Abstract
Accumulation of reactive oxygen species (ROS) in cells damages resident proteins, lipids, and DNA. In order to overcome the oxidative stress that occurs with ROS accumulation, cells must balance free radical production with an increase in the level of antioxidant enzymes that convert free radicals to less harmful species. We identified two antioxidant enzymes, thioredoxin (Trx) and Trx reductase (TrxR), in a complex associated with the DNA-bound estrogen receptor α (ERα). Western analysis and immunocytochemistry were used to demonstrate that Trx and TrxR are expressed in the cytoplasm and in the nuclei of MCF-7 human breast cancer cells. More importantly, endogenously expressed ERα, Trx, and TrxR interact and ERα and TrxR associate with the native, estrogen-responsive pS2 and progesterone receptor genes in MCF-7 cells. RNA interference assays demonstrated that Trx and TrxR differentially influence estrogen-responsive gene expression and that together, 17β-estradiol, Trx, and TrxR alter hydrogen peroxide (H2O2) levels in MCF-7 cells. Our findings suggest that Trx and TrxR are multifunctional proteins that, in addition to modulating H2O2 levels and transcription factor activity, aid ERα in regulating the expression of estrogen-responsive genes in target cells.
Introduction
Eukaryotic cells consume oxygen and produce reactive oxygen species (ROS) as by-products of normal cellular metabolism (Powell et al. 2005). ROS include a number of chemically reactive oxygen derivatives including superoxide and hydrogen peroxide (H2O2), which are less reactive, and hydroxyl radical, which is highly reactive. The initial product of oxygen metabolism, superoxide, is dismutated to H2O2 in cells by superoxide dismutase (SOD). The H2O2 is then converted to H2O and O2 by catalase, glutathione peroxidase, and peroxiredoxins, which include thioredoxin (Trx) peroxidases (Beckman et al. 1990, Webster et al. 2001, Yoshida et al. 2003, Smart et al. 2004, Hashemy et al. 2006).
ROS are needed to serve as molecular messengers in cell-signaling pathways and in the immune system to target pathogens (Lehnert & Iyer 2002, Feinendegen 2005, Goldstein et al. 2005). At low concentrations, superoxide and H2O2 are effective stimulators of cell growth (Burdon 1995). However, if H2O2 is not effectively eliminated, hydroxyl radicals can accumulate and damage proteins, lipids, and DNA (Halliwell & Gutteridge 1985, Storz et al. 1990). Increased ROS accumulation has also been linked to tumorigenesis and age-related diseases (Kirkwood & Austad 2000, Toussaint et al. 2002) as well as decreased cell survival (Salganik 2001). Thus, oxygen radicals have beneficial as well as detrimental effects.
In order to avoid ROS accumulation and its damaging effects, cells express a battery of oxidative stress response proteins that dissipate oxygen radicals (Halliwell & Gutteridge 1985, Storz et al. 1990). Trx is an oxidative stress response protein that activates i) transcription factors in order to alter gene expression and ii) peroxiredoxins, so that cellular H2O2 can be diminished (Webster et al. 2001, Arner & Holmgren 2006). Like the proteins that it reduces, Trx itself must be reduced in order to activate other proteins. Trx reductase (TrxR) utilizes NADPH to reduce and activate Trx as well as other proteins (Mustacich & Powis 2000). By maintaining protein thiols in the reduced state, Trx and TrxR help to maintain a reduced cellular environment and active transcription factors (Holmgren 1979, 1985, Das et al. 1997).
Zinc finger proteins are particularly susceptible to oxidation. Oxidation of zinc finger proteins diminishes the ability of these proteins to interact with their target DNA sequences and ultimately alters gene expression. Oxidation of two nuclear receptor superfamily members, the glucocorticoid receptor and estrogen receptor α (ERα), diminishes the ability of these proteins to bind to DNA (Makino et al. 1996, Hayashi et al. 1997).
ERα binds to hormone, dimerizes, interacts directly with its recognition sequences in DNA, estrogen-response elements (EREs), and recruits coregulatory proteins that influence estrogen-responsive gene expression. Because we had previously shown that three other oxidative stress proteins, SOD1, protein disulfide isomerase (PDI), and apurinic/apyrimidinic endonuclease 1 or redox factor-1 (Ape1/Ref-1) influence ERα-mediated gene expression (Schultz-Norton et al. 2006, Rao et al. 2008, Curtis et al. 2009), we were intrigued by the identification of Trx and TrxR in a complex of proteins associated with the DNA-bound ERα (Schultz-Norton et al. 2008, 2009) and were interested in determining whether Trx and TrxR might also influence ERα-mediated gene expression. We now show that Trx and TrxR alter estrogen-responsive gene expression and that together, 17β-estradiol (E2), Trx, and TrxR modulate H2O2 levels in MCF-7 human breast cancer cells.
