Abstract
A cell-penetrating peptide consisting of the second intracellular loop (IC2) of the Angiotensin II (AngII) type I receptor (AT1) linked to the HIV transactivating regulatory protein (TAT) domain was used to identify the role of this motif for intracellular signal transduction. HEK-293 cells stably transfected with AT1R cDNA and primary cultures of human pulmonary artery smooth muscle cells expressing endogenous AT1 receptor were exposed to the cell-penetrating peptide construct and the effect on angiotensin II signaling determined. The AT1 IC2 peptide effectively inhibited AngII stimulated phosphatidylinositol turnover and calcium influx. It also limited the activation of Akt/PKB as determined by an inhibition of phosphorylation of Akt at Ser473 and completely abolished the AngII dependent activation of the transcriptional factor NFκB. In contrast, the AT1 IC2 peptide had no effect on AngII/AT1 receptor activation of ERK. These results illustrate the potential of using cell penetrating peptides to both delineate receptor-mediated signal transduction as well as to selectively regulate G protein coupled receptor signaling.
Introduction
Cell-penetrating peptides (CPPs) are typically short cationic amino acid sequences that have been demonstrated to mediate intracellular delivery of a range of biological cargos (1). These peptides facilitate the movement of a variety of molecules into various compartments of intact cells (2, 3). The CPPs are now being used to transport proteins into cells in culture and into in situ organs. For example, Jasmin and coworkers coupled a cell penetrating peptide, antennapedia (AP), to a peptide corresponding to the scaffolding domain of caveolin-1 to ameliorate the development of monocrotaline-induced pulmonary hypertension in rats (4). In another study, a peptide corresponding to the last seven amino acids of the carboxyl terminus of the glutamate receptor 2 (GluR2) coupled to a modified tetrapeptide (Phe-Arg-Phe-Lys) previously shown to cross cell membranes (5) was able to reverse cocaine addiction in rats (6). Thus the cell penetrating peptides have potential in both experimental approaches and in therapeutic procedures.
Hypertension and its complications are major contributors to mortality and morbidity both nationally and globally. Angiotensin II (AngII), a vasoactive peptide, acting through its type I receptor (AT1), has been shown to have far reaching effects on vascular tone, structure, growth and fibrosis, and is a key regulator of vascular remodeling. Treatments which ameliorate the pathologic effects of AngII have been shown to limit organ damage in hypertension and limit morbidity and mortality (7, 8). In fact, ACE (angiotensin converting enzyme) inhibitors, which block the production of AngII from angiotensin I, are very effective in the treatment of hypertension.
The AT1 receptor for AngII is seven transmembrane G-protein coupled. The detailed signal transduction pathway of AT1 receptor in cardiovascular system was thoroughly reviewed by Mehta and Griendling (9). Our laboratory has been actively identifying motifs and their interactions within the intracellular face of the angiotensin II AT1 receptor which regulates specific signaling actions. Our mutagenesis studies suggest that the second intracellular loop (IC2) of the AT1 receptor is important in both G-protein and kinase related signal transmission (10). In the series of experiments described herein we covalently attach a HIV transactivation regulatory protein (TAT) domain, a cell penetrating peptide (11), to a peptide consisting of the IC2 sequence of the AT1R to determine whether with this approach angiotensin II regulated signaling can be controlled. Our results show that these cell penetrating peptides are a potentially powerful tool for elucidation of angiotensin II/AT1 receptor signaling and for limiting the signaling ability of the AT1 receptor.
Materials and Methods
Materials
[3H] AngII (52.5 Ci/mmol), and myo-[1,2-3H] inositol (60 Ci/mmol) were obtained from Perkin Elmer Life Sciences (Boston, MA). Antibodies for detection of phospho-ERK1/2(Thr202/Tyr204), ERK1/2, phospho-Akt (Ser473), Akt, NFκB p65 and phosphor-NFκB p65 (Ser536) were purchased from Cell Signaling Technologies (Beverly, MA). Protease inhibitor cocktail was from Roche Diagnostics (Indianapolis, IN). BCA Protein Assay Kit was from Pierce (Rockford, IL). ECL Western Blotting Detection Reagents were from Amersham Biosciences (Piscataway, NJ). The cDNA for AT1 receptor (pcDNA3.1-AGTR1) was purchased from Missouri S&T cDNA Resource Center (Rolla, MO 65409).
Cell Culture
Cell culture and transfections in HEK-293 cells were performed as described by Yu et al (10). HEK-293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), containing 10% fetal bovine serum supplemented with 50 units/ml penicillin and 50 ug/ml streptomycin at 37 °C in a humidified CO2 (5%) incubator. HEK-293 cells were transfected using Lipofectamine 2000 according to the protocol of the manufacturer (Invitrogen, Carlsbad, CA) and selected in the presence of 0.5 mg/ml G418. The G418 resistant cell culture was tested for specific binding to [3H] Ang II.
Smooth muscle cells isolated from the pulmonary arteries obtained from lung transplants were a generous gift from Dr. Serpil Erzurum (Cleveland Clinic, Cleveland, OH). Cells were maintained in 15 mM Hepes buffered DMEM/F12 medium (Invitrogen) supplemented with 10% fetal bovine serum (Lonza) and 250 units/ml penicillin, 250 ug/ml streptomycin and 0.625 ug/ml amphotericin B (Invitrogen) in an atmosphere of 95% air/5% CO2 at 37°C. In our studies, cells were used at passages 5 to 9.
