Abstract
Purpose
The anti-cancer effect of carnosol was investigated in human prostate cancer PC3 cells.
Methods
Biochemical analysis and protein array data of carnosol treated PC3 cells were analyzed.
Results
We evaluated carnosol for its potential anti-cancer properties in the PC3 cells. Using an MTT assay we found that carnosol (10 – 70 µM) decreases cell viability in a time and dose dependent manner. Next, we evaluated the effect of carnosol (20–60 uM) effect using flow cytometry as well as biochemical analysis and found induction of G2-phase cell cycle arrest. To establish a more precise mechanism, we performed a protein array that evaluated 638 proteins involved in cell signaling pathways. The protein array identified 5'-AMP-activated protein kinase (AMPK), a serine/threonine protein kinase involved in the regulation of cellular energy balance as a potential target. Further downstream effects consistent with cancer inhibition included the modulation of the mTOR/HSP70S6k/4E-BP1 pathway. Additionally, we found that carnosol targeted the PI3K/Akt pathway in a dose dependent manner.
Conclusions
These results suggest that carnosol targets multiple signaling pathways that include the AMPK pathway. The ability of carnosol to inhibit prostate cancer in vitro suggests carnosol may be a novel agent for the management of PCa.
Keywords: carnosol, prostate cancer, chemoprevention, rosemary, AMPK, mTOR, Akt, PC3
INTRODUCTION
It was estimated in 2007 that 218,890 new cases of prostate cancer (PCa) will be diagnosed with 27,000 deaths expected.(1) Unfortunately there are limited options for the successful management of this disease. Prostate cancer is generally a slow growing process with well defined stages of initiation, promotion, and progression occurring over many years beginning with clinically visible cancers and progressing to metastatic cancers. The development of PCa is often sporadic with <10% inherited, suggesting that multiple genetic factors as well as environmental insults dictate disease development and progression of PCa.(2) Most notably the major cause of mortality of PCa is metastasis of hormone refractory cancer cells that fail to respond to androgen ablation pharmacotherapy that include bicalutamide, flutamide, and niluatmide.(3) Further overwhelming evidence is suggesting that normal homeostatic control mechanisms are being evaded in prostate cancer that deregulate the fine balance between cell proliferation and cell death.
Chemoprevention is the use of natural or synthetic agents to block or delay the process of carcinogenesis in order to prevent or delay the development of invasive cancer. On a practical note we define chemoprevention as slowing the process of carcinogenesis. Typically PCa is diagnosed in men over the age of 50 suggesting that even a minimal delay in PCa development could greatly reduce the incidence of clinically detectable disease making it an ideal candidate for chemoprevention. In addition, it is becoming increasingly appreciated that cancer chemoprevention through the use of naturally occurring dietary agents may be an effective strategy for the management of PCa. (2, 4, 5). Consistent with the idea of chemoprevention there has been a growing increase to use dietary supplements by PCa patients to alleviate disease.
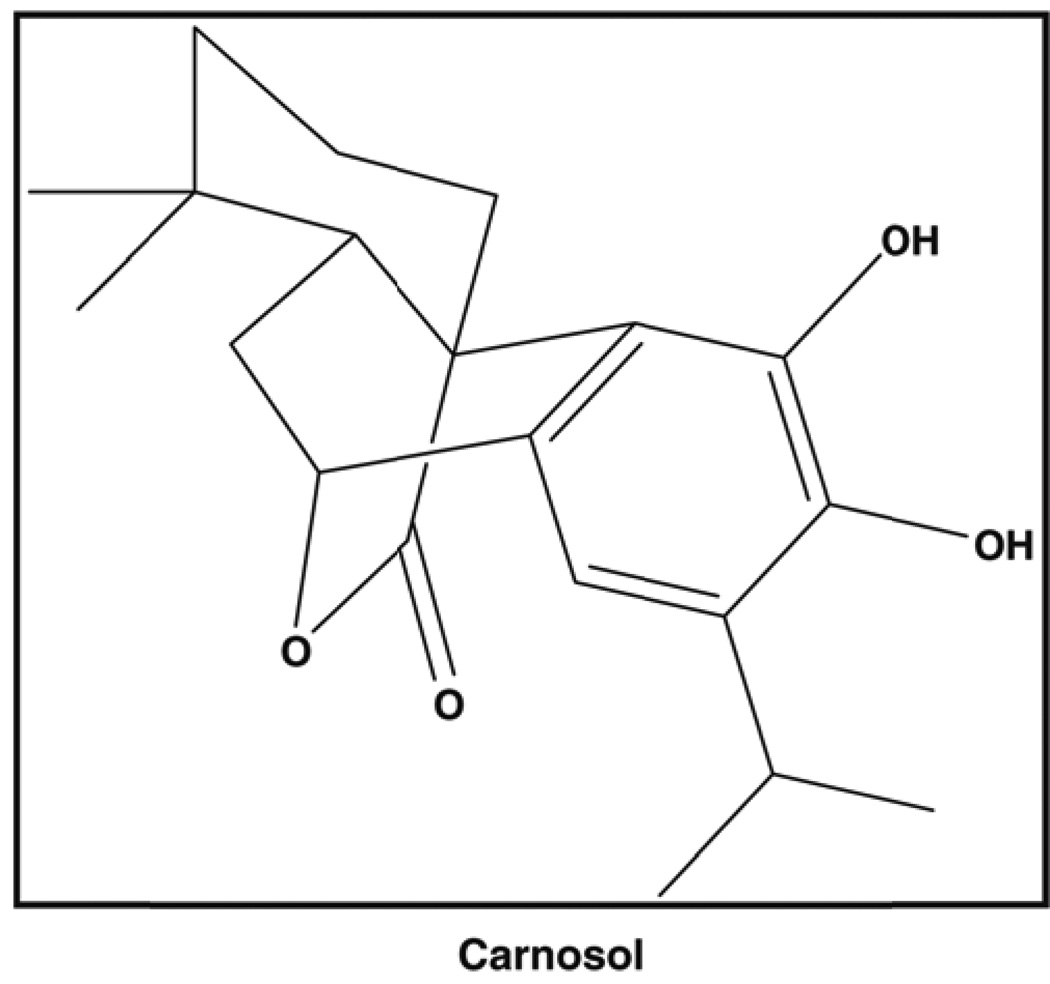
Carnosol (Figure 1) is a dietary diterpene isolated from culinary herbs that include rosemary, sage, and oregano and has been noted for its potent antioxidant activity, however, carnosol has received little attention for its cancer chemoprevention properties.(6) Approximately 5% of the dry weight of rosemary leaves is comprised of the antioxidant phenolic diterpenese carnosol and carnosic acid.(7) Carnosol and carnosic acid have been shown to be responsible for 90% of the antioxidant activity found in rosemary.(6, 8) In traditional Chinese medicine rosemary extracts are given orally that contain high amounts of diterpenes and triterpenes have been used to treat inflammatory conditions. Additionally, a variety of dietary supplements of rosemary extracts are available that have been standardized to carnosol and/or carnosic acid. An in vivo study performed evaluated the antimutagenic activity of rosemary and carnosol was associated with a significant decrease, 74% and 65%, respectively, in the number of DMBA-induced mammary adenocarcinomas when compared to controls.(9) Another in vivo study showed that dietary carnosol (0.1%) decreased APC associated adenoma formation by 46% in the C57BL/6J/Min/+ (Min/+) mouse when compared to controls (10). Recently, in vitro work has been performed and it has been shown that carnosol induces G2/M cell cycle arrest that targets cyclin A and cyclin B1 with an IC50 of 23 µM in Caco-2 colon adenocarcinoma cells which are representative of precancerous lesions.(11) Additional in vitro work has shown that carnosol significantly inhibited the highly metastatic mouse melanoma B16 cells through down regulation of matrix metalloproteinase 2, c-jun, as well as the redox sensitive transcription factor nuclear factor-kappa B (Nf-κB).
