Although the overall strategy for the use of FK 506 remains the same as when this T-cell-directed drug was introduced clinically in 1989,1,2 there have been modifications. As with cyclosporine (CyA), the nephrotoxicity of FK 506 imposes a dose ceiling, necessitating its use in drug cocktails that include adrenal corticosteroids. We recently reported that liver transplant recipients with the immunological disadvantage of a positive cytotoxic crossmatch with their donors3 could have their prognosis converted to that in crossmatch-negative cases when FK 506 was combined with high induction doses of prednisone.4 A subgroup of these patients who also were treated perioperatively with prostaglandin E1 (PGE1) had superior renal function, suggesting an amelioration of nephrotoxicity by PGE1 without a loss of immunosuppression, or conceivably with a gain. In the trial reported here, PGE1 was evaluated in crossmatch-negative liver recipients.
METHODS
Case Material
Primary adult liver recipients (>16 years of age) from three consecutive periods beginning June 10, 1990, were entered into the study, with exclusion only if there was a positive cytotoxic crossmatch or if the patient had renal failure pretransplantation. Renal failure was defined by dialysis dependence or by multiple preoperative serum creatinines of at least 2 mg/dL. Patients in all three study groups (Table 1) were started postoperatively on 20 mg/d prednisone after being given a single 1-g bolus of methylprednisolone intraoperatively. The variables were in FK 506 (high vs low induction dose) and in PGE1 (inclusion or not).
Table 1.
Patient Profile and Outcome
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Number | 124 | 38 | 41 |
| Date began | 6/10/90 | 8/27/91 | 11/1/91 |
| FK 506 (mg/kg/d) | 0.1 | 0.05 | 0.05 |
| Prednisone (mg/d) | 20 | 20 | 20 |
| PGE1 | No | No | Yes |
| Age | 44.9 ± 1.4 | 51.9 ± 1.7 | 50.4 ± 1.6 |
| Male/female | 76/48 | 27/11 | 24/17 |
| Liver disease | |||
| Idiopathic | 16 | 3 | 6 |
| Alcoholic | 25 | 6 | 13 |
| Viral | 24 | 19 | 11 |
| Cholestasis | 29 | 5 | 4 |
| Malignancy | 17 | 5 | 4 |
| Fulminant hepatic failure | 5 | 0 | 1 |
| Miscellaneous | 8 | 0 | 2 |
| Retransplantation within 1 month | 12 | 3 | 5 |
| Death in 1 month | 4 | 1 | 2 |
| (Patients died after retransplant) | (1) | (0) | (2) |
| Graft survival at 1 month | 109 (87.9%) | 34 (89.5%) | 36 (87.8%) |
| Patient survival at 1 month | 120 (96.8%) | 37 (97.4%) | 39 (95.1%) |
| Cause of graft failure | |||
| Preservation injury | 3 | 3 | 2 |
| Technical | 8 | 1 | 3 |