[3H]AngII Ligand Binding
Receptor binding studies of the AT1 receptors in intact HEK293 and in human pulmonary artery smooth muscle cells were carried out as described by Yu et al.(10). Briefly, confluent cell monolayers in 24-well plates were incubated in binding buffer (50 mmol/L Tris, 120 mmol/L NaCl, 4 mmol/L KCl, 10 μg/ml bacitracin, 10 mmol/L glucose, 0.1% BSA, 1 mmol/L CaCl2, 5 mmol/L MgCl2, 10 mmol/L HEPES pH7.35) containing various concentrations of [3H]-AngII ranging from 0.04 to 10nM in the absence (total binding) or presence of 100nM unlabeled AngII (nonspecific binding) for 2 hours at 4°C. After the incubation the cells were washed three times with ice-cold buffer and then solubilized with 0.2% SDS. Radioactivity was determined in a PACKARD 1900 TR β counter after addition of 2ml of Ecolite scintillation fluid. Equilibrium binding data (Kd and Bmax) were analyzed by best fit to a single site model using the SigmaPlot® 8 program (SPSS Inc.).
Peptide Synthesis
The cell penetrating peptides were synthesized by 21st Century Biochemicals, Marlboro, MA, using Fmoc/t-Bu solid-phase peptide chemistry. The crude products were purified to homogeneity by preparative high-performance liquid chromatography and converted to an acetate salt to avoid the exposure of cells to TFA (trifluoroacetate). The final peptides were characterized by analytical high-performance liquid chromatography (purity >95%), nanospray mass spectrometry and the sequence confirmed via collision-induced fragmentation. The peptide sequences consisted of the AT1 receptor IC2 (underlined) and the TAT sequence (not underlined). The sequence is illustrated in Fig. 1. GRKKRRQRRRPPQGGVHPMKSRLRRT and a negative control peptide which was targeted against the IC2 of the human bradykinin B2 receptor also attached distally to the TAT sequence (negative control), GRKKRRQRRRPPQGGVKTMSMGRMRG). A 5-FAM (5-fluorescein coupled via an amide linkage) fluorophore linked to the N-terminal of TAT peptide (5-FAM-Ahx-GRKKRRQRRRPPQ-amide) was also synthesized. The peptides were dissolved in endotoxin-free water to obtain 5mM stock solutions.
Figure 1.
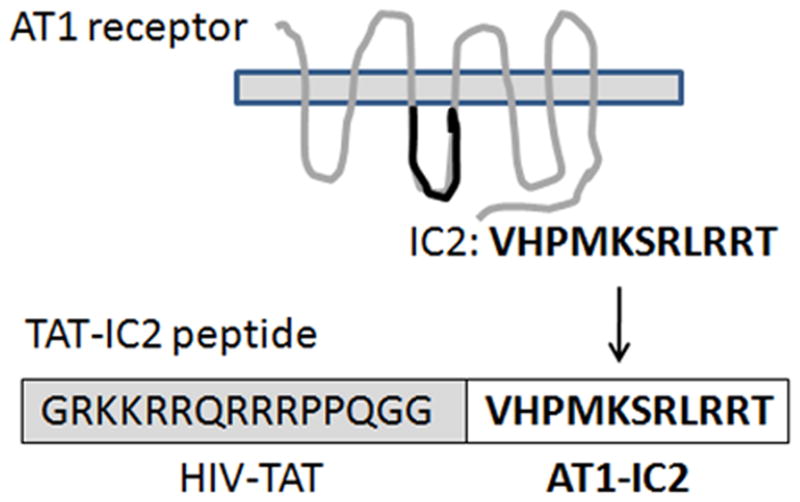
Cartoon demonstrates the construction of the TAT-AT1 IC2 peptide.
Determination of Peptide Cell Penetration by Fluorescence Activated Cell Sorting (FACS)
5-FAM tagged TAT peptide (10 μM) in serum free media was added to either HEK-293 or human pulmonary smooth muscle cell cultures grown in 6 well plates. After 30 min at 37°C, to cleave adhering CPPs from the cell membranes and to detach the cells from the wells, the cells were washed twice with PBS and then trypsinized and resuspended in PBS as a single cell suspension. The single cell suspension was transferred into FACS tubes at a concentration of 1.5 × 106 cells per ml. Cells were analyzed by FACS on a FacsCalibur (Becton Dickinson, Franklin Lakes, NJ) within 1 h after trypsinization. A total of 8,000 gated cells per sample were counted. Data were analyzed using Cytomation Summit software (Cytomation Inc., Fort Collins, USA). As controls, HEK or HPASMCs were incubated with peptide-free medium.
Phosphoinositide (PI) Turnover
HEK-293 cells were incubated with 1μCi/ml myo-[3H] inositol in 1ml of growth medium and the levels of inositolphosphates (IPs) determined 24 hours later as described by Prado et al. (12). Briefly, ten minutes prior to ligand stimulation, cells were exposed to DMEM containing 20mM LiCl2 and 20 mM HEPES, pH 7.4. The cells were then exposed to 10 nM AngII for 30 minutes at 37°C, and the incubations were terminated by removal of the media and addition of 0.5ml of 10mM ice-cold formic acid. The cells were scraped and the formic acid soluble material isolated by centrifugation and neutralized by the addition of 10ml 5mM sodium tetraborate. Total [3H]-IPs were extracted using a Dowex AG 1-X8 formate resin in an anion exchange column and eluted with 2M ammonium formate, pH 5.0, as described. Radioactivity was determined in a Packard liquid scintillation counter.