Figure 1.
Carnosol is found in a variety of culinary herbs that include basil, rosemary, sage, and oregano.
We hypothesized that carnosol may provide chemopreventive as well as chemotherapeutic effects against PCa. In this study, we first demonstrated a decrease in cell viability against the human PCa PC3 cell line treated with carnosol. We observed a decreased cell viability that we found to target the G2 phase of the cell cycle as well as the cyclins and cyclin dependent kinases (cdks). To further understand the modulation of multiple signaling pathways by carnosol we analyzed a protein array specific for 638 proteins that identified the AMP-activated protein kinase that is targeted by carnosol in human PC3 cells.
MATERIALS AND METHODS
Cell culture
The androgen insensitive human prostate cancer cells PC3 obtained from the American Type Culture Collection (ATCC, Manassa, VA) were used. Cells were maintained in RPMI 1640 media (ATCC) supplemented with 10% FBS (ATCC) and 1% penicillin-streptomycin (Hyclone, Logan, Utah) in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Cell Viability
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For cell viability assays cells were counted and plated in a 24 well tissue culture plate and used for the following treatment times 24 hrs (4×104 cells/ml), 48 hrs (3×104 cells/ml) and 72 hrs (2x104 cells/ml). After 24 hours of growth the complete media was removed and replaced with media containing carnosol with the appropriate assay concentrations for the MTT assay (10 – 70 µmol/L). The final concentration of DMSO was 0.07%. Each test dose was performed in triplicate on each 24 well plate. After incubation for the appropriate time intervals cell viability was determined. MTT reagent was prepared by dissolving 5 mg/mL in PBS and then diluted with complete media to a final volume of 10 mL. MTT reagent was added to each well (300 µL) and allowed to incubate for 2–4 hours, after which the MTT was removed and DMSO (300 µL) was added to each well and shaken for 15 minutes. Each sample was transferred to a 96 well microtiter plate in duplicate (i.e. 100 µl each) and the absorbance was recorded at 540 nm. The effect of carnosol on growth inhibition was assessed as percent cell viability where vehicle-treated cells (i.e. control) were taken as 100% viable.
Cell cycle and apoptosis analysis
The APO-DIRECT™ kit (Phoenix Flow Systems, San Diego CA) was used for measuring apoptosis and cell cycle of treated cells by flow cytometry. Briefly, PC3 cells plated to ~50% confluence and were serum depleted for 12 hours to promote synchronization and then were treated with complete media containing carnosol (20 – 60 µM) for 24 and 48 hrs respectively. The kit was followed per protocol directions.
Protein array
The evaluation of multiple signaling pathways was characterized by the Kinex Antibody Microarray service (Kinexus, Vancouver, BC, Canada). The antibody microarray service assays over 600 different cell signaling proteins in duplicate for more than 250 different phosphor-sites, 240 protein kinases, and over 100 other cell signaling proteins in the treated (carnosol) and untreated samples (vehicle). PC3 cells were grown to ~50% confluence and treated with vehicle (DMSO) and 40 µM carnosol for 48 hours. Following the treatment, cell lysates were prepared per the manufacturer’s protocol (Kinexus). Briefly, cells were washed twice with ice-cold phophsphate buffered saline (PBS) and homogenized with a buffer containing 20 mM MOPS (pH 7.0), 2 mM EGTA, 5 mM EDTA, 30 mM sodium fluoride, 60 mM b-glycerophosphate (pH 7.2), 1 mM sodium orthovanadate, 20 mM sodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, 3 mM benzamidine, 5 µM pepstatin A, 10 µM leupeptin, 0.5% Triton X-100, and 1 mM dithiothreitol. Cells were lysed by scraping homogenate and passing homogenate through 22g syringe 8 times. Homogenate was then transferred to a 1.5 mL centrifuge tube and spun for 30 minutes at 14,000 RPM in a 4° microcentrifuge. Supernatant fractions were removed and total cell protein was estimated using the Bradford Assay (Pierce, Rockford, Illinois). Samples were then assayed by the Kinex antibody microarray services at Kinexus.
