUC(0−96 h) by 25% (P=0.363). This is in contrast to results from patients on DL 0, in which no change in the mean AUC(0−96 h) of sirolimus was observed (P=0.955) on simultaneous administration of both drugs, and Cmax was only non-significantly reduced by 18% (P=0.515) on average (Table 4).
Table 4. Pharmacokinetics of sirolimus expressed as geometric means with associated 95% CI.
|
AUC μg h l−1 (95%CI)
|
Cmax
μg l−1 (95%CI)
|
t ½ h (95%CI)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
AUC(0–96 h)
|
AUC(0–12 h)
|
||||||||||||
|
Cycle 1
|
Cycle 2
|
Cycle 1
|
Cycle 2
|
Cycle 1
|
|||||||||
| DL | Dose | Day 1 | Day 16 | Ratio d1/d16 | Day 1 | Day 15 | Day 1 | Day 16 | Ratio d1/d16 | Day 1 | Day 15 | Day 1 | Day 16 |
| 1 | Sor 200 mg b.i.d., sir 2 mg q.d. | 124 (102–151) (n=6) | 78.2 (55–111) (n=6) | 0.63 (0.39–1.03) | 88.2 (50–155) (n=4) | 84.6 (n=1) | 5.74 (4.8–6.8) (n=6) | 2.60 (1.9–3.6) (n=6) | 0.45 (0.33–0.63) | 10.0 (6.4–15.7) (n=5) | 17.6 (n=2) | 50.5 (43.7–58) (n=6) | 52.5 (42.9–64.3) (n=6) |
| 0 | sor 200 mg b.i.d., sir 1 mg q.d. | 91.1 (59–141) (n=6) | 91.7 (67–125) (n=6) | 1.01 (0.81–1.26) | 59.4 (47–75) (n=6) | 57.8 (41–81) (n=6) | 3.81 (2.6–5.6) (n=6) | 3.14 (2.4–4.2) (n=6) | 0.82 (0.48–1.42) | 6.30 (4.8–8.2) (n=6) | 6.36 (4.7–8.5) (n=6) | 45.5 (27–75) (n=6) | 56.2 (39–82) (n=6) |
| IM | sor 400 mg b.i.d., sir 1 mg q.d. | 139 (98–239) (n=3) | 104 (100–113) (n=3) | 0.75 (0.46–1.14) | 101 (55–115) (n=2) | 113 (n=2) | 7.99 (6.0–13.6) (n=3) | 3.62 (2.4–4.3) (n=3) | 0.45 (0.41–0.58) | 11.2 (5.7–13.0) (n=2) | 13.7 (10.5–18.0) (n=2) | 38.30 (29.9–50.6) (n=3) | 32.9 (24.5–52.7) (n=3) |
Abbreviations: CI=confidence interval; IM=intermediate; sir=sirolimus; sor=sorafenib.
Cycle 1, day 1: single-dose sir, day 16: effect of continuous dosing sor on single-dose sir; cycle 2, days 1 and 16: effect of continuous dosing sor on continuous dosing sir.
Anti-tumor activity
In all, 14 patients were evaluated for response after two cycles. No objective responses were observed. Six patients had PD at the first evaluation after 8 weeks (two cycles). Of the eight remaining patients, two patients with stable disease decided to stop after 9 and 18 weeks of study participation because of the considerations on the quality of life based on multiple grade 1–2 toxicities. One other patient had to stop after 8 weeks because of cardiac ischaemia, which was a DLT in the last few days of the DLT period. Of the remaining four patients, two showed PD after 16 weeks (four cycles) and two after 24 weeks (six cycles).
Discussion
This phase I study investigates the combination of two oral small molecule signal transduction inhibitors each targeting different pathways, namely the RAS-RAF-MEK-ERK and VEGF pathway (sorafenib) and the phosphatidyl-inositol 3-kinase/Akt pathway (sirolimus). In this study, the combination of sorafenib and sirolimus caused enhanced toxicity with only modest clinical activity.
The recommended dose was established on sorafenib 200 mg b.i.d. and sirolimus 1 mg q.d. This is 50% below the recommended dose for single-agent therapy with sorafenib. For sirolimus, as an anti-cancer therapy, no regular dosing is known, although reports mention effective doses between 0.5 and 10 mg daily (Stallone et al, 2005; Reardon et al, 2006; Rizell et al, 2008). The PK results did not provide a clarification for the observed enhanced toxicity.