Calcium Mobilization
Mobilization of Ca2+ was determined as reported previously with some modifications (13). The HEK-293 cells were trypsinized and washed two times in physiological buffer solution (140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L glucose, 0.9 mmol/L CaCl2, 15 mmol/L HEPES, 0.1% BSA). The HEK-293 cells were resuspended at 1.5 × 107 cells/ml and incubated with Fura-2/AM for 30 min (2 μmol/L final concentration). After 30 min, the cell suspension was diluted 5 times with physiological buffer solution and incubated for another 15 min. Cells were pelleted and resuspended at 1 × 106 cells/ml. Ca2+ mobilization experiments were performed using a Hitachi F-2500 Fluorescence Spectrophotometer.
Western Blot Analysis
Human pulmonary smooth muscle cells were incubated with 10nM AngII for 5 min. The cells were then washed twice with ice-cold PBS. Cell lysates were prepared by addition of ice-cold RIPA buffer, 150 mM NaCl, 1.0% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0 (Sigma, St Louis, MO) and 1× complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and sedimented at 12,000 rpm in a microcentrifuge at 4°C for 20 minutes. The proteins were fractionated on 10% SDS-PAGE gels and western blots were carried out using antibodies against phosphorylated or unphosphorylated ERK1/2, NFκB (p65 subunit) or Akt. Proteins were detected by chemiluminescence and the film scanned with an Epson Perfection 3170 scanner using Epson Scan (version 1.22A) software. The image was then analyzed using Sigma Scan10 (Jandel Scientific, San Rafael, CA) to determine the intensity of each band.
Statistical analysis and data analysis
Statistical evaluation of the data was carried out using the Student t-test. Probability values less than 0.05 were considered significant.
Results
Expression of the AT1 Receptor
The human AT1 receptor cDNA (AGTR1) was stably transfected into the HEK-293 cells. The human pulmonary artery smooth muscle cells (HPASMCs) express the AT1 receptor endogenously. Real time PCR showed the expression of AT1 mRNA in both the HEK-293 and the HPASMCs. Binding studies showed 738,800 receptors/cell in the HEK-293 cells and a much smaller number, 7461 receptors/cell in the HPASMCs.
Cell Penetration of the TAT Cell Penetrating Peptide
The cell penetrating TAT sequence was distally attached to a fluorescent FAM molecule. Its penetration of HEK-293 and HPASMCs was determined using Fluorescence Activated Cell Sorting (FACS). 10 μM FAM tagged TAT peptide in serum free media was added to either HEK-293 or human pulmonary smooth muscle cell cultures. Results are shown in Fig. 2A and 2B. 96% of the HEK-293 and 92% of the HPASMCs contained the fluorescent TAT peptide. To insure that no extracellular, adhering CPPs remained at the cell surface the cells were treated with 0.5% trypsin after a 30 minutes incubation with the peptides at 37°C.