Western Blot
After 48 hours of cell growth the complete media was removed and replaced with media containing carnosol with the appropriate assay concentrations for the whole cell lysates (20 – 60 µmol/L). The final concentration of DMSO was 0.06% (whole cell lysates). Cells were lysed as previously described above and centrifuged at 14,000×g for 25 minutes, and the supernatant fraction was used for immunoblotting. Proteins were resolved on a 12% tris glycine gel and transferred onto a nitrocellulose membrane. After blocking with 5% nonfat dry milk in blocking solution the membrane was incubated with the desired primary antibody for 2 hours at room temperature. The membrane was then incubated with the appropriate peroxidase-conjugated secondary antibody and the immunoreactive bands were visualized using the SuperSignal® West Pico Chemiluminescent Substrate (Pierce, Rockford, Illinois) kit. To ensure equal protein loading, each membrane was stripped and reprobed with β-actin antibody to normalize for differences in protein loading.
RESULTS
Effect of carnosol on cell viability of PC3 cells
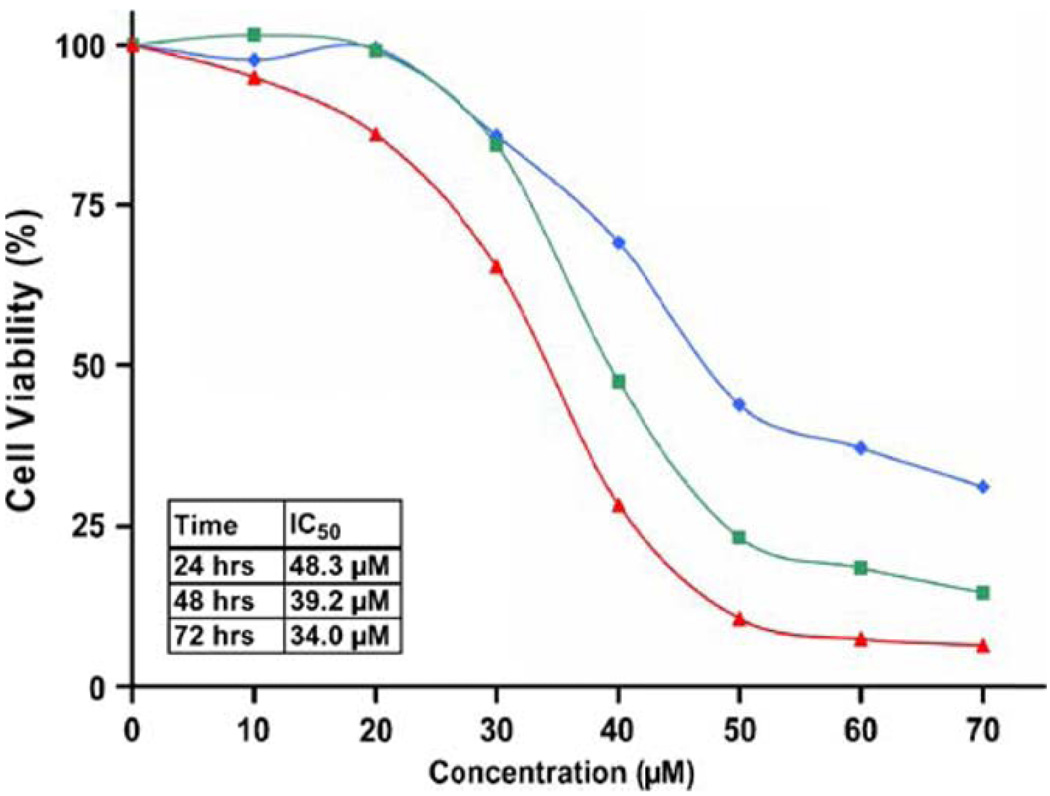
PC3 cells were treated for 24, 48, and 72 hours displaying a dose and time dependent decrease in cell viability (Figure 2). The IC50 was estimated to be 48.3 µmol/L, 39.2 µmol/L, and 34 µmol/L at 24, 48 and 72 hours respectively. These observations led to the selection of doses for further mechanistic studies that ranged from 20 to 60 µmol/L over 48 hours. A dose dependent effect of carnosol on cellular morphology was observed by phase contrast microscopy and can be visualized in Figure 3A.
Figure 2.
Effect of carnosol on androgen insensitive prostate cancer cell line PC3. Cells were treated for 24 (◆), 48 (■) and 72 (▲) hours and cell viability was determined by MTT assay. Each concentration was repeated in three wells using a 24 well plate. Percent viable cells, where control (i.e. vehicle) were considered 100% viabile.
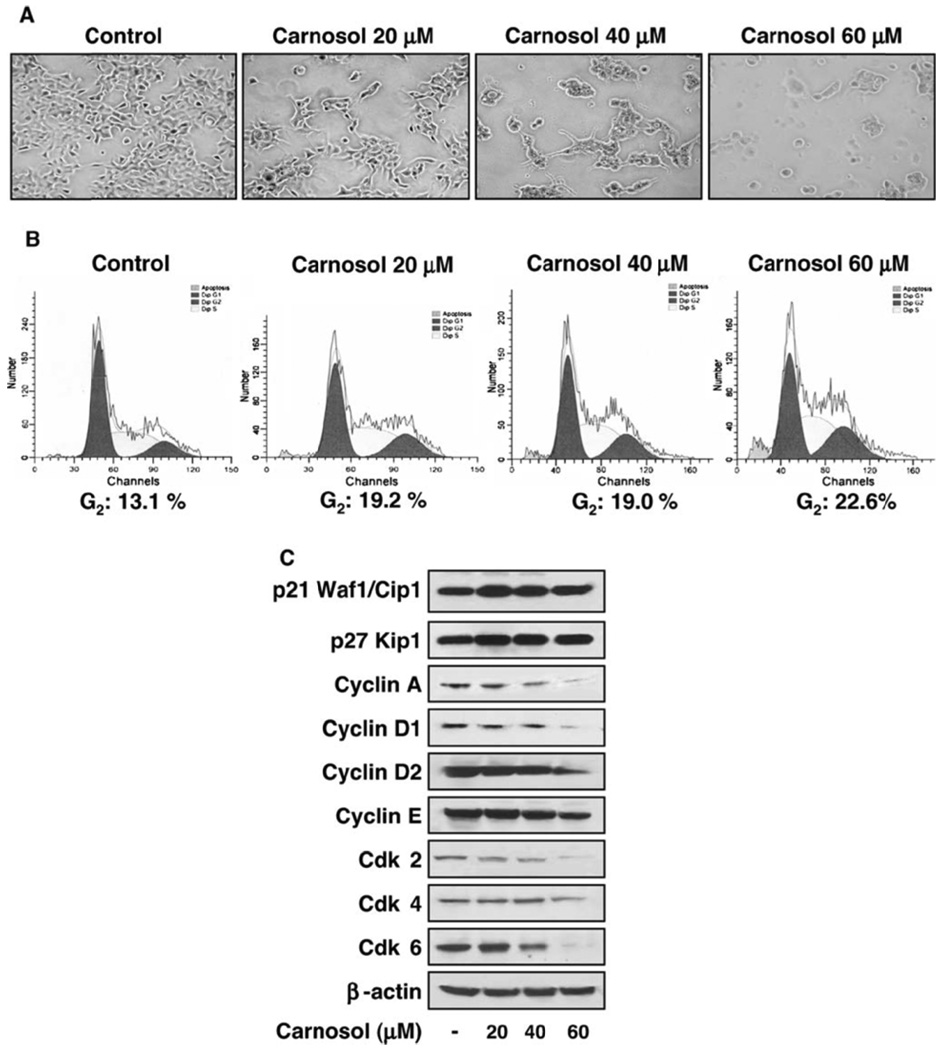
Figure 3.