| Infection | 4 | 0 | 0 |
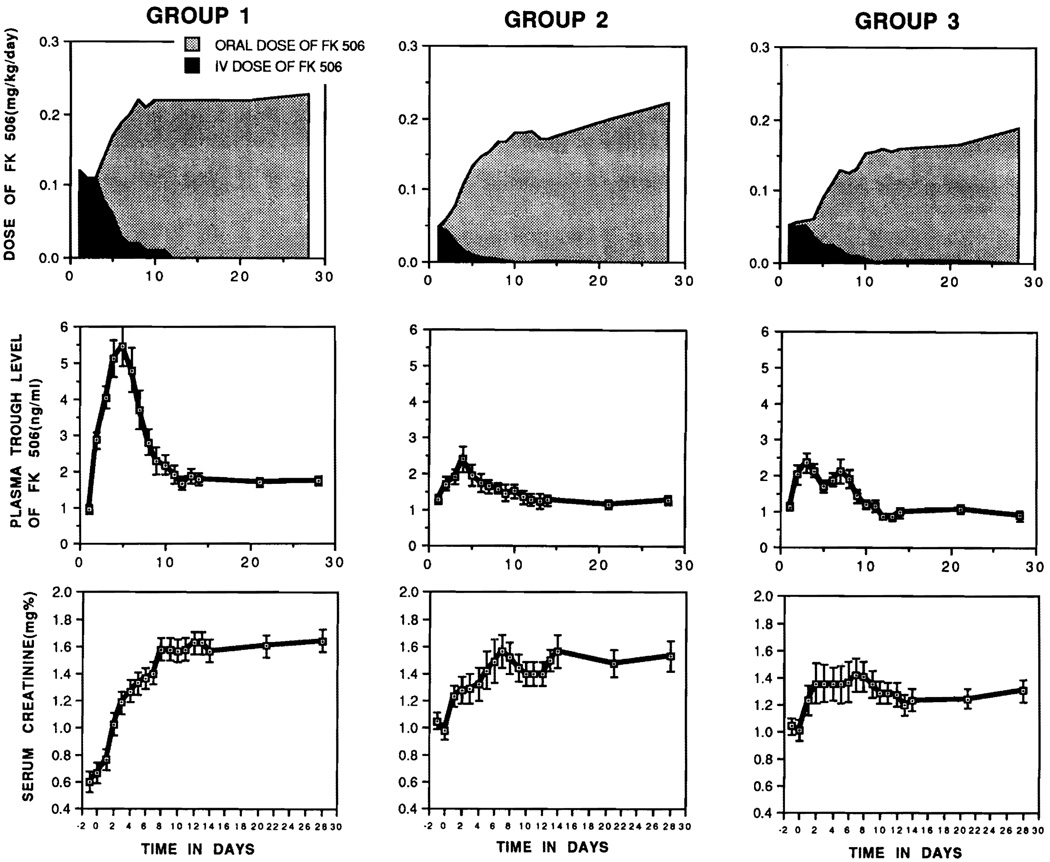
FK 506 dosing was controlled throughout by measurement of plasma levels.5 Trough levels over 1 ng/mL were considered to reflect a potential toxicity at all times after the first week. The starting daily intravenous FK 506 doses of 0.1 mg/kg/d in group 1 and half this amount in groups 2 and 3 were given by 24-hour continuous infusion. When the conversion to oral FK 506 was made (Fig 1), dosing as close to 0.3 mg/kg/d was attempted. However, it usually was not possible to give this much of the drug without driving up the plasma trough levels beyond the desired 1 to 2 ng/mL range. The heavy initial FK 506 dosing as a policy in group 1 was reflected in significantly higher plasma levels than in either group 2 or 3 for each of the first 4 weeks and the total month.
Fig 1.
Dose of intravenous and oral FK 506, plasma trough levels, and serum creatinine levels in patients surviving 30 days (excluding hemodialysis patients). The mean dose of group 1 was significantly higher than those of group 2 or group 3 (P < .001). The mean FK 506 plasma level of group 1 for the first 30 days was significantly higher than those of group 2 or group 3 (P < .001). No statistically significant difference was observed in the dosage of intravenous and oral FK 506 regimens or plasma FK 506 trough levels between group 2 and group 3. The mean values of serum creatinine in group 3 were significantly lower than those of group 1 patients at the second (P = .22), third (P = .0046), and fourth postoperative weeks (P = .0004). Values are expressed as means ± SE.
There were no significant FK 506 dosage or plasma level differences between groups 2 and 3 (Fig 1). Patients in group 3 were given intravenous PGE1 (Prostin VR) beginning during the operation or after its completion. The starting dose was 0.2 µg/kg/h which was increased to 0.6 µg/kg/h and maintained there for the next 5 to 7 days unless hypotension or cardiovascular instability interdicted the increase. When the patients resumed diet, they were switched to the oral PGE1 analog misoprostol (Cytotec) at doses of 400 to 800 µg/d in four divided doses. Two of the 41 patients in group 3 could not complete the PGE1 therapy, one because of poor graft function, norepinephrine dependence, and the need for retransplantation after 7 days. Therapy was foreshortened in the other because of a fatal myocardial infarction 3 days posttransplantation. These two patients were included in survival calculations but excluded from the analyses of renal function.