Figure 2. The TAT peptide penetrates the intact HEK-293 and pulmonary artery smooth muscle cells.
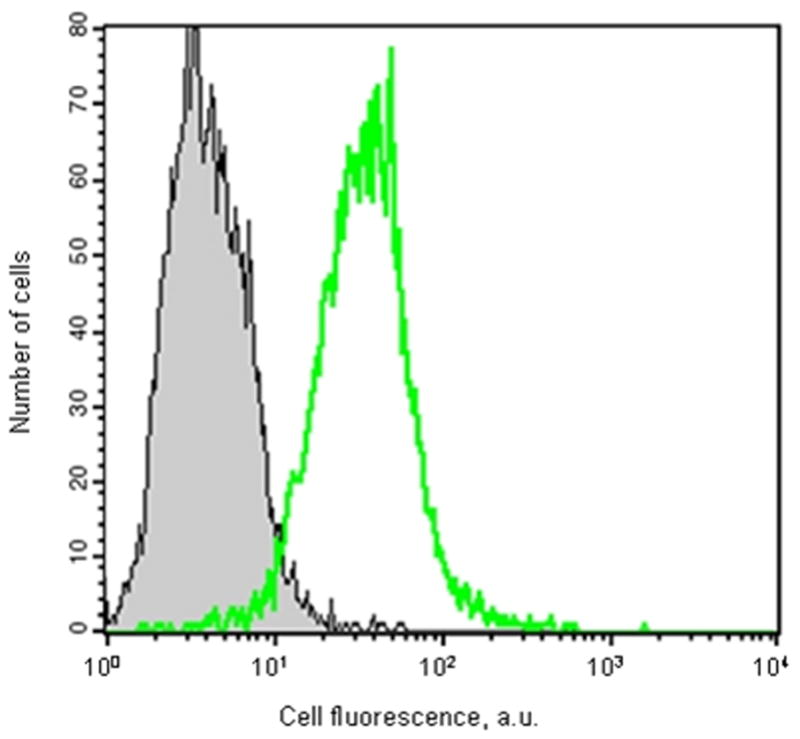
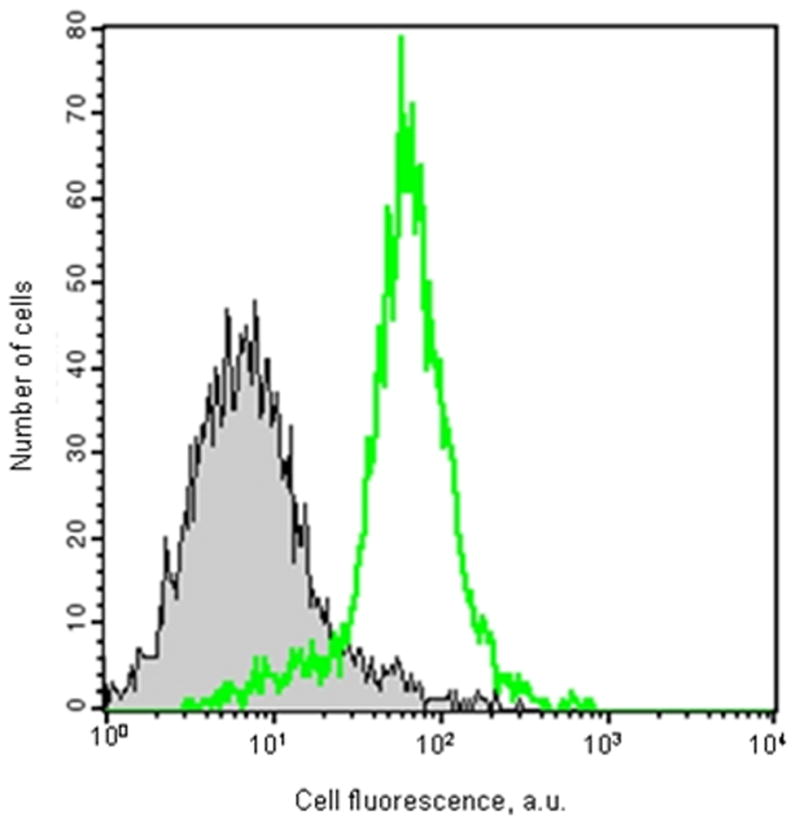
Flow cytometry was conducted on HEK-293 (A) or human pulmonary artery smooth muscle (B) cells that were incubated for 30 minutes with 10 μM 5-FAM labeled TAT peptide (no filling under the curve) or peptide free medium (grey filling under the curve). Cells were then trypsinized and resuspended in PBS as a single cell suspension before flow cytometry.
Effect of the Peptide on AT1R Activated Gαq Signaling
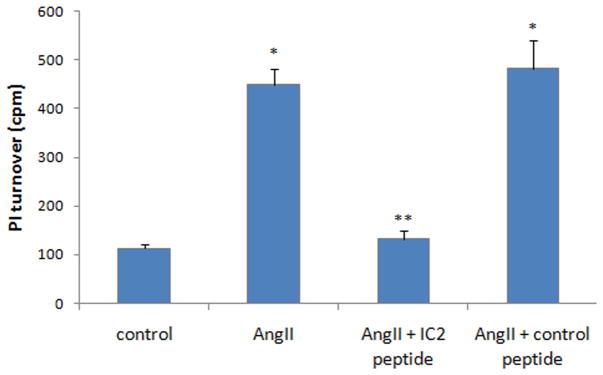
To study the role of the secondary intracellular domain of the AT1 receptor on its signal transduction, we synthesized a HIV-Tat conjugated peptide as shown in Fig. 1. The AT1 receptor is known to couple to the G protein Gαq. The activation of phospholipase C (PLC) by AngII occurs through this mechanism. PLC in turn catalyzes the PI turnover reaction. To obtain a robust result the PI turnover in response to AngII was measured in the HEK-293 cells. As shown in Fig. 3 AngII induced a marked PI turnover. This was unhindered in the presence of a control peptide (10 μM), which contains the IC2 of another G protein coupled receptor, bradykinin B2 receptor. On the contrary, the AT1 IC2 mimicking peptide (10 μM) inhibited AngII promoted PI turnover by 94%.
Figure 3. AT1 IC2 peptide blocks the PI turnover in the HEK-293 cells.
Inositol phosphate production was measured in myo-[3H] inositol-labeled cells as described in Materials and Methods. 10 μM AT1 IC2 peptide or control peptide was incubated for 30 minute before the PI turnover assay after 10 nM AngII stimulation. Data represent the average of triplicate samples ± S.D. from a representative experiment of at least three experiments. * p< 0.05 compared with control. ** p< 0.05 compared with AngII induced PI turnover.
Effect of Peptide on Intracellular Calcium Mobilization
As shown in Fig. 4 AngII caused a strong calcium influx in the AT1 transfected HEK-293 cells. The peptide targeted against the AT1 IC2 (10 μM) inhibited the AngII mobilized influx by 81%. The 10 μM control peptide, which did not contain the AT1 IC2 amino acid sequence, had no effect on AngII promoted Ca2+ entry.
Figure 4. Effect of the AT1 peptide on the calcium mobilization.