The observed toxicity of the combination of sorafenib and sirolimus was impressive. This is illustrated by Table 2, showing high frequencies of toxicities, which accumulated during prolonged study participation. The impact of the ongoing combination of several toxicities, even when relatively mild as grade 1 or 2, on the quality of life of the participating patients was high. Enhanced toxicity is observed in several other combinations of targeted therapies. A recent phase I study, which combined sorafenib with monoclonal VEGF antibody bevacizumab reported enhanced toxicity, including transaminitis grade 2–4 in 13 out of 39 patients (33%) and PPE in 31 out of 39 patients (79%, in 23 patients grade ⩾2; Azad et al, 2008) Like in our study, the MTD of this combination was below single-agent therapy doses with sorafenib 200 mg b.i.d. and bevacizumab 5 mg kg−1 intravenously once in every 2 weeks. The same group studied intermittent dosing of sorafenib with bevacizumab, which was tolerated better (Lee et al, 2010). In two preliminary reports of studies combining sorafenib with mTOR inhibitor temsirolimus, significant toxicity was described including mucocutaneous toxicity, serum transaminase elevations, hypertrygliceridemia and thrombocytopenia (Patnaik et al, 2007; Kim et al, 2009). In the first study, the MTD was sorafenib 400 mg and 200 mg daily with temsirolimus 25 mg intravenously every week. (Kim et al, 2009) In the second study, the MTD was not yet determined, although the DLs combining sorafenib 400 mg b.i.d. with temsirolimus 25 mg intravenously every week were not tolerated and lower doses of sorafenib were under study (Patnaik et al, 2007). In contrast to these observations, three preliminary studies reported the combination of sorafenib and mTOR inhibitor everolimus as tolerable and safe, as did one preliminary report of a phase I study with the combination of sorafenib and temsirolimus (Giessinger et al, 2008; Cen et al, 2009; Harzstark et al, 2009; Wen et al, 2009). Two studies combined sunitinib with temsirolimus. In one of them, PPE and fatigue were common AEs. The other study was terminated because of substantial toxicity (Li et al, 2009; Patel et al, 2009).
We can only speculate about the reasons for the enhanced toxicity. Toxicities of targeted therapies might be because of the inhibition of multiple so-called ‘off-targets’ elements in the pathways that are not the primary target of a tyrosine kinase inhibitor. Both specific targets as well as off-targets do have a normal physiological function, for example the survival of endothelial cells and the maintenance of vascular integrity. The combination of several targeted agents enhances the number of elements within the pathways that are influenced, which might explain the enhanced toxicity (Gotink and Verheul, 2009). Furthermore, in a ‘vertical strategy’, drugs are combined to inhibit a cascade of signalling molecules. Here applied combination of mTOR inhibitor sirolimus and VEGFR inhibitor sorafenib is an example for this approach (Sosman and Puzanov, 2009). By inhibition in a ‘vertical’ strategy, feedback loops in the network of signalling pathways might be involved in unexpected and undesirable outcomes of targeted therapies (Gotink and Verheul, 2009). Another interesting factor involved in toxicity might be an altered pharmacodynamic effect of a tyrosine kinase inhibitor owing to polymorphisms in specific genes encoding for metabolizing enzymes, efflux transporters and drug targets, as is observed for sunitinib (van Erp et al, 2009). The combination of several targeted therapies with overlapping metabolic profiles may enhance gene vulnerability.
In this study, the most occurring dose-limiting toxicities were serum transaminase elevations and PPE. Both toxicities have been reported before in combination strategies with mTOR inhibitors and VEGF(R) inhibitors (Patnaik et al, 2007; Azad et al, 2008, 2009; Kim et al, 2009). However, for both sorafenib and sirolimus, serum transaminase elevations are not a frequently reported AE (Bhojani et al, 2008). The timing of the rise in serum transaminases 2–3 weeks after start of the combination and the recovery after discontinuation of the combination, suggest a causal relationship. Vascular endothelial growth factor has a role in structural, functional integrity of the liver. Most consistently, growth stimulating, regenerative and cytoprotective effects of VEGF and a VEGFR agonist have been found in pre-clinical models of ischaemia and reperfusion-induced hepatic toxicity, indicating a causal relationship between VEGFR inhibitors and transaminitis (Eskens and Verweij, 2006). Analysis of skin biopsies of PPE patients indicate that epidermal cells are swollen, capillaries are dilated and apoptotic endothelial cells are present. This suggests that the skin toxicity is a direct consequence of the biological activity of VEGFR inhibitors (Faivre et al, 2006). Other observed cutaneous toxicities were maculopapular rash, acne, pruritis and dry skin. These are well known AEs of oral VEGFR inhibitors. (Lee et al, 2010).
The clinical efficacy observed in our study was only moderate, with stable disease in 53% of patients but only in 14% persisting for more than 4 months. The patients participating in this study were heavily pre-treated and had PD at the time of enrolment.
In conclusion, the MTD of the combination is sorafenib 200 mg b.i.d. and sirolimus 1 mg q.d. This combination showed enhanced toxicity, which could not be explained by the influence of the PK of each targeted agent. We do not recommend further exploration of this dosing schedule. Pre-clinical research focusing on the causes of enhanced toxicity of these combination therapies is highly warranted.
