Abstract
The National Institute on Drug Abuse established the National Drug Abuse Treatment Clinical Trials Network (CTN) in 1999 to improve the quality of addiction treatment using science as the vehicle. The network brings providers from community-based drug abuse treatment programs and scientists from university-based research centers together in an alliance that fosters bi-directional communication and collaboration. Collaboration enhanced the relevance of research to practice and facilitated the development and implementation of evidence-based treatments in community practice settings. The CTN’s 20 completed trials tested pharmacological, behavioral, and integrated treatment interventions for adolescents and adults; more than 11,000 individuals participated in the trials. This paper reviews the rationale for the CTN, describes the translation of its guiding principles into research endeavors, and anticipates the future evolution of clinical research within the Network.
1. Introduction
Significant investments in basic and clinical science discovery produce a staggering amount of empirical literature intended to alleviate suffering, reduce mortality, and improve the quality of our lives. Despite these investments, the United States continues to struggle to deliver high-quality healthcare and improve patient outcomes – due in part to the systemic failure of the dissemination, adoption, and implementation of evidence-based discoveries in a timely fashion (Dougherty & Conway, 2008). This phenomenon is especially pronounced in the field of substance abuse treatment.
Prolonged translational periods are associated with many causal factors. In the substance abuse field, clinical research primarily occurs in academic centers while the vast majority of patients receive addiction care in community-based drug abuse treatment programs (Miller, Sorensen, Selzer, & Brigham, 2006). Academic and community settings operate under very different conditions. Consequently, research conducted in academic settings without attention to the specific needs of community-based treatment programs is likely to result in new treatments that will never be implemented (Westfall, Mold, & Fagnan, 2007).
In an attempt to address these deficiencies, the National Institute on Drug Abuse (NIDA) and the Substance Abuse and Mental Health Services Administration (SAMHSA) jointly commissioned the Institute of Medicine (IOM) in 1997 “to determine mechanisms for the effective transfer of information from the research communities to community-based drug treatment centers” (Lamb, Greenlick, & McCarty, 1998). The IOM committee’s report, Bridging the Gap Between Practice and Research: Forging Partnerships with Community-Based Drug and Alcohol Treatment, presented recommendations to increase communication, interaction, and activities, especially research activities, to enhance knowledge transfer between community-based drug treatment organizations and the research community. In response to the recommendations, NIDA established the National Drug Abuse Treatment Clinical Trials Network (CTN) (Hanson, Leshner, & Tai, 2002). The CTN brought together academic researchers and community-based providers to develop and execute clinical trials that generate and validate treatment interventions that fulfill the practical needs of community-based drug abuse treatment programs. Currently, the CTN supports sixteen centers or Regional Nodes. Participating providers include directors of community-based treatment programs and their front-line clinicians and counselors. The roster of researchers includes leaders in drug abuse treatment research from prominent academic medical centers (National Drug Abuse Treatment Clinical Trials Network, 2008).
2. CTN Infrastructure and Governance
The most fundamental undertaking in building the CTN into an effective research partnership was to establish an infrastructure that fostered bi-directional communication between researchers and treatment providers. Bi-directional communication guided every step taken to meet the CTN mission: the design of the infrastructure, the development of operational processes and the research portfolio, and the implementation of clinical trials. Collaboration between researchers and providers allowed the development of research projects that address practice-relevant questions. Providers in the network, through their participation in the design and conduct of clinical trials, acquired a better awareness of how research answers pressing clinical questions and improves the effectiveness of their services. Researchers developed an appreciation of how practice-relevant clinical research improves the significance of research questions and the potential for research findings to influence clinical practice.
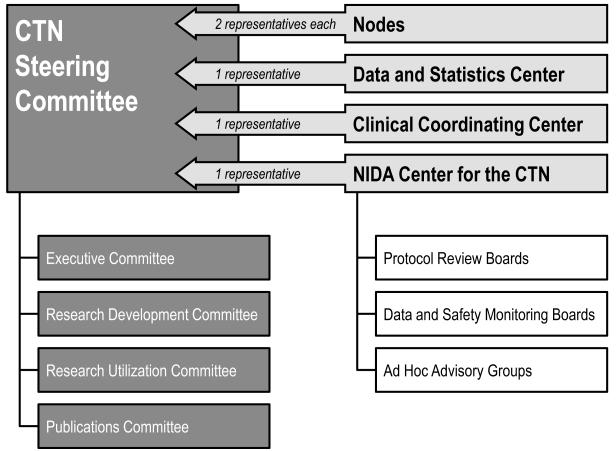
An effective research partnership requires an infrastructure with fair and transparent processes in addition to a governance body that values bi-directional communication and collaboration. As illustrated in Figure 1, the amalgamation of CTN infrastructure components—Regional Nodes, the Center for the Clinical Trials Network at NIDA, and coordinating centers—with governance by the Steering Committee and its sub-committees, facilitates effective communication and successful collaboration among researchers, providers, and NIDA.
Figure 1.
CTN governance structure (p5)
2.1 Regional Nodes
‘Nodes’ are the basic organizational, functional, and funding units of the CTN. The close working relationship between researchers and treatment providers within a Node makes it the primary location of bi-directional communication. Each Node includes a Regional Research and Training Center (RRTC) and five or more Community-based Treatment Programs (CTPs). The RRTC is the “academic” center of the Node and is situated within a major university. CTPs are primarily located in the geographical vicinity of the RRTC, but may include facilities in distant locations that provide access for special patient populations. CTPs were chosen to represent different addiction treatment modalities and to provide access to a diverse (in terms of age, race/ethnicity/gender and primary drug of addiction) clinical population for potential inclusion as study participants.
2.2 NIDA’s Center for the Clinical Trials Network
NIDA’s Center for the Clinical Trials Network (CCTN) manages the CTN. Nodes are supported via cooperative agreements that require collaboration between NIDA and the Nodes. Thus, NIDA participates actively in the design and management of trials. The CCTN supports CTN research activities and oversees investigator performance and trial progress. To centralize support activities in the network, the CCTN contracts for two CTN coordinating centers: 1) the Clinical Coordinating Center (CCC), and 2) the Data and Statistics Center (DSC).