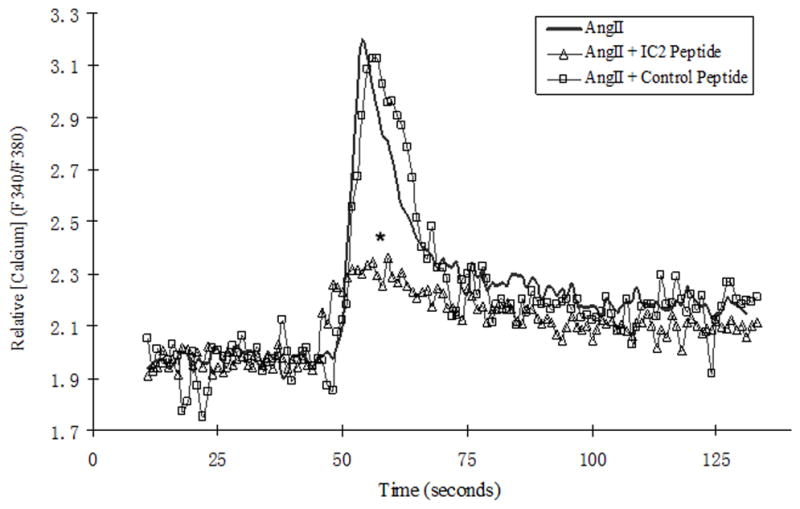
Calcium mobilization in response to 10 nM AngII was measured in AT1 transfected HEK-293 cells as described in Materials and Methods. The results of AngII (10nM) stimulated calcium mobilization in the non-peptide, 10 μM AT1 IC2 peptide and 10 μM control peptide treated cells were drawn as thick lines, thin line with triangle and thin line with square respectively. * p< 0.05 compared with AngII induced calcium mobilization.
Effect of Peptide on the AngII Signaling in Human Pulmonary Artery Smooth Muscle Cells
We proceeded to determine the effect of the AT1 IC2 peptide on AngII signaling through its endogenous AT1 receptor in primary human smooth muscle cells obtained from pulmonary arteries of lung transplants. A sizable activation of Akt/PKB in these cells by AngII is demonstrated in Fig. 5A. Quantization of the western blot shows that the IC2 peptide (10 μM) reduced this phosphorylation by 61%. The control peptide (10 μM) had no effect.
Figure 5. Effect of AT1 peptide in human pulmonary artery smooth muscle cells.
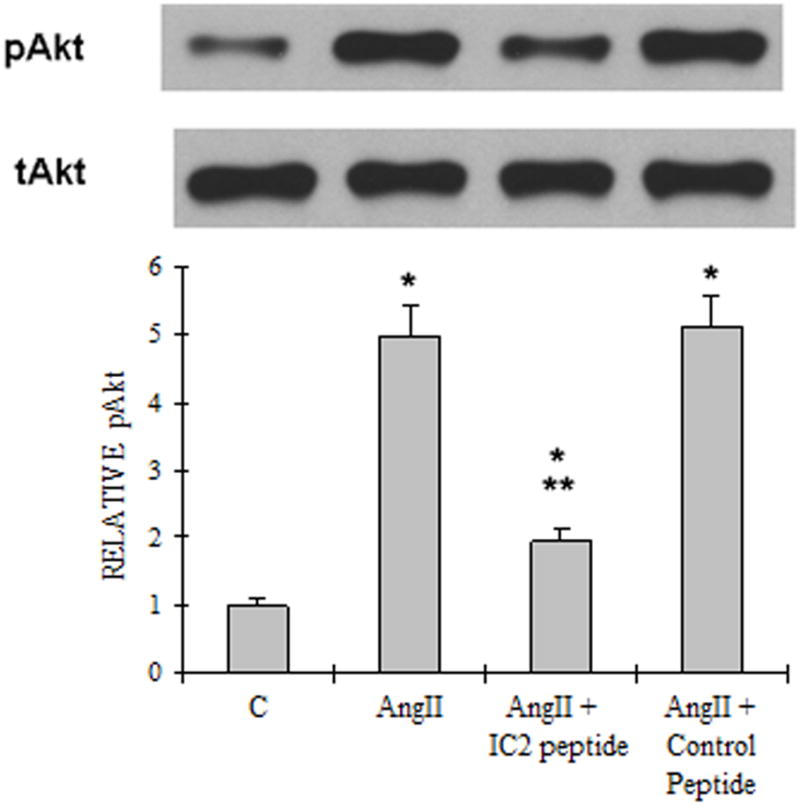
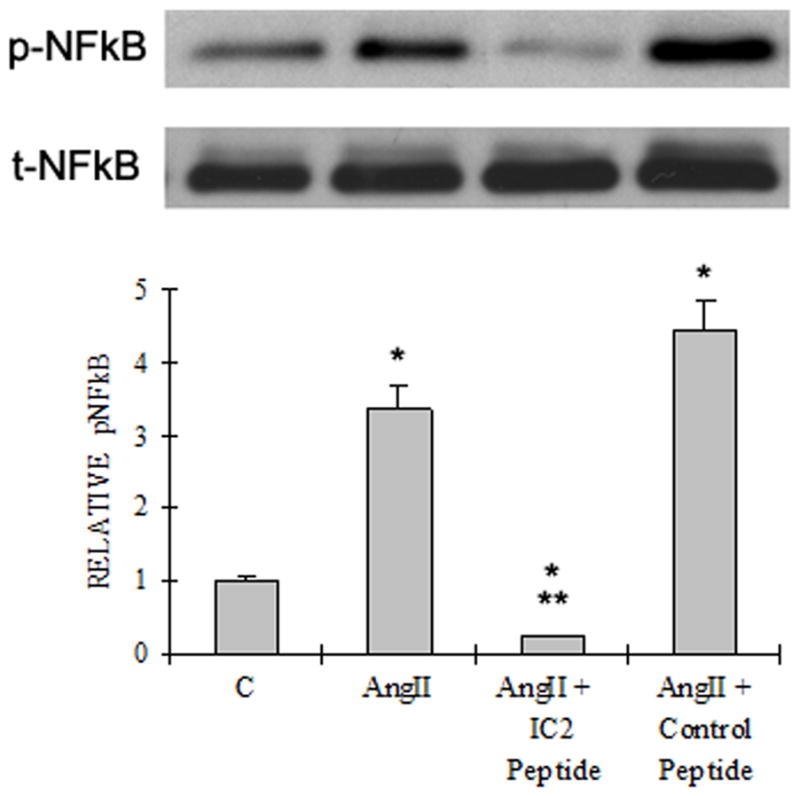
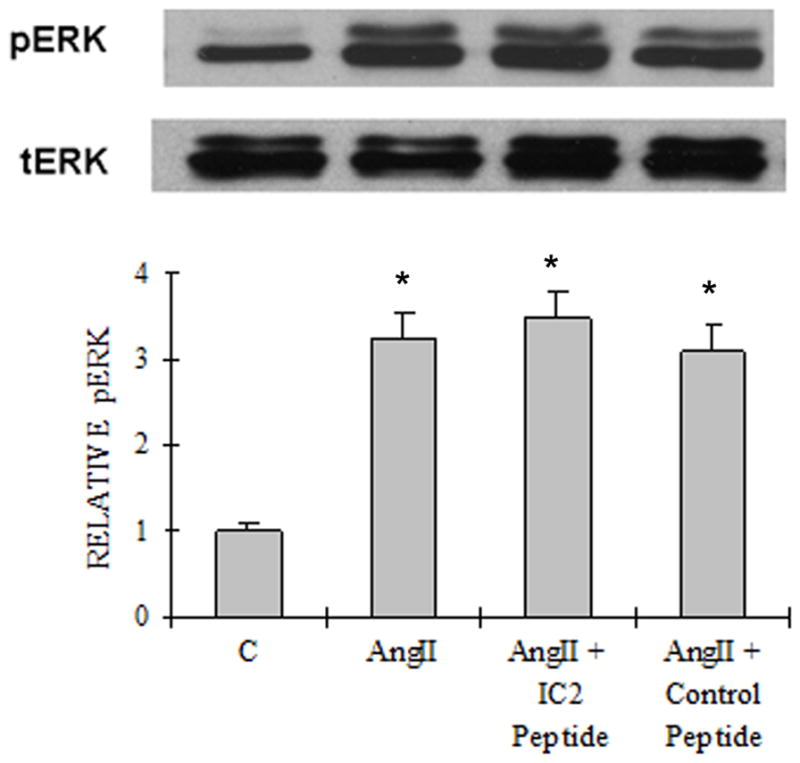
A). Akt/PKB activation Western blot analysis of cell lysates of the PASMCs treated with or without AngII (10 nM), IC2 peptide (10 μM) or control peptide (10 μM). The upper bands are phosphor-Akt (Ser 473) and the lower bands are total Akt. The results are representative of at