References
- Abraham RT, Gibbons JJ (2007) The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res 13: 3109–3114 [DOI] [PubMed] [Google Scholar]
- Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, Minasian L, Sarosy G, Kotz HL, Premkumar A, Cao L, McNally D, Chow C, Chen HX, Wright JJ, Figg WD, Kohn EC (2008) Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol 26: 3709–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad NS, ragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, Steinberg SM, Turner ML, Kohn EC, Kong HH (2009) Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res 15: 1411–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojani N, Jeldres C, Patard JJ, Perrotte P, Suardi N, Hutterer G, Patenaude F, Oudard S, Karakiewicz PI (2008) Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol 53: 917–930 [DOI] [PubMed] [Google Scholar]
- Cen P, Daleiden A, Doshi G, Amato R (2009) A phase I study of everolimus plus sorafenib in patients with renal cell carcinoma (mRCC). J Clin Oncol 27(e16056): Ref Type: Abstract [Google Scholar]
- Dent P, Curiel DT, Fisher PB, Grant S (2009) Synergistic combinations of signaling pathway inhibitors: mechanisms for improved cancer therapy. Drug Resist Updat 12: 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: 125–134 [DOI] [PubMed] [Google Scholar]
- Eskens FA, Verweij J (2006) The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer 42: 3127–3139 [DOI] [PubMed] [Google Scholar]
- Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24: 25–35 [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessinger S, Amato RJ, Jac J, Saxena S, Willis JP, Chiang SB (2008) A phase I study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) plus sorafenib for patients with metastatic renal cell cancer (mRCC). J Clin Oncol 26[14603]: Ref Type: Abstract [Google Scholar]
- Gotink KJ, Verheul HM (2009) Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis 13(1): 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzstark AL, Rosenberg JE, Weinberg VK, Sun J, Ryan J, Lin AM, Fong L, Brocks DR, Small EJ (2009) A phase I study of sorafenib and RAD001 for metastatic clear cell renal cell carcinoma. J Clin Oncol 27(5104): Ref Type: Abstract [Google Scholar]
- Kim KB, Davies MA, Papadopoulos NE, Bedikian AY, Hwu W, Woodard KW, Ashington EW, Dancey JE, Wright J, Hwu P (2009) Phase I/II study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. J Clin Oncol 15s(9026): Ref Type: Abstract [Google Scholar]
- Lee JM, Sarosy GA, Annunziata CM, Azad N, Minasian L, Kotz H, Squires J, Houston N, Kohn EC (2010) Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer 102: 495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kluger H, Saif MW, Murren JR, Lee JJ, Kelly WK, Rink L, Devine L, Sznol MA (2009) Phase I study of sunitinib in combination with sirolimus in adults with advanced refractory malignancies. J Clin Oncol 15s[3554]: Ref Type: Abstract [Google Scholar]
- MacDonald A, Scarola J, Burke JT, Zimmerman JJ (2000) Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther 22(Suppl B): B101–B121 [DOI] [PubMed] [Google Scholar]
- Meric-Bernstam F, Gonzalez-Angulo AM (2009) Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 27: 2278–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PH, Senico PL, Curiel RE, Motzer RJ (2009) Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer 7: 24–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik A, Ricart A, Cooper J, Papadopoulos K, Beeram M, Mita C, Mita MM, Hufnahel D, Izbicka E, Tolcher A (2007) A phase I, pharmacokinetic and pharmacodynamic study of sorafenib (S), a multi-targeted kinase inhibitor in combination with temsirolimus (T), an mTOR inhibitor in patients with advanced solid malignancies. J Clin Oncol 18s(3512): Ref Type: Abstract [Google Scholar]
- Reardon DA, Quinn JA, Vredenburgh JJ, Gururangan S, Friedman AH, Desjardins A, Sathornsumetee S, Herndon JE, Dowell JM, McLendon RE, Provenzale JM, Sampson JH, Smith RP, Swaisland AJ, Ochs JS, Lyons P, Tourt-Uhlig S, Bigner DD, Friedman HS, Rich JN (2006) Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res 12: 860–868 [DOI] [PubMed] [Google Scholar]
- Rizell M, Andersson M, Cahlin C, Hafstrom L, Olausson M, Lindner P (2008) Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. Int J Clin Oncol 13: 66–70 [DOI] [PubMed] [Google Scholar]
- Sosman J, Puzanov I (2009) Combination targeted therapy in advanced renal cell carcinoma. Cancer 115: 2368–2375 [DOI] [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B, Di PS, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G (2005) Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med 352: 1317–1323 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van GM, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- van Erp NP, Eechoute K, van der Veldt AA, Haanen JB, Reyners AK, Mathijssen RH, Boven E, van der ST, Baak-Pablo RF, Wessels JA, Guchelaar HJ, Gelderblom H (2009) Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol 27: 4406–4412 [DOI] [PubMed] [Google Scholar]
- Wen PY, Cloughesy T, Kuhn J, Lamborn K, Abrey LE, Lieberman F, Robins HI (2009) Phase I/II study of sorafenib and temsirolimus for patients with recurrent glioblastoma (GBM)(NABTC 05–02). J Clin Oncol 27[2006]: Ref Type: Abstract [Google Scholar]