The CCC supports protocol implementation, including research training and adherence to federal standards. In 2008, the CCC established a Web Seminar Series to provide on-line training on the fundamentals of clinical research. The faculty of this training program includes providers and research associates from the CTN Nodes. This training program allows for the dissemination of research skills throughout the network through the assistance of research-savvy peers.
Initially, trial data management was decentralized within each Node and the process varied by trial. In 2005 the CTN established a single network Data and Statistics Center (DSC) that manages CTN data, centralizes statistical expertise and enhances the efficiency and effectiveness of network operations. One notable improvement was a reduction of the time from last patient visit to database lock from 8 months to 3 months (Pan, et al., 2009). In 2005, the DSC, on behalf of NIDA, launched a data-sharing website, (http://www.ctndatashare.org/) to make data from completed CTN studies available for secondary analyses and meta-analyses.
The CCTN provides oversight during the research life cycle to ensure process integrity. Program officers in the CCTN offer scientific input on study design. With NIDA’s input and approval, CTN researchers and providers propose and select research topics and identify a research team to lead each study. Independent Protocol Review Boards and Data and Safety Monitoring Boards review and approve proposed protocols and monitor ongoing protocols for public health significance, scientific integrity, design adequacy, as well as participant safety and ethical considerations. In addition, NIDA oversees compliance with federal regulations for research conduct and human subject protection (Department of Health and Human Services, 2005).
2.3 Steering Committee
In order to mold the CTN infrastructure into a functional, open and transparent research forum that promotes inter-Node and bi-directional communication and decision-making, the CTN, upon its inception, wrote by-laws and formed a governing body—the CTN Steering Committee (National Drug Abuse Treatment Clinical Trials Network, 2008). The by-laws mandate equal representation of researchers and providers with two voting seats for each Regional Node—one filled by an RRTC representative (typically the grant Principal Investigator), and one filled from one of the affiliated CTPs. In addition, the CCTN and the two coordinating centers each hold one voting seat. The Steering Committee met frequently during the CTN’s first few years to develop consensus on all research and operational issues. With growing trust among members and a need to improve operational efficiency, the frequency of face-to-face meetings declined to two or three per year. During the past ten years, network governance activities have been progressively streamlined, with most operational work currently being completed through four committees that report to the Steering Committee: the Executive Committee, the Research Development Committee, the Research Utilization Committee, and the Publications Committee. Additionally, the CTN’s treatment providers and investigators meet as separate caucuses to address issues unique to each group.
2.4 Special Interest Groups
Special Interest Groups (SIGs) composed of investigators and providers promote bi-directional exchange of scientific ideas and practice expertise. SIGs form spontaneously and voluntarily among researchers and providers, with communication occurring via face-to-face meetings and tele-conferencing. During the past ten years, more than twenty SIGs have been formed. Table 1 provides a sampling of SIG tasks and identifies the CTN SIGs. SIGs define and develop many of the research questions for CTN studies. For example, the HIV/AIDS SIG was the first to form and has generated all of the CTN HIV/AIDS research concepts. In addition, SIGs serve as expert panels and advocate for scientific and clinical issues across CTN trials. A notable example is the Minority Interest Group. This group has been instrumental in ensuring that CTN studies are designed, implemented, analyzed and reported in a manner that addresses the specific needs of ethnic minorities. This SIG also works to increase training opportunities for ethnic minority scholars and clinicians. This is one of many examples of the invaluable contributions that the ‘blended expert panels’ of SIGs have made to CTN studies and publications (CTN Dissemination Library, 2009).
Table 1.
CTN Special Interest Groups—Where Science and Practice Blend
| Tasks | |
|---|---|
| Discuss the unmet needs of treatment | Serve as expert resources |
| Generate snapshots of current practice | Develop research concepts |
| Review empirically supported literature | Strategize long-range research plans |
| Identify, assess and develop special research tools |
| Groups (2000-now) | |
|---|---|
| HIV/AIDS | Smoking |
| Common assessment battery | Homelessness |
| Design and analysis | Spirituality |
| Adolescent | Eating disorder |
| Women and gender | Pain |
| Co-morbidity | Alcohol treatment |
| Court-involved patients | Genetics |
| Ethnic minority | Treatment algorithm |
| Behavioral therapy | Health services research |
| Pharmacotherapy (including Buprenorphine) | Residential treatment |
| Treatment matching | Cost analysis |
In 2006, a report from the NIH Roadmap Inventory and Evaluation of Clinical Research Networks (IECRN) acknowledged the CTN for its best practice in its internal interactivity by building trust and mutual respect through intensive interaction backed by strong sponsor support for the network mission (Inventory and Evaluation of Clinical Research Networks, 2006). A decade after the CTN made bidirectional communication a cornerstone of its endeavors, other Practice Based Research Networks (PBRNs) have begun to promote bi-directional communication in community-based participatory research (Horowitz, Robinson, & Seifer, 2009).
3. Influence of Bi-Directionality on Protocol Development and Implementation
Protocol development can be challenging in a bi-directional partnership. While academic investigators are trained to focus on scientific innovation and the validity of research methodology, providers attend to the quality of patient care, the constraints of clinical budgets and resources and the challenge of implementing evidence-based treatment in clinical practice. These perspectives conflict at times but discussion often leads to research innovation. Furthermore, translating research from well-controlled academic environments to the context of community-based “real-world” clinical care settings may strengthen the external validity of interventions and enhance generalization of study results (Glasgow, Davidson, Dobkin, Ockene, & Spring, 2006; Glasgow, Magid, Beck, Ritzwoller, & Estabrooks, 2005; Tunis, Stryer, & Clancy, 2003). The nature of “real-world settings” calls for a better understanding of cultural, social, economic and psychological variations in order to develop research projects that meet scientific standards, are feasible to implement and hold promise for adoption. These factors influence the determination of target population, characteristics needed in a research site, and realistic outcome measures in trial designs suitable for research in community settings (Hohmann & Shear, 2002).