least three experiments. The quantitation of the western is illustrated as a graph which shows fold change in spot intensity of the bands with basal level as 1. B) NFκB activation The experiment was performed as described in the legend for Figure. 4A except that specific antibodies against NFκB p65 subunit and phospho-NFκB p65 subunit (Ser536) were used. C) ERK1/2 activation. The experiment was performed as described in the legend for Figure. 4A except that specific antibodies against MAPK p42/p44 (ERK1/2) and phospho-MAPK p42/p44 (ERK1/2) (Thr202/Tyr204) were used. (A)–(C) are representative blots from three independent experiments. The bar graph represents the average of the three gels. * p< 0.05 compared with control. ** p< 0.05 compared with AngII stimulated activation.
Phosphorylation of the p65 subunit of nuclear factor κB (NFκB) is an event required for maximal transcriptional activity of NFκB (14). As illustrated, AngII clearly enhanced the Ser536 phosphorylation of p65 (Fig. 5B). This phosphorylation was inhibited approximately 93% by the AT1 IC2 peptide at 10 μM, whereas the control peptide (10 μM) has no inhibitory effect.
With regard to the activation of ERK1/2, we previously showed that alternation within the IC2 of AT1 receptor had no effect on AngII activated ERK phosphorylation (10). Consistent with this observation, neither the control peptide nor the IC2 peptide at 10 μM affected the activation of this MAP kinase by AngII (Fig. 5C).