(A) Effect of carnosol on cellular morphology in PC3 cells. Cells were grown to approximately 50% confluence and treated with vehicle (control) and carnosol (20 – 60 µM) for 48 hours using phase contrast microscopy. (B) Effect of carnosol on cell cycle regulation leading to G2 cell cycle arrest in PC3 cells. Cells were treated with carnosol for 48 hours, collected, and labeled using the APODIRECT kit per vendor’s protocol as detailed in materials and methods. (C) Carnosol displays a dose dependent effect on cell cycle regulatory proteins in PC3 cells. As detailed in materials and methods, whole cell lysates were prepared and the protein was subjected to SDS-PAGE followed by immunoblot anlaysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin.
Carnosol induces cell cycle arrest in PC3 cells
The treatment of PC3 cells with carnosol for 48 hours resulted in a significantly higher number of cells in G2 phase at the concentrations used. As summarized in Figure 3B, after 48 hours an even higher number of cells in G1 phase was observed at the following concentrations 20 µmol/L (19.22 %), 40 µmol/L (18.98 %), and 60 µmol/L (22.63 %) compared to control (13.1 %). These results suggest that an inhibition in cell viability in androgen insensitive prostate cancer PC3 cells by carnosol may be associated with an induction of G2 arrest. The regulation of cell cycle is critical in the growth and development of cancer explaining why cell cycle arrest is targeted as an intervention against cancer.
Carnosol induces p21 (Waf1/Cip1) leading to decreased cyclin and cdk activity in PC3 cells
As our study demonstrated that carnosol promotes G2 cell cycle arrest in PC3 cells we wanted to understand the role of carnosol on cell regulatory molecules. We assessed the effect of carnosol on the induction of p21 (Waf1/Cip1) that is known to block cell cycle induction and found a marked increase in a dose dependent manner compared to control levels (Figure 3C). Another member of the Cip/Kip family of cyclin dependent kinase inhibitors that was evaluated was p27 (Kip1). We found a dose dependent increase in p27 protein expression when PC3 cells were treated with carnosol. To further understand the role of G2 cell cycle arrest we analyzed proteins regulated by p21 (Waf1/Cip1) that included the cyclins A, D1, D2, and E as well as the cdks (cyclin dependent kinases) 2, 4, and 6 by immunoblot (Figure 3C). Carnosol treatment led to a dose dependent decrease in cyclins A, D1, D2, as well as a decrease in protein expression with cdks 2 and 6 was observed.
Carnosol affects the expression of mitochondria-dependent apoptotic proteins
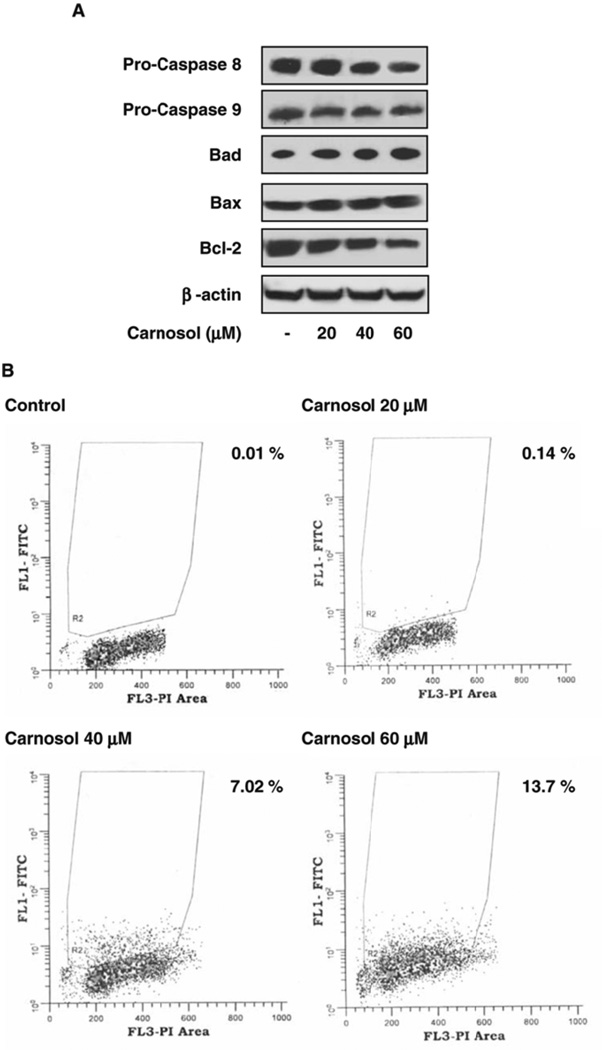
To determine if apoptosis was occurring we analyzed immunoblots for the expression of mitochondriadependent apoptotic proteins. As shown in Figure 6A we found that carnosol resulted in a modest decrease of pro-caspase 8 at 40 µmol/L and 60 µmol/L with no effect on pro-caspase 9. Additionally, we found a dose dependent increase of the pro-apoptotic protein Bax as well as a dose dependent decrease of the anti-apoptotic protein Bcl-2 (Figure 4A).