Within the CTN, bi-directional communication and collaboration result in study designs that meet the needs of both academic and clinical settings and ultimately increase the likelihood that treatment providers will make use of research findings. For example, the CTN held a Steering Committee meeting in early 2000 to identify and develop research topics suitable for CTN partners. Each RRTC worked with its CTPs to craft research concepts for review and selection. Together, they identified research projects that not only addressed important scientific questions but also met the needs of providers in their daily practices. It became clear during these interactions that interventions studied within the CTN must be evidence-based treatments that are easy to adopt, have large effect sizes, and, in order to be sustained, fit within program clinical budgets.
To ensure that CTN studies meet the highest scientific standards, independent scientific experts review each study protocol to strengthen the science and protect participant safety. While it is important that protocol development procedures are streamlined and efficient, in the CTN it is equally important that: 1) only properly vetted research of the highest scientific rigor and relevance for the practice of substance abuse treatment is conducted, 2) all necessary safeguards are in place to guarantee the safety of study participants and the integrity of data collected (Rosa, Campbell, Kleppinger, Sampson, Tyson, & Mamay-Gentilin, 2009), and 3) the work of the CTN is always a true collaboration among researchers, treatment providers, and NIDA. A helpful step during the development of many CTN protocols is the circulation of a study questionnaire. CTPs respond to indicate interest in participating in the protocol and capacity to recruit the specified patient population, and may provide comment on study feasibility. This step facilitates implementation planning.
The generation of evidence-based interventions that are fully adopted in community-based treatment facilities depends on successfully addressing several ancillary research questions (Green & Glasgow, 2006). These include, but are not limited to: 1) the cost/benefit ratio of the intervention, 2) the treatment effects within various populations, including small and hard-to-reach subgroups, and 3) the validity of diagnostic and outcome measurements (Kilbourne, Neumann, Pincus, Bauer, & Stall, 2007). To address these questions, the CTN has been a fertile platform for a wide range of ancillary studies funded by R01 grants and other research mechanisms, particularly at the local level. Bi-directionality is implicit in these efforts because they are “sponsored” by the Nodes with the willing and able participation of the CTPs. The effect has been to highly leverage the investment of direct CTN funding through its facilitation of other funded research (Brown, et al., 2007; Forman, et al., 2007; McCarty, et al., 2008).
4. The “First Wave” of Research Studies
The first CTN studies tested brief, simple-to-implement interventions including: 1) short-term buprenorphine/naloxone-aided opioid detoxification, 2) one-session Motivational Interviewing and three-session Motivational Enhancement therapies, and 3) low-cost contingency management programs. The interventions in these initial studies had great potential for adoption in many different treatment settings and patient populations. The development processes for these trials, described below, illustrate the bi-directional give and take between pragmatic concerns and considerations of research methodology and validity.
4.1 Buprenorphine
In 2002, buprenorphine HCl sublingual tablets (Subutex®) and the combination product buprenorphine HCl and naloxone HCl dihydrate sublingual tablets (Suboxone®) became the first medications to gain FDA approval as physician office-based therapies for opiate dependence under the Drug Addiction Treatment Act of 2000 (Substance Abuse and Mental Health Services Administration, 2000). However, despite promising study findings, these novel treatments faced resistance in acceptance and adoption in clinical practice. At the time, providers trained in the use of buprenorphine and willing to prescribe it were rare, and many substance abuse treatment programs and providers remained reluctant to use pharmacotherapies in the treatment of addiction (Saxon & McCarty, 2005).
The CTN developed and implemented a series of studies to address important research and clinical questions regarding buprenorphine, with the added benefit of creating buprenorphine early adopters among participating community-based treatment programs. These studies evaluated: 1) the use of buprenorphine to facilitate short-term detoxification initiated in both inpatient and outpatient settings (Ling, et al., 2005), 2) the design of tapering regimens for patients stabilized on buprenorphine (Ling, et al., 2009), and 3) the use of buprenorphine to treat opioid-dependent adolescents and young adults (Woody, et al., 2008). During protocol development and implementation, researchers at each Node worked closely with staff at community treatment programs to complete the trials. This collaborative approach yielded findings with important scientific and clinical implications, and demonstrated that treatment programs could use buprenorphine safely and effectively (Amass, et al., 2004; Fiellin, 2008). Furthermore, most of the CTPs involved in these trials, including some that had previously been opposed to medication-assisted detoxification and treatment, continued to use buprenorphine in their clinical practice. Trial participation spurred several CTPs to initiate their own buprenorphine research projects (Brigham, Amass, Winhusen, Harrer, & Pelt, 2007; Collins, Horton, Reinke, Amass, & Nunes, 2007).
Publication of these research findings, along with other dissemination efforts, both formal and informal, extended the CTN’s impact on the adoption of buprenorphine well beyond the treatment programs directly involved in the network’s buprenorphine studies. A comparison of all 240 CTN CTPs to a national sample of non-CTN-affiliated treatment facilities indicated that CTN member programs were five times more likely to begin using buprenorphine (Ducharme, Knudsen, Roman, & Johnson, 2007). Furthermore, under the NIDA-SAMHSA Blending Initiative, the two agencies collaborated to develop a training package for treatment providers based on study findings from CTN trials on short-term buprenorphine detoxification (NIDA-SAMHSA-ATTC Blending Team, 2007).