Materials and methods
Cell culture
MCF-7 human breast cancer cells, which express ERα, were maintained in phenol red-containing minimum essential medium (MEM, Invitrogen) with 1× non-essential amino acids (NEAA, Invitrogen), 20 mM HEPES, and antibiotics (penicillin–streptomycin and gentamicin) with 5% calf serum. Cells were switched to phenol red-free MEM with 5% charcoal/dextrantreated calf serum (CDCS, Eckert & Katzenellenbogen 1982), NEAA, and antibiotics for 1–3 days before experiments were initiated. MDA-MB-231 human breast cancer cells, which do not express ERα, were maintained in Leibovitz’s L-15 medium (Invitrogen) with the same additives as used for MCF-7 cells. U2 osteosarcoma (U2OS) cells were maintained in MEM with 15% heatinactivated FCS and with the same additives as used for MCF-7 cells. HeLa cervical cancer cells were maintained in DMEM /Nutrient Mixture F-12 Ham with 5% heatinactivated FCS and penicillin–streptomycin.
Isolation and identification of Trx and TrxR
Trx and TrxR were isolated as proteins associated with the ERE-bound ERα using agarose gel shift assays and identified using mass spectrometry analysis as previously described (Schultz-Norton et al. 2008, 2009). Three peptides that contained amino acid sequences unique to Trx1 (TAFQEALDAAGDKLVVVDF-SATWCGPCK, PFFHSLSEK, and EKLEATINELV) and 11 peptides that contained amino acid sequence identical to TrxR1 (VMVLDFVTPTPLGTRWGLGGTCVNVGCIPKKLMHQAALLGQALQDSR, MIEAVQNHIGSLNWGYR, KVVYENAYGQFIGPHR, FLIATGERPR, IGEHMEEHGIK, QFVPIKVEQIEAGTPGR, VVAQSTNSEEIIEGEYNTVMLAIGR, IPVTDEEQTNVPYIYAIGDILEDKVELTPVAIQAGR, FGEENIEVYHSYFWPLEWTIPSR, VVGFHVLGPNAGEVTQGFAAALK, and QLDSTIGIHPVCAEVFTTLSVTK) were identified. Together, these peptides account for 45.7 and 49.7% of the total Trx and TrxR amino acid sequences respectively.
Western-blot experiments
Nuclear extracts from human breast (MCF-7 and MDA-MB-231), bone (U2OS), and cervical (HeLa) cancer cells were prepared as described (Wood et al. 2001). Ten micrograms of nuclear extract were fractionated on 10–18% SDS-polyacrylamide gels, and transferred to a nitrocellulose membrane, which was probed with a Trx-, TrxR-, ERα- (sc-20146, sc-28321, sc-8002 respectively, Santa Cruz Biotechnologies, Santa Cruz, CA, USA), PR-A- and PR-B- (RM-9102-S1, Lab Vision, Fremont, CA, USA), or GAPDH- (TAB1001, Open Biosystems, Huntsville, AL, USA) specific antibody and a HRP-conjugated secondary antibody. Proteins were detected by the SuperSignal West Femto Maximum Sensitivity Substrate chemiluminescent system (Pierce, Rockford, IL, USA).
Immunocytochemistry
MCF-7 cells were plated onto coverslips in six-well plates containing phenol red-free MEM with NEAA, antibiotics, and 5% CDCS. Three wells were treated with ethanol vehicle or with 10 nM E2. After 24 h, cells were washed with PBS, fixed in PBS with 4% formaldehyde for 10 min, washed with PBS, permeabilized with PBS containing 0.1% Triton X-100 for 20 min, and washed with PBS containing 0.1% Tween 20 (PBST). Samples were blocked with PBST containing 2% BSA and 2% fetal bovine serum for 30 min, incubated with a Trx- or TrxR-specific antibody (sc-20146 and sc-28321 respectively, Santa Cruz Biotechnologies) for 1 h in a humidified chamber, washed with PBST, incubated with donkey anti-rabbit biotin-SP-conjugated antibody (Trx, 711-066-152) or donkey anti-mouse biotin-SP-conjugated antibody (TrxR, 715-066-150, Jackson Immuno-Research, West Grove, PA, USA) for 30 min, washed with PBST, incubated with DyLight 549-conjugated Streptavidin (016-500-084, Jackson ImmunoResearch) for 30 min in the dark, and washed with PBST. Primary antibodies were omitted in control slides and run in parallel to demonstrate the specificity of the Trx and TrxR antibodies. Samples were mounted with Vectashield (Vector Laboratories Inc., Burlingame, CA, USA) and visualized with a 40X objective using a Leica DM4000 B confocal microscope (Leica Microsystems, Inc., Bannockburn, IL, USA) with the Leica TCS SPE system and Application Suite Advanced Fluorescence software. Three fields were examined in three independent experiments so that nine fields were examined for each treatment.