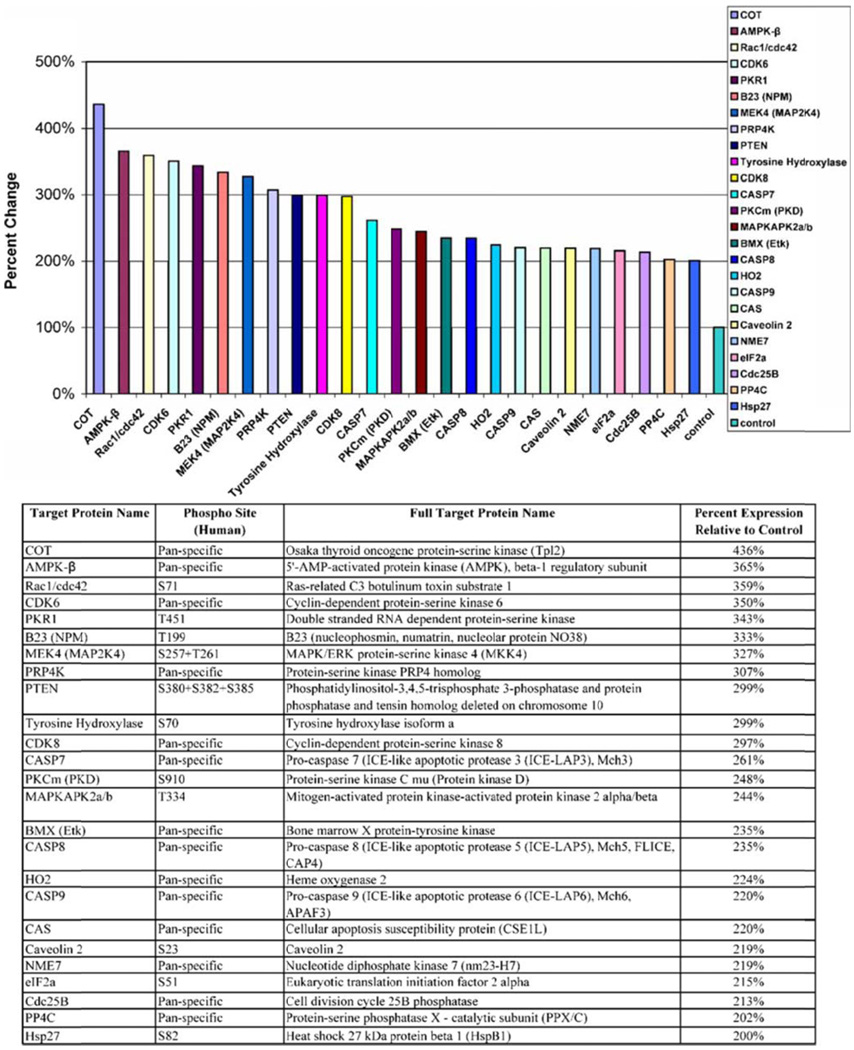
Figure 6.
PC3 cells were treated with carnosol (40 µM) and a protein array was analyzed for proteins that are up regulated at least 200% compared to control.
Figure 4.
(A) Effect of carnosol on mitochondrial dependent apoptotic proteins in PC3 cells. As detailed in materials and methods, whole cell lysates were prepared and the protein was subjected to SDS-PAGE followed by immunoblot anlaysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. (B) PC3 cells treated with carnosol were labeled as described in materials and methods and apoptosis was quantified by flow cytometry.
To determine if cell cycle deregulation leading to apoptosis was occurring when PC3 cells were treated with carnosol we quantified the extent of apoptosis by flow cytometry over a 48 hour period. As shown in Figure 4B, we we observed apoptosis in a dose dependent manner with 0.01 %, 0.14 %, 7.02 %, and 13.7 % at 0 µmol/L, 20 µmol/L, 40 µmol/L and 60 µmol/L respectively. A modest degree of apoptosis was observed suggesting that other multiple signaling pathways may be involved in carnosol induced cell death.