4.2 Motivational Interviewing and Motivational Enhancement Therapy
Motivational Interviewing (MI) is based on methodologies that evoke a person’s intrinsic desire and ability to change, rather than imposing a directive approach in initiating treatment and in reducing use of alcohol, cigarettes, and abused drugs. A considerable body of research has shown strong support for the usefulness of motivational interviewing interventions (Martins & McNeil, 2009). Within the CTN, the initial protocol concept was to test a three-session intervention. However, treatment providers in some states reported that they could not be reimbursed for three individual sessions. Therefore, the protocol evolved into two separate trials: 1) a trial of a one-session motivational interviewing intervention, and 2) a trial of a three-session motivational enhancement therapy intervention (Carroll, et al., 2002). Both trials revealed that enhanced motivation improved treatment retention. Most importantly, these trials demonstrated the significant impact of clinical supervision in ensuring effective treatment delivery (Ball, et al., 2007; Carroll, et al., 2006). This evidence prompted the recognition and acceptance of rigorous training and supervision in MI delivery in the field. The findings from these studies led to the development of a NIDA-SAMHSA Blending Initiative product of supervisory tools for enhancing counselors’ proficiency in MI (Martino, et al., 2006).
4.3 Motivational Incentives
Motivational incentives (also referred to as contingency management) programs for the treatment of substance use disorders have been extensively studied, and their efficacy has been demonstrated in opioid, marijuana, alcohol, cocaine and methamphetamine dependent patients (Bickel, Amass, Higgins, Badger, & Esch, 1997; Budney, Higgins, Radonovich, & Novy, 2000; Higgins, Alessi, & Dantona, 2002; Higgins, Budney, Bickel, Foerg, Donham, & Badger, 1994; Knapp, Soares, Farrell, & Silva de Lima, 2007; Petry, Martin, Cooney, & Kranzler, 2000). However, two major apprehensions have prevented widespread adoption of motivational incentives by substance abuse treatment providers. The first concern is the potential negative consequences of providing tangible reinforcements to patients/clients with substance use disorders (Kellogg, Burns, Coleman, Stitzer, Wale, & Kreek, 2005). The second concern is the cost associated with offering incentives in clinics with limited budgets (Higgins, Silverman, & Heil, 2008). Many treatment providers perceive incentive interventions as too costly.
In order to overcome these barriers to adoption, the CTN developed and implemented two clinical trials testing low-cost motivational incentive interventions. One trial was carried out in methadone clinics, and the other was conducted in outpatient psychosocial treatment programs. Extensive discussions between participating researchers and treatment providers resulted in study designs utilizing a relatively low-cost, prize-based motivational incentives intervention that is acceptable in a broad range of treatment programs (Petry, 2000).
Findings from these trials and the associated cost-effectiveness analyses demonstrated the feasibility and clinical utility, in terms of both abstinence and treatment retention outcomes, of relatively low-cost motivational incentive interventions: methadone clinic participants received an average of $120 in prizes, and outpatient psychosocial program participants averaged $203 in prizes (Olmstead, Sindelar, & Petry, 2007; Sindelar, Olmstead, & Peirce, 2007). CTN treatment providers, however, worried that a prize system might trigger increased gambling. Study teams, therefore, recorded gambling behaviors as adverse events and measured potential problems related to gambling. These data provided objective evidence to document the safety of the intervention—incentives did not increase gambling (Peirce, et al., 2006; Petry, et al., 2005; Petry, et al., 2006). As with the CTN buprenorphine clinical trials, participation in the motivational incentives studies inspired some treatment providers to promote the wider use of this treatment approach. New York City, for example, promoted the intervention in all of its municipally funded treatment centers (Kellogg, Burns, Coleman, Stitzer, Wale, & Kreek, 2005). To reach a larger audience of providers, the NIDA-SAMHSA Blending Initiative used the CTN’s findings to develop a dissemination package, Promoting Awareness of Motivational Incentives (Albright, et al., 2006).
5. CTN Accomplishments
5.1 Research
The CTN fosters collaboration between researchers and treatment providers to generate scientific findings that enhance clinical practice. By June 2009, the CTN completed 20 clinical trials testing pharmacological and behavioral interventions for the treatment of substance use disorders. An additional four trials were recruiting participants or completing follow-up data collection. Four new trials were in protocol development. Table 2 lists the current and completed CTN trials (Calsyn, et al., 2009; Hien, et al., 2009; Hubbard, et al., 2007; Reid, et al., 2008; Tross, et al., 2008; Winhusen, et al., 2008). The trials have consented and randomized more than 11,000 individuals and have generated more than 80 papers in peer-reviewed journals.
Table 2.