Diagnosis and Treatment of Rejection
A positive biopsy was a condition for the diagnosis of liver allograft rejection. The histopathological grading system of rejection, as developed by Demetris et al,6 was: grade 1 (early or consistent with rejection), grade 2 (mild), grade 3 (moderate), and grade 4 (severe). In addition to the biopsy, a Doppler ultrasound also was required with intact liver vascularization and no dilatation of intrahepatic bile ducts. A cholangiogram and an arteriogram were performed if the ultrasound findings were equivocal. One gram of intravenous methylprednisolone was given for biopsy-proven rejection, followed, if necessary, by an additional 5-day “burst” of methylprednisolone, beginning with 200 mg the first day, followed by a daily decrease of 40 mg until 20 mg/d was reached on the sixth day. If rejection persisted, a 3- to 5-day course of 5 to 10 mg/d of OKT3 was given.
Statistical Analysis
Biochemical parameters were followed for 30 days and included hepatic and renal function, and dosage and plasma trough levels of FK 506. Statistical analysis was performed by Mann-Whitney U test or chi-square testing with Yates correction. Results were expressed as means ± SEM.
RESULTS
Patient and Graft Survival
Patient survival was essentially the same in all groups (95.1% to 97.4%), as were the graft survival rates (87.5% to 89.5%) and the need for retransplantation (Table 1). Liver grafts were lost to various causes (Table 1). Primary nonfunction leading to retransplantation was observed in three patients in group 1 (2.4%), three patients in group 2 (7.9%), and two patients in group 3 (4.8%). Sepsis caused four deaths in group 1 but was not responsible for any mortality in groups 2 and 3 (Table 1).
Incidence and Treatment of Rejection
Although no grafts were lost to acute rejection in any of the groups (Table 1), rejection was diagnosed within 1 month in 53%, 62%, and 44% of the recipients in groups 1 through 3, respectively. The time of onset, frequency, and histopathological severity of these episodes were also similar in the three groups (Tables 2 and 3). However, the mean number of steroid boluses given per patient during the first 30 days was 0.55 for group 1, significantly lower than 0.97 for group 2, and 0.86 for the group 3. In addition, the high induction dose of FK 506 (group 1) significantly reduced the need for steroid recycling vs group 2 (P = .024) but not group 3 (P = .27). Differences in OKT3 usage among the groups were not statistically significant; actually no patient in group 3 required OKT3 in the first 30 days (Table 2).
Table 2.
Episodes of Acute Cellular Rejection and Supplemental Immunosuppression in Grafts Surviving 30 Days
| Group 1 | Group 2 | Group 3 | P Value | |
|---|---|---|---|---|
| Number of primary grafts | 109 | 34 | 36 | |
| Number of grafts with rejection in month 1 | 58 (53.2%) | 21 (61.8%) | 16 (44.4%) | NS |
| Onset of first episode (day)* | 9.4 ± 0.8 | 8.8 ± 0.9 | 10.7 ± 2.1 | NS |
| Number of rejection episodes | ||||
| 1 | 32 (29.4%) | 12 (35.3%) | 11 (30.6%) | NS |
| 2 | 19 (17.4%) | 8 (23.5%) | 3 (8.3%) | NS |
| ≥3 | 7 (6.4%) | 1 (2.9%) | 2 (5.6%) | NS |
| Supplemental immunosuppression | ||||
| 1 g methylprednisolone | 0.55 ± 0.07† | 0.97 ± 0.15 | 0.86 ± 0.13 | .012 |
| Steroid cycling | 16 (14.7%)‡ | 11 (32.4%) | 8 (22.2%) | .024 |
| OKT3 | 4 (3.7%) | 2 (5.9%) | 0 (0%) | NS |
| T. Bilirubin at first month (mg/dL) | 1.85 ± 0.29 | 1.26 ± 0.14 | 1.23 ± 0.17 | NS |
Biopsy verified, mean ± SE.
P = .012 vs group 2, P = .044 vs group 3.
P = .024 vs group 2.
Table 3.