AMPK signaling in human prostate cancer PC3 cells identified via an antibody array-based proteomics approach
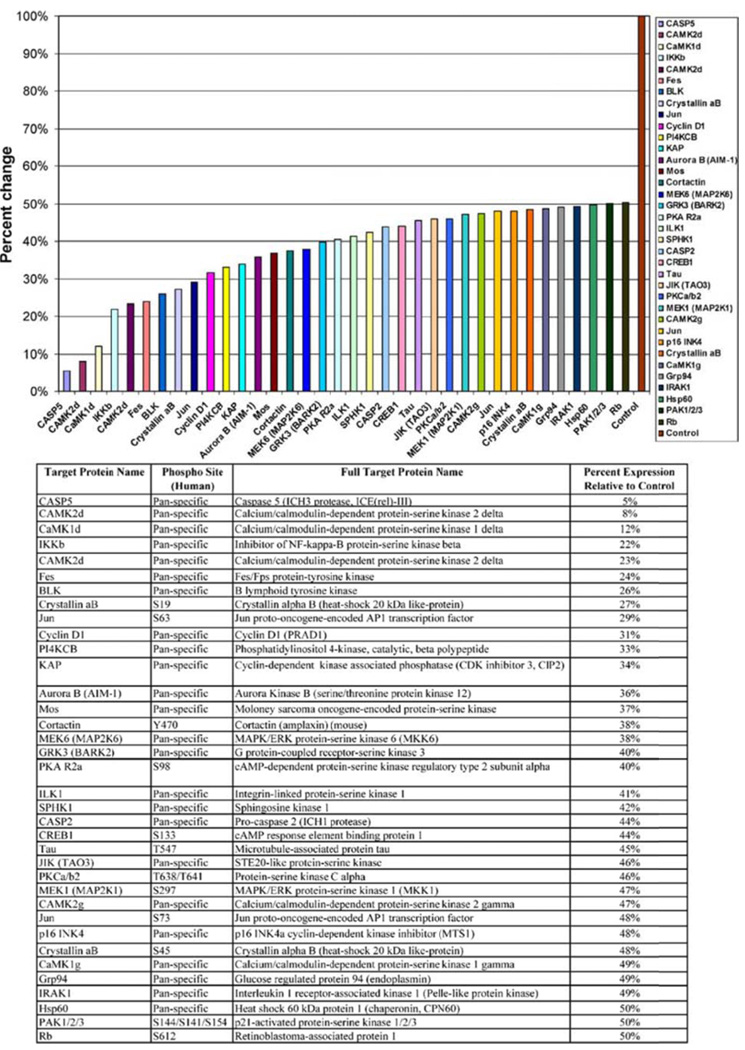
To further understand the multiple signaling pathways that are targeted with carnosol we performed a antibody array to identify target proteins of interest. To focus on proteins of interest we chose the concentration of 40 µmol/L over 48 hours for the treatment of PC3 cells which is near the IC50 of carnosol treated PC3 cells. The protein array identified 36 proteins that were down regulated at least 50% (Figure 4). In addition, there were 24 proteins that were upregulated at least 200% (Figure 5). The protein array identified that AMPK beta-1 regulatory subunit was upregulated 365% compared to control. Recently several articles have suggested that the 5’-AMP-activated protein kinase (AMPK) regulates the growth and/or survival of cancer cells suggesting that targeting AMPK may be beneficial in the treatment of cancer. (12) Further studies performed focused on understanding the role of carnosol on the AMPK pathway.
Figure 5.
PC3 cells were treated with carnosol (40 µM) and a protein array was analyzed for proteins that are down regulated at least 50% compared to control.
Carnosol targets the AMPK pathway
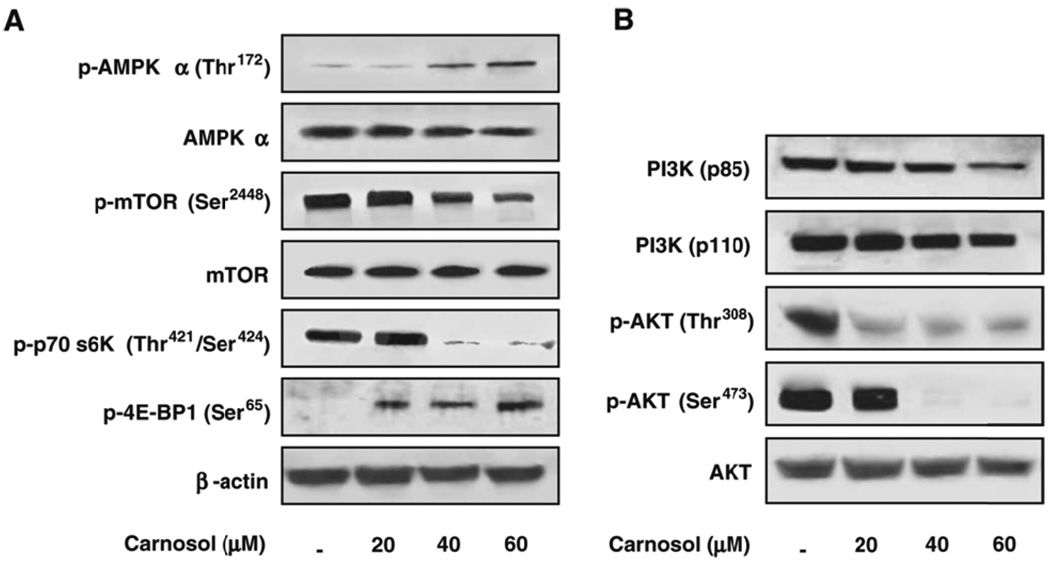
Earlier we described how we analyzed a protein array to identify a 365% upregulation of the AMPK beta-1 regulatory subunit when PC3 cells were treated with 40 µmol/L of carnosol. AMPK is a heterotrimeric complex that consists of a catalytic α subunit and regulatory β and γ subunits that are being evaluated as a drug target for cancer. Therefore, we determined the effect of carnosol on the phosphorylated forms of AMPK-α (Thr172). We found that a dose dependent decrease in the phosphorylated form of AMPK-α (Thr172) as shown in Figure 7A. AMPK has been shown by ourselves as well as others to be an upstream regulator of the mammalian target of rapamycin (mTOR). To understand the downstream effects of AMPK modulation by carnosol we evaluated the mammalian target of rapamycin (mTOR) which is upregulated in PCa. Immunoblot analysis showed a dose dependent decrease in the phosphorylation of mTOR (Ser2448) that can be seen in Figure 7A. Since mTOR activation stimulates the phosphorylation of other down stream kinases, we next determined the effect of carnosol on p70 S6K and 4E-BP1 expression. We observed a dose dependent decrease in p70 S6K and a dose dependent increase in the expression of the translator repressor protein 4E-BP1 as shown by immunoblot anlaysis (Figure 7A).
Figure 7.
(A) Effect of Carnosol on phosphorylation of AMPK/mTOR/p70 S6K pathway. As detailed in materials and methods, whole cell lysates were prepared and the protein was subjected to SDS-PAGE followed by immunoblot anlaysis and chemiluminescence detection.
(B) Next we evaluated the effects of carnosol on the PI3K/Akt pathway. As detailed in materials and methods, whole cell lysates were prepared and the protein was subjected to SDS-PAGE followed by immunoblot anlaysis and chemiluminescence detection.