Discussion
Angiotensin II through its AT1 receptor participates in the etiology of a number of diseases, such as hypertension and fibrotic diseases. Although its overall actions can be regulated by preventing its formation with ACE inhibitors or blocking its overall action with AT1 receptor blockers, the mechanisms of it actions are essentially unknown. A very proficient way to study the signaling by the AT1 receptor is through its signal motif manipulation by mutagenesis. This approach provides information as to the actions and interactions of the motifs but does not provide an opportunity to regulate the action of the wild type receptor. This communication is an initial attempt to make use of cell penetrating peptides to both delineate and regulate the action(s) of the AT1 receptor.
In previous studies we demonstrated that AngII, through the AT1 receptor, increases Akt phosphorylation in AT1 cDNA tranfected HEK-293 cells while bradykinin decreases it in bradykinin B2 receptor cDNA transfected cells (10). In HEK-293 cells transfected with a mutant AT1 receptor containing the IC2 of the BKB2R receptor AngII decreased pAkt (10) thus assigning regulation of Akt phosphorylation. In contrast, these receptor manipulations at the level of the IC2 of the AT1R had no effect on the activation of ERK by the mutant receptor as compared to the WT AT1 receptor. Thus the IC2 of the AT1R is an important signaling motif with respect to both G-protein associated signaling as exemplified by PI turnover and Akt activation, whereas IC2 seems not involved in ERK activation. It has been shown that AngII activates ERK by a Gαq-independent action (15). Studies using carboxyl terminal-truncated AT1 receptors indicate that the amino acid sequence between 312 and 337 is required for the activation of ERK. The YIPP motif within this sequence appears to be the important motif in this action (16). Mutations in the highly conserved D125R126Y127 sequence of the AT1R result in a receptor that is not coupled to Gαq, but still activates ERK (17).
In the past few years there has been a progressive development of membrane-penetrating peptides which has allowed for the delivery of otherwise impermeable bioactive molecules across the plasma membrane. Numerous studies using 10–100μM CPP concentrations have demonstrated that once accumulated in specific compartments (i.e., the cytosol, nucleus and mitochondria) the specific cargo can regulate targeted cellular activities through protein-protein interactions within the intact cells (18). The results obtained here utilizing the AT1-IC2/HIV TAT cell penetrating peptide confirmed our previous observations and illustrated that the introduction of competing peptide into the cytosol mimicking a receptor motif (IC2) can indeed alter receptor signaling ability. In predominant number of reports 10–100 μM CPP concentrations have been used to demonstrate the effect of these peptides. We chose the lowest concentration of this span in order to limit as much as possible any non specific effects of TAT. TAT was shown to rapidly transduce large cargos such as CRE recombinase into primary cells (19). Thus the size of the cargo does not limit the cell penetrating ability of TAT. As shown by the 5-FAM/FACS experiment carried out as part of these studies the TAT peptide clearly crossed into the cytosol of both the HEK-293 and the pulmonary smooth muscle cells. The AT1 IC2 peptide effectively inhibited Gαq related signaling such as PI turnover and Ca2+ influx in HEK-293 cells. In addition, it limited the AngII dependent activation of Akt/PKB and NFκB, but had no effect on ERK activation in HPASMCs. We used the TAT-linked second intracellular loop of the bradykinin B2 receptor as the control peptide. This peptide had no effect on AngII induced signaling actions. Thompson et al showed that cotransfecting second and third loops inhibit AT1 receptor activation of PLC in HEK-293 cells (20). Our peptide approach fits their results. Vazquez et al reported that the synthesized third intracellular loop of the AT1 receptor linked to HIV-TAT was able to be transduced into the hypothalamus and brainstem neurons and increased neuronal firing rate, an effect similar to that observed with angiotensin II stimulation of the neuronal AT1R (21). A more recent study demonstrated that a peptide based on the C-terminus of the AT1 receptor with native cell-penetrating sequences elicits G-protein mediated blood vessel contraction (22), though our IC2 peptide seems evoking an inhibitory effect. Therefore, the activating or inhibiting effects of the intracellular motifs in GPCRs are subtle and complicated. Further structural studies such as molecular modeling or NMR could elucidate the accurate mechanisms of those effects including possible “off target” effects of the peptides.
In summary, our results with cell/tissue penetrating peptides are very encouraging, and are leading us to anticipate that these peptides will contribute to the control of receptor initiated signaling and blood vessel function.
Acknowledgments
This work was supported in part by NIH, NHLBI Grant HL25776.
The smooth muscle cells isolated from the pulmonary arteries obtained from human lung transplants were a generous gift from Dr. Serpil Erzurum (Cleveland Clinic, Cleveland, OH).