Evaluation of Biopsy Specimens in Grafts Surviving 30 Days
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Number of primary grafts | 109 | 34 | 36 |
| Total number of biopsy specimens* | 179 | 53 | 52 |
| Biopsy specimens per graft | 1.64 | 1.56 | 1.44 |
| No ACR† | 88 (49.2%) | 20 (37.7%) | 27 (51.9%) |
| Consistent with or early ACR (grade 1) | 15 (8.4%) | 9 (17.0%) | 7 (13.5%) |
| Mild ACR (grade 2) | 59 (33.0%) | 19 (35.8%) | 13 (25.0%) |
| Moderate ACR (grade 3) | 14 (7.8%) | 5 (9.4%) | 5 (9.6%) |
| Severe ACR (grade 4) | 3 (1.7%) | 0 | 0 |
During the first 30 days.
ACR, Acute cellular rejection.
Renal Function
Hemodialysis was required in 13.8% of the group 1 patients, 14.7% of the group 2 patients, and in only one (2.8%) of the 41 patients in group 3 (Table 4). The exceptional patient in group 3 had two treatments for volume overload on the second and third postoperative days with slow continuous ultrafiltration with Dianeal (Table 4). By the end of the first month, 9.2%, 11.8%, and 0% of the patients in groups 1 through 3, respectively, were still on hemodialysis (Table 4).
Table 4.
Hemodialysis and Renal Function in Primary Grafts Surviving More Than 30 Days
| Group 1 | Group 2 | Group 3 | P Value | |
|---|---|---|---|---|
| Number of patients | 109 | 34 | 36 | |
| Hemodialysis for first month | 15 (13.8%) | 5 (14.7%) | 1 (2.8%)* | NS |
| Hemodialysis at first month | 10 (9.2%) | 4 (11.8%) | 0 (0%) | NS |
| Preoperative creatinine (mg/dL) | 0.72 ± 0.04 | 1.03 ± 0.06 | 1.06 ± 0,06† | <.001 |
| Creatinine at 1 week‡ | 1.41 ± 0.09 | 1.57 ± 0.16 | 1.42 ± 0.12 | NS |
| Creatinine at 2 weeks | 1.57 ± 0.08 | 1.50 ± 0.13 | 1.24 ± 0.08§ | .022 |
| Creatinine at 3 weeks | 1.63 ± 0.08 | 1.48 ± 0.11 | 1.25 ± 0.08§ | .0046 |
| Creatinine at 4 weeks | 1.68 ± 0.07 | 1.53 ± 0.12 | 1.31 ± 0.09§ | .0004 |
| Creatinine ≥2.0 mg/dL at first month | 18 (19.1%) | 3 (10.3%) | 3 (8.6%) | NS |
Patients required two times of slow continuous ultrafiltration with Dianeal. b, c, d; Group 1 vs group 3.
Group 1 vs group 2, group 1 vs group 3.
Excludes the hemodialysis patients, mean ± SE.
Group 1 vs group 3.
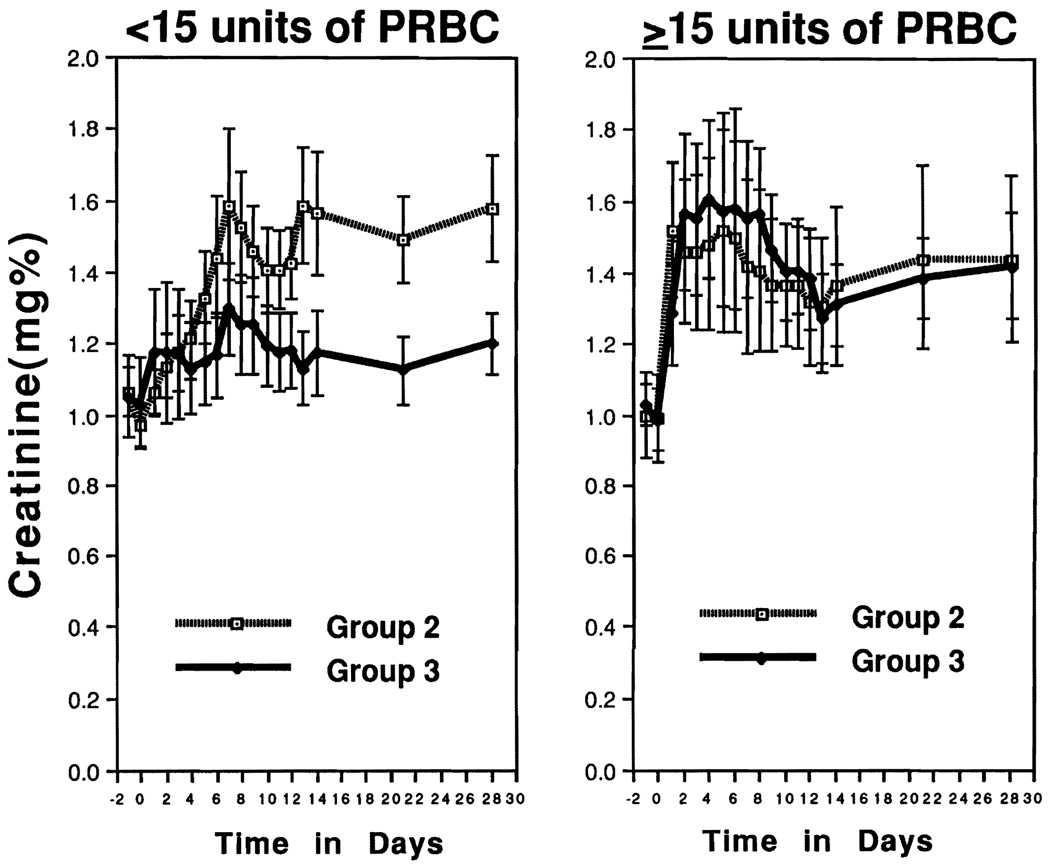
Even excluding those patients on dialysis, the mean serum creatinine during the first 30 days was elevated in all groups. These levels decreased slowly after the second postoperative week in group 3 (Fig 1), but they remained significantly higher in groups 1 and 2 relative to group 3 (Table 4). The beneficial kidney-protective effect of PGE1 in the group 3 patients vs those in group 2 was most pronounced in those recipients who were given <15 units of packed red blood cells vs those with a blood transfusion requirement greater than this (P = .0087) (Fig 2).
Fig 2.