Carnosol inhibits the PI3K/Akt pathway
The PI3K/Akt pathway has an important role in a various cellular process, that include cell growth, survival, and motility. Additionally, Akt has been shown to phosphorylate tuberous sclerosis complex (TSC2) at sites Ser-939 and Thr-1462 leading to the inhibition of TSC2 and promoting cell growth. Analysis of our protein array found that PTEN, a PI3K/AKT inhibitor, was upregulated 299 % compared to control (Figure 6). Therefore, we determined the effect of carnosol on the PI3K and the phosphorylated forms of Akt (i.e. Thr-308 and Ser-473). We found a dose dependent decrease in expression of PI3K (p85) and PI3K (p110). Additionally, carnosol was found to significantly inhibit the phosphorylation of Akt at both sites (i.e. Thr308 and Ser473) as shown by a dramatic decrease in protein expression (Figure 7B). We found these results to be consistent with data that were shown previously when B16 mouse melanoma cells were treated with carnosol.(11).
DISCUSSION
Prostate cancer is the most common cancer among men in the USA and represents the second leading cause of death in all males.(1) The initial strategy for treatment of prostate cancer (PCa) is through androgen blockade and is heavily dependent on the stage of disease (i.e. Gleason Score), symptoms, and life expectancy of the patient. Depending on the staging clinical guidelines often recommend more invasive strategies such as radiotherapy or radical prostatectomy as initial interventions. Patients and providers may often be reluctant to initiate these interventions. As a result, pharmacologic therapies have been developed to inhibit androgen activity such as LH-RH agonists (leuprolide), anti-androgens (flutamide, bicalutamide, and nilutamide), and even estrogens. Each of these interventions has a unique array of adverse effects that are often viewed as irritating and annoying by patients and can lead to non-compliance and limits their usefulness. Therefore, it is essential that further agents be developed for their use in PCa. Chemoprevention by the use of naturally occurring dietary substances is an attractive option in prostate cancer due to its prevalence in men over the age of fifty, and long latency between pre-malignant lesions and clinically evident cancer.(2) An intensive effort to develop agents with high therapeutic potential coupled with less systemic toxicity is being searched for worldwide. The development of agents that display chemopreventive properties for the treatment of cancer is gaining interest and popularity because it may have the potential to greatly improve the quality of life of prostate cancer patients. We evaluated the growth inhibitory effects of carnosol on human PCa cells and found a dose and time dependent inhibitory effect in PC3 cells. In this study, we provide evidence that carnosol, a diet based agent, can inhibit the human PCa cell line PC3. Several proteins that promote cell cycle blockade as well as apoptosis were shown to be modulated by carnosol in PC3 cells, however, flow cytometric analysis determined that cell death was through a unique mechanism. We propose that carnosol induced cell death of PC3 cells is through the AMPK pathway.
Recent studies have suggested that activated AMP protein kinase (AMPK) acts as a regulator to several signaling pathways and should be evaluated as a therapeutic target for cancer (Figure 8). (12, 13) AMPK is a heterotrimeric complex of a catalytic α, and regulatory β and γ subunits and is found in all eukaryotes.(14) Activated AMPK occurs when conditions deplete cellular ATP and elevate AMP levels such as glucose deprivation, hypoxia, ischemia and heat shock which have been associated with an increased AMP/ATP ratio.(15) When AMPK is activated it phosphorylates a variety of metabolic enzymes involved in energy dependent events including synthesis of fatty acids, cholesterol, and protein.
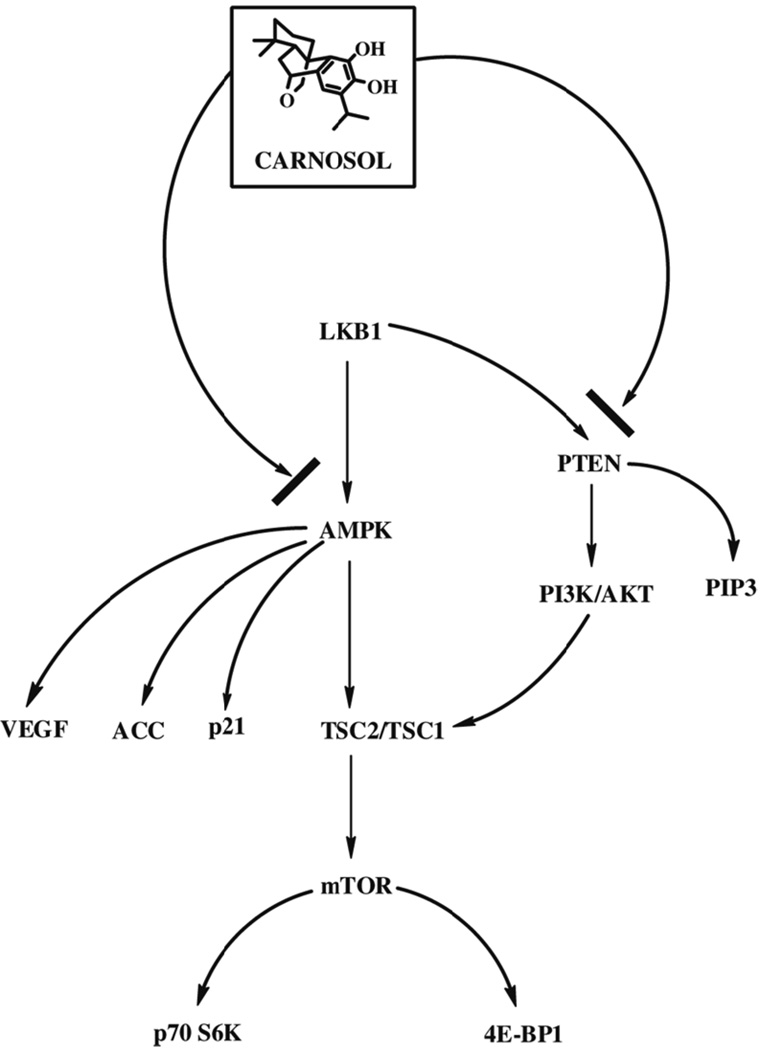
Figure 8.
Proposed mechanism of carnosol in human prostate cancer PC3 cell line.