STATUS OF CTN RANDOMIZED, CONTROLLED CLINICAL TRIALS (Fall 2009)
| Protocol # (Referenceb) |
Study | CTPs | N | Statusa |
|---|---|---|---|---|
| CTN 0001 | Buprenorphine/naloxone for inpatient detoxification | 6 | 113 | Completed (Ling, et al., 2005) |
| CTN 0002 | Buprenorphine/naloxone for outpatient detoxification | 6 | 230 | Completed (Ling, et al., 2005) |
| CTN 0003 | Comparison of two buprenorphine/naloxone taper schedules | 11 | 516 | Completed (Ling, et al., 2009) |
| CTN 0004 | Motivational enhancement therapy for patients in treatment for substance use disorders | 6 | 496 | Completed (Ball, et al., 2007) |
| CTN 0005 | Motivational interviewing for patients in treatment for substance use disorders | 5 | 423 | Completed (Carroll, et al., 2006) |
| CTN 0006 | Low cost motivational incentives for stimulant-abusing patients in outpatient psychosocial treatment programs | 8 | 454 | Completed (Petry, et al., 2005) |
| CTN 0007 | Low cost motivational incentives for stimulant-abusing patients in methadone maintenance treatment | 6 | 403 | Completed (Peirce, et al., 2006) |
| CTN 0009 | Incorporating smoking cessation treatment into substance abuse treatment programs | 12 | 225 | Completed (Reid, et al., 2008) |
| CTN 0010 | Buprenorphine/naloxone-facilitated rehabilitation for opioid-dependent adolescents and young adults | 6 | 154 | Completed (Woody, et al., 2008) |
| CTN 0011 | Telephone enhancement procedure for long-term engagement in continuing care | 4 | 339 | Completed (Hubbard, et al., 2007) |
| CTN 0013 | Motivational enhancement therapy for pregnant women in treatment for substance use disorders | 4 | 200 | Completed (Winhusen, et al., 2008) |
| CTN 0014 | Brief Strategic Family Therapy for adolescents in treatment for substance use disorders | 8 | 457 | Manuscript in Preparation |
| CTN 0015 | Seeking Safety therapy for women with PTSD in treatment for substance use disorders | 7 | 353 | Completed (Hien, et al., 2009) |
| CTN0017 | HIV HCV risk reduction interventions for injection substance users | 8 | 632 | Manuscript in Preparation |
| CTN 0018 | HIV/STD risk reduction interventions for men in treatment for substance use disorders | 14 | 594 | Completed (Calsyn, et al., 2009) |
| CTN 0019 | HIV/STD risk reduction interventions for women in treatment for substance use disorders | 12 | 517 | Completed (Tross, et al., 2008) |
| CTN 0020 | Job seekers training for patients in treatment for substance use disorders | 12 | 628 | Manuscript in Preparation |
| CTN 0021 | Motivational enhancement therapy for Spanish-speaking patients in treatment for substance use disorders | 6 | 463 | Completed (In Press) |
| CTN 0027 | Liver function in patients maintained on buprenorphine/naloxone or methadone | 9 | 1269 | Follow-up |
| CTN 0028 | Osmotic-release methylphenidate for ADHD in adolescents in treatment for substance use disorders | 11 | 303 | Manuscript in Preparation |
| CTN 0029 | Osmotic-release methylphenidate for ADHD in patients receiving smoking cessation treatment | 6 | 255 | Completed (In Press) |
| CTN 0030 | Treatment of prescription opioid addiction | 11 | 648 | Follow-up |
| CTN 0031 | Twelve-step engagement for patients in treatment for stimulant use disorders | 10 | 471 | Follow-up |
| CTN 0032 | HIV rapid testing and counseling in substance abuse treatment programs | 12 | 1281 | Follow-up |
| CTN 0037 | Exercise as an adjunctive treatment for substance use disorders | 10c | 330c | Protocol Development |
| CTN 0044 | Web-delivered treatment for substance use disorders | 10c | 500c | Protocol Development |
| CTN 0046 | Smoking cessation intervention for patients in treatment for stimulant use disorders | 12c | 528c | Protocol Development |
Status terminology
Completed: All protocol-defined research is finished and the primary paper has been accepted
Manuscript in preparation: All protocol-defined research is finished and the primary paper is in process
Follow-up: Participant enrollment is finished. Participant follow-up is ongoing.
See Reference section for full citation
Planned study parameters
In many disciplines, the process of research utilization is slow and marked by tension between the research “center” and the “field” (Rogers, 2003). Recognition of this barrier emphasizes the importance of the CTN infrastructure and bi-directional operational principle. Many CTPs joined the CTN because it allowed them to participate in cutting-edge scientific research, develop evidence-based treatments, use these treatments to better serve their patients/clients and help shape the research agenda to answer “real world” clinical problems. Providers reported disappointment in previous research experiences and felt that researchers had used them as patient recruitment machines and rarely engaged them as partners. To avoid this pitfall, the CTN abides by the policy that study results are discussed and reported to participating CTPs prior to any public presentations and/or publications (National Drug Abuse Treatment Clinical Trials Network, 2008). CTP providers also collaborate in the publication process, which may provide recognition for the CTP among peers while also providing an opportunity for CTPs to test emerging interventions and become early adopters. CTN CTPs are opinion leaders among addiction treatment programs and influence the research utilization and adoption process in the treatment community. Furthermore, the CTN research experience has opened opportunities for CTPs to successfully secure their own research and treatment funding from federal, state and private funders.
5.2 Dissemination and Research Utilization
The results of CTN research have a broad audience and are disseminated in a variety of ways. CTN publications and presentations are available through the CTN Dissemination Library (http://www.disseminationlibrary.org), maintained at the Pacific Northwest Node, and study findings have been translated into training packages (i.e., Blending Products) for providers. The CTN’s Research Utilization Committee (RUC) promotes the use of CTN research results. The RUC is composed of a Research Utilization Coordinator from each Node; a Chair is elected from the members. The Committee leads, plans, and reviews research utilization efforts including: 1) sharing of information and resources for intra-Node training activities (e.g., clinical skills training and writing skills training for CTP affiliated scientists to draft research papers), 2) monitoring the utilization of the CTN Dissemination Library, 3) serving as a point of contact with NIDA on Blending Conferences, and 4) serving as a point of contact with other agencies (e.g., SAMHSA in the preparation of the Blending Initiative dissemination packages and training sessions).
Much research utilization is conceived and delivered at the Node level. RRTCs from Regional Nodes sponsor all intra-Node research utilization activities with the Node’s CTPs and other facilities within the community. Routine intra-Node training sessions are a common practice for many Regional Nodes. For example,
The Southwest Node conducted a series of 13 workshops during 2006 and 2007 on evidence-based treatments derived from CTN research and Blending Initiative products. In addition, this Node is currently conducting a study with attendees of one of the thirteen workshops to assess the effectiveness of the instructional techniques and curricula.
The California-Arizona Node has extended its training efforts to treatment programs in the greater San Francisco Bay Area. The Node has developed an e-mail distribution list to: 1) broadcast upcoming training events and conferences, 2) collect post-training evaluation survey forms to gauge the needs of frontline treatment providers, and 3) design and plan training activities that meet the providers’ needs.
Furthermore, some Nodes serve as resources to outside institutions and organizations.
The Mid-Atlantic Node hired a full time Dissemination Specialist, who serves as the Node Research Utilization Coordinator and chairs the Motivational Incentives Workgroup within the RUC. She coordinates with the Central East Addiction Technology Transfer Center (ATTC) and with the Office of Education and Training for Addiction Services (OETAS), a state-wide training arm of the Maryland Alcohol and Drug Abuse Administration. The Mid-Atlantic Node also participates in Baltimore Substance Abuse Services (BSAS), the quasi-governmental agency that receives and distributes federal block grant funding for substance abuse treatment.