Division of blood usage during liver transplantation in group 2 (n = 23) and group 3 (n = 19) with low transfusion group [<15 units of packed red blood cells (PRBC)] and high transfusion group (≥15 units of PRBC) (n = 6 in group 2, n = 16 in group 3, excluding hemodialysis patients). In the lower transfusion group, the mean creatinine in group 3 was significantly lower than that of group 2 for the first 30 days (1.48 ± 0.08 mg/dL vs 1.20 ± 0.05 mg/dL, P = .0087). Values are expressed as mean ± SE.
DISCUSSION
The pathogenesis of FK 506 nephrotoxicity and other side effects has been thought from the outset to be similar to that of CyA.2,7–9 With both drugs, a decrease in effective renal plasma flow and glomerular filtration rate and an increase in renal vascular resistance may be associated with the secretion of endothelin-110 or thromboxane A211 and other products of arachidonic acid metabolism. Because prostaglandins of series I2, E1 and E2 are potent vasodilators and modulators of tone in the pre- and post-glomerular arterials, they were evaluated for the pharmacological interdiction of these undesirable effects of CyA in the studies by Makowka et al12 and Ryffel et al.13 In Makowka’s original study, CyA blood levels were not obtained.
In the present investigation, in which this supporting data of FK 506 were available, the dose and plasma levels were essentially the same in groups 2 and 3, making it possible in these two groups to make a discriminative assessment of the kidney-sparing effect of PGE1. The PGE1 appeared to have substantially reduced the FK 506 nephrotoxicity. This was particularly evident in patients with the least troublesome perioperative courses, as reflected by their minimum needs for blood transfusion. In contrast, the value of PGE1 was obscured in patients who required more than 15-unit transfusions.
The reduced nephrotoxicity in the PGE1-treated group 3 was reflected not only in the better average postoperative renal function, but also by the nearly complete elimination of the need for posttransplant dialysis. Only a single patient of the 36 in group 3 required such support, and in this instance the dialysis intervention for correction of volume overload was very brief. It has long been known that the liver recipient is especially vulnerable to renal failure, partly because occult renal dysfunction so often already is present in the preoperative period.14 Causes of further renal damage perioperatively are multifactorial and, in addition to suspect immunosuppressive drugs, include cardiovascular instability, endotoxemia, and nephrotoxic antibiotics. In spite of the complexity of this background in the liver transplant recipients, our conclusion that PGE1 protects the kidney from concomitantly administered nephrotoxic drugs was essentially the same as that reached by Moran et al15 in less complicated clinical renal transplant trials.