One of the more studied cancer pathways in regard to AMPK modulation is the mTOR signaling pathway. When sufficient nutrients are available mTOR responds to a phosphatidic acid mediated signal leading to activation of p70 S6 kinase and inactivates the the eIF4E inhibitor, 4E-BP1.(16) Agents such as the AMP-mimetic 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) have been shown to activate AMPK by energy starvation leading to phosphorylation of TSC2 at Thr-1277 and Ser-1345.(13, 17) Phosphorylation at these sites increases the activity of the TSC1-TSC2 complex to inhibit mTOR.(12, 18). In the current study we confirmed the results of a protein array and found that carnosol induced the AMPK-α subunit. We found that AMPK-α activation by carnosol inhibits the phosphorylation of mTOR at Ser-2448 leading to cancer inhibition. We also observed a decreased expression of p70 S6K as well as in induction of the eIF4E inhibitor, 4E-BP1.
AMPK activation by the AMP mimetic AICAR has been shown to cause G1 cell cycle arrest through the p53-p21 axis in hepatoma HepG2 cells.(19) In this study we found that carnosol promoted G2 cell cycle arrest in the PC3 cell line and was independent of p53 status since the PC3 cell line does not carry the p53 tumor suppressor gene. The induction of cyclin dependent kinase inhibitors p21 as well as p27 was observed along with dose dependent inhibition of cyclins D1, D2, and E along with cdks 2 and 6. It is interesting that flow cytometric analysis found that limited apoptosis occurred in PC3 cells, however, proteins involved in apoptosis were found to be modulated suggesting that other cancer cell lines may display apoptosis when treated with carnosol.
Akt is activated by various growth and survival factors and promotes cell survival by inhibiting apoptosis through several targets that include Bad, TSC2, and the mTOR pathway.(12, 20–23) When Akt is activated it phosphorylates TSC2 at phosphorylation sites Ser-939 and Thr-1462 that leads to the inhibition of TSC2 thereby activating the mTOR pathway.(12, 23) We observed a significant dose dependent decrease in the activated forms of Akt at phosphorylation sites Thr-308 and Ser-473 as well as an inhibition of PI3K (p85) and PI3K (p110).
Conclusions
Taken together, our findings show that carnosol when used in vitro has anti-cancer activity with a mechanistic rationale in the androgen insensitive human prostate cancer PC3 cell line. Carnosol promoted G2 cell cycle arrest with inhibition of cyclins A, D1, D2 and cdks 2 and 6. Evaluation of a protein array identified the AMPK pathway as a target of carnosol. Activation of the AMPK pathway resulted in downstream effects that are consistent with cancer inhibition. These observations warrant further in vitro studies as well as in vivo oral delivery studies in an animal model that mimics human prostate cancer. Future studies will help estimate the potential of carnosol as a cancer chemoprevention and/or chemotherapeutic agent for the prevention of human PCa.
Acknowledgements
This work was supported by a Clinical and Translational Science Award (CTSA) training grant (J.Johnson) through the Institute for Clinical and Translation Research (ICTR) at the University of Wisconsin. (NIH 1KL2RR025012-01 and K12 RR023268)
Abbreviations
- AICAR
(5-aminoimidazole-4-carboxamide ribonucleoside)
- AMPK
(5'-AMP-activated protein kinase)
- cdks
(cyclin dependent kinases)
- DMSO
(dimethyl sulfoxide)
- FBS
(fetal bovine serum)
- LH-RH
(luteinizing hormone-releasing hormone)
- mTOR
(mammalian target of rapamycin)
- PBS
(phophsphate buffered saline)
- PCa
(prostate cancer)
- PI3K
(phosphatidylinositol 3-kinase)
- PTEN
(phosphatase and tensin homologue deleted on chromosome ten)
- TSC2
(tuberous sclerosis complex 2)
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of Prostate Cancer through Dietary Agents: Progress and Promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 3.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Johnsonand JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Kakizoe T. Chemoprevention of cancer--focusing on clinical trials. Jpn J Clin Oncol. 2003;33:421–442. doi: 10.1093/jjco/hyg090. [DOI] [PubMed] [Google Scholar]
- 6.Aruoma OI, Spencer JP, Rossi R, Aeschbach R, Khan A, Mahmood N, Munoz A, Murcia A, Butler J, Halliwell B. An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provencal herbs. Food Chem Toxicol. 1996;34:449–456. doi: 10.1016/0278-6915(96)00004-x. [DOI] [PubMed] [Google Scholar]
- 7.Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, Conney AH. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–708. [PubMed] [Google Scholar]
- 8.Aruoma OI, Halliwell B, Aeschbach R, Loligers J. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- 9.Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBADNA adduct formation. Cancer Lett. 1996;104:43–48. doi: 10.1016/0304-3835(96)04227-9. [DOI] [PubMed] [Google Scholar]
- 10.Moran AE, Carothers AM, Weyant MJ, Redston M, Bertagnolli MM. Carnosol inhibits beta-catenin tyrosine phosphorylation and prevents adenoma formation in the C57BL/6J/Min/+ (Min/+) mouse. Cancer Res. 2005;65:1097–1104. [PubMed] [Google Scholar]
- 11.Visanji JM, Thompson DG, Padfield PJ. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006;237:130–136. doi: 10.1016/j.canlet.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 12.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 14.Hardieand DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 15.Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 16.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 17.Guan TJ, Qin FJ, Du JH, Geng L, Zhang YY, Li M. AICAR inhibits proliferation and induced S-phase arrest, and promotes apoptosis in CaSki cells. Acta Pharmacol Sin. 2007;28:1984–1990. doi: 10.1111/j.1745-7254.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 18.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 19.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5'-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 20.Khan N, Afaq F, Kweon MH, Kim K, Mukhtar H. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007;67:3475–3482. doi: 10.1158/0008-5472.CAN-06-3941. [DOI] [PubMed] [Google Scholar]
- 21.Xiaoand D, Singh SV. Diallyl trisulfide, a constituent of processed garlic, inactivates Akt to trigger mitochondrial translocation of BAD and caspase-mediated apoptosis in human prostate cancer cells. Carcinogenesis. 2006;27:533–540. doi: 10.1093/carcin/bgi228. [DOI] [PubMed] [Google Scholar]
- 22.Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427–431. [PMC free article] [PubMed] [Google Scholar]
- 23.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]