6. Future Directions
Having built, over the past ten years, a capable and bi-directional infrastructure for conducting research that is relevant to community-based practice, the CTN is adapting to new challenges. The network’s continuing relevance and utility depends on its ability to address emerging public health issues and research opportunities in the field of substance abuse treatment. Areas for future study may include the integration of primary care and substance abuse treatment systems into effective chronic care models for addiction treatment (McLellan, 2009) and the assessment of holistic approaches in treating patients with co-occurring substance use, mental health and somatic disorders. The use of health information technology (e.g., web-based interventions, SMS/text messaging, etc.) to improve treatment delivery efficiency and expand treatment capacity is another urgent and yet under-researched area that the CTN must address.
The next decade is likely to bring increasingly sophisticated pharmacotherapies and behavioral therapies to the research and addiction treatment arenas. The CTN’s alliance of treatment providers and investigators is well positioned to conduct rigorous tests of these innovations in practice environments throughout their development and to conduct economic evaluations to help guide potential adopters of these interventions. As the CTN completes research on interventions, the network reaches providers through research utilization efforts and engages stakeholders to disseminate therapeutic tools and improve substance abuse treatment.
The randomized controlled trial remains the gold standard for generating evidence-based therapies; however, randomized trials are both costly and time consuming. The CTN must consider adopting more innovative and practical strategies for developing evidence-based substance abuse treatment interventions. A recent Institute of Medicine (IOM) report suggested a meta-experimental approach for building a foundation for evidence-based medicine. It may include some or all of the following tactics:
Utilization of existing patient databases (large survey databases, treatment reimbursement databases, etc.), registry databases, and electronic medical records;
Development of statistical tools and techniques such as adaptive design, simulation and modeling and methods to analyze large databases;
Attention to practical clinical trials and subgroup analysis; and
Exploration of reasonable approaches to incorporate physiologic and genetic information into evidence-based medicine (Institute of Medicine, 2008).
The CTN has great potential to broaden its research portfolio to realize this meta-experimental approach in generating evidence-based addiction treatment interventions.
A flexible and comprehensive research strategy is critical for the CTN to better serve as the home for Comparative Effectiveness Research (CER) in substance abuse treatment. CER, a centerpiece of the current healthcare reform initiative, provides healthcare professionals with evidence-based treatment options (Institute of Medicine, 2007; Institute of Medicine, 2009). These options should minimize unnecessary and inappropriate health interventions and reduce the frequency of medical errors and adverse consequences, with the ultimate goal of improving care and containing costs (Garber & Tunis, 2009). CER in substance abuse treatment is of particular importance, as the development of research information compatible with the broader goals of healthcare reform will further the integration of substance abuse treatment into the mainstream of medical practice. With its durable research infrastructure and experienced workforce, the CTN is well positioned to undertake CER projects that address substance abuse treatment within the context of the larger healthcare system. To do so, the CTN must expand to incorporate clinical settings that frequently see patients with co-morbid substance use disorders. These may include, but are not limited to, primary care clinics, dental clinics, pain management clinics, emergency care centers and HIV/STD clinics. With this broadened approach to addiction treatment research, the CTN will continue to serve as a forum for bi-directional communication and collaboration within its research and dissemination efforts.
In the next decade, expeditiously translating scientific advances into patient care is a top priority for the NIH (NIH Roadmap Initiative, 2008). Conducting research directly within the community and involving community providers in research activities is critical to achieve this goal (Horowitz, Robinson, & Seifer, 2009). The CTN has learned a number of important lessons, reflected in the foregoing descriptions, about how to practice bi-directionality and how to conduct research in real world treatment settings. This work needs to continue, while at the same time we hope the lessons learned may encourage similar efforts in other related fields.
Acknowledgments
The authors thank John Rotrosen, M.D., and Edward V. Nuñes, M.D. for their critical reading and comments of the manuscript, and Katia Delrahim-Howlett, M.B.A., M.A., for her expert assistance in preparation of this manuscript. The opinions are those of the authors and do not represent the official position of the U.S. government. Awards from the National Institute on Drug Abuse supported the participation of Ron Jackson (U10 DA13714) and Dennis McCarty (U10 DA13036) in the development of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albright L, Hamilton JA, Kellogg S, Kileen T, Petry NM, Rosenfield J, Shanahan A, Peters C, Skinstad AH, Stitzer ML. Promoting Awareness of Motivational Incentives. 2006 Addiction Tehcnology Transfer Center Network: http://www.attcnetwork.org/exlore/priorityareas/science/blendinginitiative/pami/
- Amass L, Ling W, Freese TE, Reiber C, Annon JJ, Cohen AJ, et al. Bringing buprenorphine-naloxone detoxification to community treatment providers: the NIDA Clinical Trials Network field experience. American Journal on Addictions. 2004;13(Suppl 1):542–566. doi: 10.1080/10550490490440807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SA, Martino S, Nich C, Frankforter TL, Van Horn D, Crits-Christoph P, et al. Site matters: multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. Journal of Consulting and Clinical Psychology. 2007;75(4):556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Higgins ST, Badger GJ, Esch RA. Effects of adding behavioral treatment to opioid detoxification with buprenorphine. Journal of Consulting and Clinical Psychology. 1997;65(5):803–810. doi: 10.1037//0022-006x.65.5.803. [DOI] [PubMed] [Google Scholar]
- Brigham GS, Amass L, Winhusen T, Harrer JM, Pelt A. Using buprenorphine short-term taper to facilitate early treatment engagement. Journal of Substance Abuse Treatment. 2007;32(4):349–356. doi: 10.1016/j.jsat.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Brown LS, Kritz S, Goldsmith RJ, Bini EJ, Robinson J, Alderson D, et al. Health services for HIV/AIDS, HCV, and sexually transmitted infections in substance abuse treatment programs. Public Health Reports. 2007;122(4):441–451. doi: 10.1177/003335490712200404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68(6):1051–61. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Calsyn DA, Hatch-Maillette M, Tross S, Doyle SR, Crits-Christoph P, Song YS, et al. Motivational and skills training HIV/sexually transmitted infection sexual risk reduction groups for men. Journal of Substance Abuse Treatment. 2009;37:138–150. doi: 10.1016/j.jsat.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C, et al. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug and Alcohol Dependence. 2006;81(3):301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Farentinos C, Ball SA, Crits-Christoph P, Libby B, Morgenstern J, et al. MET meets the real world: design issues and clinical strategies in the Clinical Trials Network. Journal of Substance Abuse Treatment. 2002;23(2):73–80. doi: 10.1016/s0740-5472(02)00255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins ED, Horton T, Reinke K, Amass L, Nunes EV. Using buprenorphine to facilitate entry into residential therapeutic community rehabilitation. Journal of Substance Abuse Treatment. 2007;32(2):167–175. doi: 10.1016/j.jsat.2006.03.018. [DOI] [PubMed] [Google Scholar]
- CTN Dissemination Library [Retrieved June 15, 2009];Publications Catalog. 2009 from The CTN Dissemination Library Website: http://ctndisseminationlibrary.org/catalog.pdf.