The second and less clearly resolved question in this study was whether PGE1 altered the efficacy of FK 506 or was itself inherently immunosuppressive. There was no clear evidence for either possibility. In the low FK 506 dose groups 2 and 3, in which the therapeutic variable was PGE1, the presence or absence of the PGE1 did not obviously influence the rate of rejection, the use of supplemental steroids or adjuvant immunosuppressive measures, or the doses and plasma levels of FK 506. In both groups 2 and 3, the need for augmented steroids was significantly greater than in the patients of group 1, who were started on high doses of FK 506.
Thus, this study, as well as our earlier one of patients who received crossmatch-positive livers,4 has shown that the greatest value of PGE1 is amelioration of nephrotoxicity. However, we emphasize that previous workers in controlled animal experiments have shown obtundation of cellular16,17 or humoral responsiveness,18 or both.19–21 We believe that an immunosuppressive effect was too subtle to be detected under the complex circumstances of these liver transplantation cases.
Acknowledgments
Supported by Project Grant No. DK 29961 from the National Institutes of Health.
REFERENCES
- 1.Starzl TE, Todo S, Fung J, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todo S, Fung JJ, Starzl TE, et al. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takaya S, Bronsther O, Iwaki Y, et al. Transplantation. 1992;53:400. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takaya S, Iwaki Y, Starzl TE. Transplantation. 1992;54:927. doi: 10.1097/00007890-199211000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura K, Kobayashi M, Hashimoto K, et al. Transplant Proc. 1987;19 Suppl 6:23. [PubMed] [Google Scholar]
- 6.Demetris AJ, Qian S, Sun H, et al. Am J Surg Pathol. 1990;14:49. [PubMed] [Google Scholar]
- 7.Kusne S, Dummer JS, Singh N, et al. Medicine. 1988;67:132. doi: 10.1097/00005792-198803000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Fung JJ. JAMA. 1990;263:2686. [PMC free article] [PubMed] [Google Scholar]
- 9.Nalesnick M, Lai HS, Murase N, et al. Transplant Proc. 1990;22:87. [PMC free article] [PubMed] [Google Scholar]
- 10.Moutabarrik A, Ishibashi M, Fukunaga, et al. Transplant Proc. 1991;23:3133. [PubMed] [Google Scholar]
- 11.Rogers TS, Elzinga L, Bennet WM, et al. Transplantation. 1988;45:153. doi: 10.1097/00007890-198801000-00033. [DOI] [PubMed] [Google Scholar]
- 12.Makowka L, Lopatin W, Gilas T, et al. Clin Nephrol. 1986;25:S89. [PubMed] [Google Scholar]
- 13.Ryffel B, Donatsch P, Hiestand P, et al. Clin Nephrol. 1986;25:S95. [PubMed] [Google Scholar]
- 14.Klintmalm GBG, Klingensmith WC, III, Iwatsuki S, et al. Radiology. 1982;142:199. doi: 10.1148/radiology.142.1.7031760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran M, Mozes MF, Maddux MS, et al. N Engl J Med. 1990;322:1183. doi: 10.1056/NEJM199004263221703. [DOI] [PubMed] [Google Scholar]
- 16.Chonaib S, Robb RJ, Welte K, et al. J Clin Invest. 1987;80:333. doi: 10.1172/JCI113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makoul GT, Robinson DR, Bhalla AK, et al. J Immunol. 1985;134:2645. [PubMed] [Google Scholar]
- 18.Makowka L, Miller C, Chapchap P, et al. Ann Surg. 1987;206:482. doi: 10.1097/00000658-198710000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rappaport RS, Dodge GR. J Exp Med. 1982;155:943. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder DS, Beller DI, Unanue ER. Nature. 1982;299:163. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- 21.Weir MR, Wang Li X, Gomolka D, et al. Transplantation. 1991;52:1053. doi: 10.1097/00007890-199112000-00021. [DOI] [PubMed] [Google Scholar]