References
- 1.Frederic H, May Catherine M, Gilles D. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. British Journal of Pharmacology. 2009;157(2):195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends in Cell Biology. 2000;10(7):290. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- 3.Murriel CL, Dowdy SF. Influence of protein transduction domains on intracellular delivery of macromolecules. Expert Opinion on Drug Delivery. 2006;3(6):739–46. doi: 10.1517/17425247.3.6.739. [DOI] [PubMed] [Google Scholar]
- 4.Jasmin J-F, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-Term Administration of a Cell-Permeable Caveolin-1 Peptide Prevents the Development of Monocrotaline-Induced Pulmonary Hypertension and Right Ventricular Hypertrophy. Circulation. 2006;114(9):912–20. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 5.Zhao K, Luo G, Zhao G-M, Schiller PW, Szeto HH. Transcellular Transport of a Highly Polar 3+ Net Charge Opioid Tetrapeptide. J Pharmacol Exp Ther. 2003;304(1):425–32. doi: 10.1124/jpet.102.040147. [DOI] [PubMed] [Google Scholar]
- 6.Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha J-HJ, et al. Phosphorylation-Dependent Trafficking of GluR2-Containing AMPA Receptors in the Nucleus Accumbens Plays a Critical Role in the Reinstatement of Cocaine Seeking. J Neurosci. 2008;28(43):11061–70. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin JE, Frank BC, Gaspard RM, Duka I, Gavras H, Quackenbush J. Cardiac transcriptional response to acute and chronic angiotensin II treatments. Physiol Genomics. 2004;18(2):152–66. doi: 10.1152/physiolgenomics.00057.2004. [DOI] [PubMed] [Google Scholar]
- 8.Duprez DA. Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: a clinical review. J Hypertens. 2006;24(6):983–91. doi: 10.1097/01.hjh.0000226182.60321.69. [DOI] [PubMed] [Google Scholar]
- 9.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292(1):C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Lubinsky D, Tsomaia N, Huang Z, Taylor L, Mierke D, et al. Activation of ERK, JNK, Akt, and G-protein coupled signaling by hybrid angiotensin II AT1/bradykinin B2 receptors expressed in HEK-293 cells. J Cell Biochem. 2007;101(1):192–204. doi: 10.1002/jcb.21161. [DOI] [PubMed] [Google Scholar]
- 11.Mann DA, Frankel AD. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991;10(7):1733–9. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prado GN, Taylor L, Polgar P. Effects of intracellular tyrosine residue mutation and carboxyl terminus truncation on signal transduction and internalization of the rat bradykinin B2 receptor. J Biol Chem. 1997;272(23):14638–42. doi: 10.1074/jbc.272.23.14638. [DOI] [PubMed] [Google Scholar]
- 13.Prado GN, Mierke DF, Pellegrini M, Taylor L, Polgar P. Motif mutation of bradykinin B2 receptor second intracellular loop and proximal C terminus is critical for signal transduction, internalization, and resensitization. J Biol Chem. 1998;273(50):33548–55. doi: 10.1074/jbc.273.50.33548. [DOI] [PubMed] [Google Scholar]
- 14.Strassheim D, Asehnoune K, Park J-S, Kim J-Y, He Q, Richter D, et al. Phosphoinositide 3-Kinase and Akt Occupy Central Roles in Inflammatory Responses of Toll-Like Receptor 2-Stimulated Neutrophils. J Immunol. 2004;172(9):5727–33. doi: 10.4049/jimmunol.172.9.5727. [DOI] [PubMed] [Google Scholar]
- 15.Miura S-i, Zhang J, Matsuo Y, Saku KS, Karnik S. Activation of Extracellular Signal-Activated Kinase by Angiotensin II-Induced Gq-Independent Epidermal Growth Factor Receptor Transactivation. Hypertension Research. 2004;27(10):765–70. doi: 10.1291/hypres.27.765. [DOI] [PubMed] [Google Scholar]
- 16.Seta K, Sadoshima J. Phosphorylation of tyrosine 319 of the angiotensin II type 1 receptor mediates angiotensin II-induced trans-activation of the epidermal growth factor receptor. J Biol Chem. 2003;278(11):9019–26. doi: 10.1074/jbc.M208017200. [DOI] [PubMed] [Google Scholar]
- 17.Seta K, Nanamori M, Modrall JG, Neubig RR, Sadoshima J. AT1 receptor mutant lacking heterotrimeric G protein coupling activates the Src-Ras-ERK pathway without nuclear translocation of ERKs. Journal of Biological Chemistry. 2002;277(11):9268–77. doi: 10.1074/jbc.M109221200. [DOI] [PubMed] [Google Scholar]
- 18.Costa-Junior HM, Suetsugu MJ, Krieger JE, Schechtman D. Specific modulation of protein kinase activity via small peptides. Regulatory Peptides. 2009;153(1–3):11–8. doi: 10.1016/j.regpep.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Peitz M, Pfannkuche K, Rajewsky K, Edenhofer F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: A tool for efficient genetic engineering of mammalian genomes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(7):4489–94. doi: 10.1073/pnas.032068699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson JB, Wade SM, Harrison JK, Salafranca MN, Neubig RR. Cotransfection of Second and Third Intracellular Loop Fragments Inhibit Angiotensin AT1a Receptor Activation of Phospholipase C in HEK-293 Cells. Journal of Pharmacology and Experimental Therapeutics. 1998;285(1):216–22. [PubMed] [Google Scholar]
- 21.Vazquez J, Sun C, Du J, Fuentes L, Sumners C, Raizada MK. Transduction of a Functional Domain of the AT1 Receptor in Neurons by HIV-Tat PTD. Hypertension. 2003;41(3):751–6. doi: 10.1161/01.HYP.0000047878.13793.41. [DOI] [PubMed] [Google Scholar]
- 22.Östlund P, Kilk K, Lindgren M, Hällbrink M, Jiang Y, Budihna M, et al. Cell-Penetrating Mimics of Agonist-Activated G-Protein Coupled Receptors. International Journal of Peptide Research and Therapeutics. 2005;11(4):237. [Google Scholar]