- Department of Health and Human Services [Retrieved June 15, 2009];Protection of Human Subjects. 2005 from HHS Office for Human Research Protections (OHRP) Website: http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm.
- Dougherty D, Conway PH. The “3T’s” road map to transform US health care: The“how” of high-quality care. Journal of the American Medical Association. 2008;299(19):2319–2321. doi: 10.1001/jama.299.19.2319. [DOI] [PubMed] [Google Scholar]
- Ducharme LJ, Knudsen HK, Roman PM, Johnson JA. Innovation adoption in substance abuse treatment: exposure, trialability, and the Clinical Trials Network. Journal of Substance Abuse Treatment. 2007;32(4):321–329. doi: 10.1016/j.jsat.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA. Treatment of adolescent opioid dependence: no quick fix. Journal of the American Medical Association. 2008;300(17):2057–2059. doi: 10.1001/jama.2008.567. [DOI] [PubMed] [Google Scholar]
- Forman R, Crits-Christoph P, Kaynak O, Worley M, Hantula DA, Kulaga A, et al. A feasibility study of a web-based performance improvement system for substance abuse treatment providers. Journal of Substance Abuse Treatment. 2007;33(4):363–71. doi: 10.1016/j.jsat.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber AM, Tunis SR. Does comparative-effectiveness research threaten personalized medicine? New England Journal of Medicine. 2009;360(19):1925–1927. doi: 10.1056/NEJMp0901355. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Davidson KW, Dobkin PL, Ockene J, Spring B. Practical behavioral trials to advance evidence-based behavioral medicine. Annals of Behavioral Medicine. 2006;31(1):5–13. doi: 10.1207/s15324796abm3101_3. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Magid DJ, Beck A, Ritzwoller D, Estabrooks PA. Practical clinical trials for translating research to practice: design and measurement recommendations. Medical Care. 2005;43(6):551–557. doi: 10.1097/01.mlr.0000163645.41407.09. [DOI] [PubMed] [Google Scholar]
- Green LW, Glasgow RE. Evaluating the relevance, generalization, and applicability of research: issues in external validation and translation methodology. Evaluation & the Health Professions. 2006;29(1):126–153. doi: 10.1177/0163278705284445. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Leshner AI, Tai B. Putting drug abuse research to use in real-life settings. Journal of Substance Abuse Treatment. 2002;23(2):69–70. doi: 10.1016/s0740-5472(02)00269-6. [DOI] [PubMed] [Google Scholar]
- Hien DA, Wells EA, Jiang H, Suarez-Morales L, Campbell AN, Cohen LR, et al. Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders. Journal of Consulting and Clinical Psychology. 2009;77(4):607–619. doi: 10.1037/a0016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Alessi SM, Dantona RL. Voucher-based incentives: a substance abuse treatment innovation. Addictive Behaviors. 2002;27(6):887–910. doi: 10.1016/s0306-4603(02)00297-6. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH. Contingency management in substance abuse treatment. Guilford Press; New York: 2008. [Google Scholar]
- Hohmann AA, Shear MK. Community based intervention research: coping with the noise of real life in study design. American Journal of Psychiatry. 2002;159(2):201–207. doi: 10.1176/appi.ajp.159.2.201. [DOI] [PubMed] [Google Scholar]
- Horowitz CR, Robinson M, Seifer S. Community-based participatory research from the margin to the mainstream: are researchers prepared? Circulation. 2009;119(19):2633–2642. doi: 10.1161/CIRCULATIONAHA.107.729863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RL, Leimberger JD, Haynes L, Patkar AA, Holter J, Liepman M, et al. Telephone enhancement of long-term engagement (TELE) in continuing care for substance abuse treatment: a NIDA clinical trials network (CTN) study. American Journal on Addictions. 2007;16(6):495–502. doi: 10.1080/10550490701641678. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . Evidence-based medicine and the changing nature of health care: 2007 IOM annual meeting summary. The National Academies Press; Washington, DC: 2008. [PubMed] [Google Scholar]
- Institute of Medicine . Initial national priorities for comparative effectiveness research. The National Academies Press; Washington, DC: 2009. [Google Scholar]
- Institute of Medicine [Retrieved June 15, 2009];Learning what works best: the nations need for evidence on comparative effectiveness in health care. 2007 from http://www.iom.edu/Object.File/Master/43/390/Comparative%20Effectiveness%20White %20Paper%20(F).pdf.
- Inventory and Evaluation of Clinical Research Networks [Retrieved June 15, 2009];IECRN Project Report. 2006 from Networks for Clinical Research: https://www.clinicalresearchnetworks.org/4f.asp#_Report.
- Kellogg S, Burns M, Coleman P, Stitzer ML, Wale JB, Kreek MJ. Journal of Substance Abuse Treatment. 1. Vol. 28. 2005. Something of value: the introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service; pp. 57–65. [DOI] [PubMed] [Google Scholar]
- Kilbourne AM, Neumann MS, Pincus HA, Bauer MS, Stall R. Implementing evidence-based interventions in health care: application of the replicating effective programs framework. Implementation Science. 2007;2(42) doi: 10.1186/1748-5908-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp WP, Soares B, Farrell M, de Lima M. Silva. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD003023.pub2. [DOI] [PubMed] [Google Scholar]
- Lamb S, Greenlick MR, McCarty D. Bridging the gap between practice and research: Forging partnerships with community-based drug and alcohol treatment. National Academy Press; Washington, DC: 1998. [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D, et al. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100(8):1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Hillhouse M, Domier C, Doraimani G, Hunter J, Thomas C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104(2):256–265. doi: 10.1111/j.1360-0443.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino S, Ball SA, Gallon SL, Hall D, Garcia M, Ceperich S, et al. Motivational Interviewing Assessment: Supervisory Tools for Enhancing Proficiency. Salem: 2006. [Google Scholar]
- Martins RK, McNeil DW. Review of Motivational Interviewing in promoting health behaviors. Clinical Psychology Review. 2009;29(4):283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- McCarty D, Fuller B, Kaskutas LA, Wendt WW, Nunes EV, Miller M, et al. Treatment programs in the National Drug Abuse Treatment Clinical Trials Network. Drug and Alcohol Dependence. 2008;92(1-3):200–207. doi: 10.1016/j.drugalcdep.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan T. Revisiting the past for a look toward future research: a final editorial. Journal of Substance Abuse Treatment. 2009;36(4):352–354. doi: 10.1016/j.jsat.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Miller WR, Sorensen JL, Selzer JA, Brigham GS. Disseminating evidence-based practices in substance abuse treatment: A review with suggestions. Journal of Substance Abuse Treatment. 2006;31(1):25–39. doi: 10.1016/j.jsat.2006.03.005. [DOI] [PubMed] [Google Scholar]
- National Drug Abuse Treatment Clinical Trials Network [Retrieved June 15, 2009];Network Organization/By-Laws. 2008 from CTN Website: http://www.nida.nih.gov/CTN/pdf/CTNByLaws.pdf.
- National Drug Abuse Treatment Clinical Trials Network [Retrieved June 15, 2009];Network Organization/Nodes. 2008 from CTN Website: http://www.drugabuse.gov/CTN/node.html.
- NIDA-SAMHSA-ATTC Blending Team . The science of treatment: dissemination of research-based drug addiction treatment findings. NIDA/SAMHSA-ATTC Blending Initiative; Rockville, MD: 2007. [Google Scholar]
- NIH Roadmap Initiative [Retrieved June 15, 2009];NIH Roadmap for medical research. 2008 from Re-engineering the Clinical Research Enterprise: http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
- Olmstead TA, Sindelar JL, Petry NM. Cost-effectiveness of prize-based incentives for stimulant abusers in outpatient psychosocial treatment programs. Drug and Alcohol Dependence. 2007;87(2/3):175–182. doi: 10.1016/j.drugalcdep.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JJ, Nahm M, Wakim P, Cushing C, Poole L, Tai B, et al. A centralized informatics infrastructure for the National Institute on Drug Abuse Clinical Trials Network. Clinical Trials. 2009;6(1):67–75. doi: 10.1177/1740774508100983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63(2):201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence. 2000;58(1-2):9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Peirce JM, Roll JM, Stitzer ML, et al. Prize-based contingency management does not increase gambling. Drug and Alcohol Dependence. 2006;83(3):269–273. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68(2):250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs : a National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2005;62(10):1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, et al. Smoking cessation treatment in community-based substance abuse rehabilitation programs. Journal of Substance Abuse Treatment. 2008;35(1):68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Rogers EM. Diffusion of Innovations. 5th Edition Free Press; New York: 2003. [Google Scholar]
- Rosa CL, Campbell ANC, Kleppinger C, Sampson R, Tyson C, Mamay-Gentilin S. Quality assurance of research protocols conducted in the community: the National Institute on Drug Abuse Clinical Trials Network experience. Clinical Trials. 2009;6(2):151–161. doi: 10.1177/1740774509102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon AJ, McCarty D. Challenges in the adoption of new pharmacotherapeutics for addiction to alcohol and other drugs. Pharmacology & Therapeutics. 2005;108(1):119–128. doi: 10.1016/j.pharmthera.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Sindelar JL, Olmstead TA, Peirce JM. Cost-effectiveness of prize-based contingency management in methadone maintenance treatment programs. Addiction. 2007;102(9):1463–1471. doi: 10.1111/j.1360-0443.2007.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration [Retrieved June 15, 2009];Public Law No. 106-310, Title XXXV. 2000 from Drug Addiction Treatment Act of 2000: http://www.buprenorphine.samhsa.gov/fulllaw.html.
- Tross S, Campbell AN, Cohen LR, Calsyn D, Pavlicova M, Miele GM, et al. Effectiveness of HIV/STD sexual risk reduction groups for women in substance abuse treatment programs: results of a NIDA Clinical Trials Network Trial. Journal of Acquired Immune Deficiency Syndromes. 2008;48(5):581–589. doi: 10.1097/QAI.0b013e31817efb6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. Journal of the American Medical Association. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- Westfall JM, Mold J, Fagnan L. Practice-based research--“Blue Highways” on the NIH Roadmap. Journal of the American Medical Association. 2007;297(4):403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Kropp F, Babcock D, Hague D, Erickson SJ, Renz C, et al. Motivational enhancement therapy to improve treatment utilization and outcome in pregnant substance users. Journal of Substance Abuse Treatment. 2008;35(2):161–173. doi: 10.1016/j.jsat.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs. short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. Journal of the American Medical Association. 2008;300(17):2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]