Immunoprecipitation assays
MCF-7 cells were treated with ethanol or 10 nM E2 for 0.75 h, washed with PBS, harvested in 20 mM Tris pH 7.4, 10 mM EDTA, 100 mM NaCl, 0.5% NP-40, 1 mM Na3VO4, 50 mM NaF, and 1× protease inhibitor cocktail (PIC, Sigma), and then pelleted at 20 800 g at 4 °C for 10 min. The protein concentration of each supernatant was determined using the Bio-Rad protein assay (Bio-Rad) with BSA as a standard. One microgram of Trx- or TrxR-specific antibody (sc-18215 or sc-31057 respectively, Santa Cruz Biotechnologies) or a control antibody directed against fluorescein (Immunological Resource Center, University of Illinois, Urbana, IL, USA) was incubated with 500 µg of extract overnight at 4 °C with rotation, incubated with 60 µl of a 50% Protein G Sepharose slurry for 1 h (GE Healthcare, Piscataway, NJ, USA), and centrifuged at 960 g at 4 °C for 2 min. Samples were washed thrice with 20 mM Tris pH 7.4, 10 mM EDTA, 100 mM NaCl, 0.1% NP-40, 1 mM Na3VO4, 50 mM NaF, and 1× PIC before fractionation on SDS-polyacrylamide gels and western analysis with an ERα-specific antibody (sc-543, Santa Cruz Biotechnologies).
Chromatin immunoprecipitation assays
MCF-7 cells were treated with ethanol or 10 nM E2 for 0.75 or 24 h and incubated with 1% formaldehyde. Cells were processed essentially as described (Curtis et al. 2007). Antibodies directed against ERα or TrxR (sc-8002 and sc-31057 respectively, Santa Cruz Biotechnologies) were used for chromatin immunoprecipitation (ChIP). Primers specific to the ERE-containing region of the pS2 gene or two regions of the progesterone receptor (PR) gene (JL Boney-Montoya, YS Ziegler, CD Curtis, JA Montoya & AM Nardulli, unpublished observations), located 205 kb (PR205) or 221 kb (PR221) upstream of the PR-B transcription start site, were used for real-time PCR with iQ SYBR Green Supermix (Bio-Rad) and the iCycler PCR thermocycler. Standard curves were generated using 1000, 5000, 10 000, 50 000, and 100 000 copies of each gene for each primer set in each experiment and run in parallel.
RNA interference experiments
MCF-7 cells were transferred to phenol red-free MEM with NEAA and antibiotics with 5% CDCS 1 day prior to plating. Cells were seeded in 12-well plates 24 h before transfection with siLentFect (Bio-Rad) and transferrin (Sigma). We combined 50 pmol small interfering RNA (siRNA) directed against Trx (117158 or 111300), TrxR (111302 or 41855), or control Renilla luciferase (4630, Ambion, Austin, TX, USA) with 500 µl phenol red-free medium and incubated this with the cells for 24 h. Cells were then incubated in phenol red-free medium containing ethanol or 10 nM E2 for 24 h. For protein analysis, cells were harvested with TNE (10 mM Tris, pH 7.5, 150 mM NaCl, and 1 mM EDTA), lysed in lysis buffer (20 mM Tris pH 8, 1 mM EDTA, 200 mM NaCl, and 0.2% NP-40), and subjected to western blot analysis with a Trx-, TrxR- (sc-20146 and sc-28321, Santa Cruz Biotechnologies), PR-A- and PR-B- (RM-9102-S1, Lab Vision), or GAPDH- (TAB1001, Open Biosystems) specific antibody. For RNA analysis, cells were resuspended in TRIzol (Invitrogen) and processed according to the manufacturer’s instructions. cDNA was prepared using the Reverse Transcription System (Promega), and real-time PCR was performed using iQ SYBR Green Supermix and the iCycler PCR thermocycler (Bio-Rad) with primers specific to Trx (5′-CTTTCTTTCATTCCCTCT TG-3′ and 5′-GCATTTGACTTCACA CTCTG-3′), TrxR (5′-TGGAATTGGTGCTTGTG-3′ and 5-′TATCTCTTGAC-GGAATCG-3′), pS2, PR, cyclin G2, cyclin D1, Bcl2, and 36B4 (Curtis et al. 2007, Creekmore et al. 2008). Standard curves were derived using cDNA equivalents of 0.02, 0.2, 2, and 20 ng of RNA and were run in duplicate with each primer set in each experiment.
H2O2 quantitation
MCF-7 cells were treated with control, Trx or TrxR siRNA, and ethanol or E2 as described for siRNA experiments. Cells were harvested and centrifuged at 960 g for 5 min at 4 °C. The pelleted cells were resuspended in lysis buffer and spun at 20 800 g for 5 min at 4 °C. The supernatants were transferred to a 96-well plate and Amplex Red (Invitrogen), which interacts with H2O2 to produce the red fluorescent oxidation product resorufin, was used to determine the level of endogenous H2O2 (Invitrogen). To derive a standard curve, duplicate samples, which included 250, 500, 750, and 1000 nM of H2O2, were run in parallel. All samples were analyzed with a fluorescence plate reader using 544 nm for excitation and 590 nm for fluorescence detection. Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad) and used to normalize each sample for any changes in protein levels that might occur in response to E2.
Statistical analysis
SAS 9.1 Basic Statistics (SAS Institute, Cary, NC, USA) was used for statistical analysis. One-way ANOVA was used to determine whether there were differences between control and experimental groups. A P-value ≤0.05 was considered to be statistically significant.
Results
Trx and TrxR are present in cytoplasmic and nuclear compartments
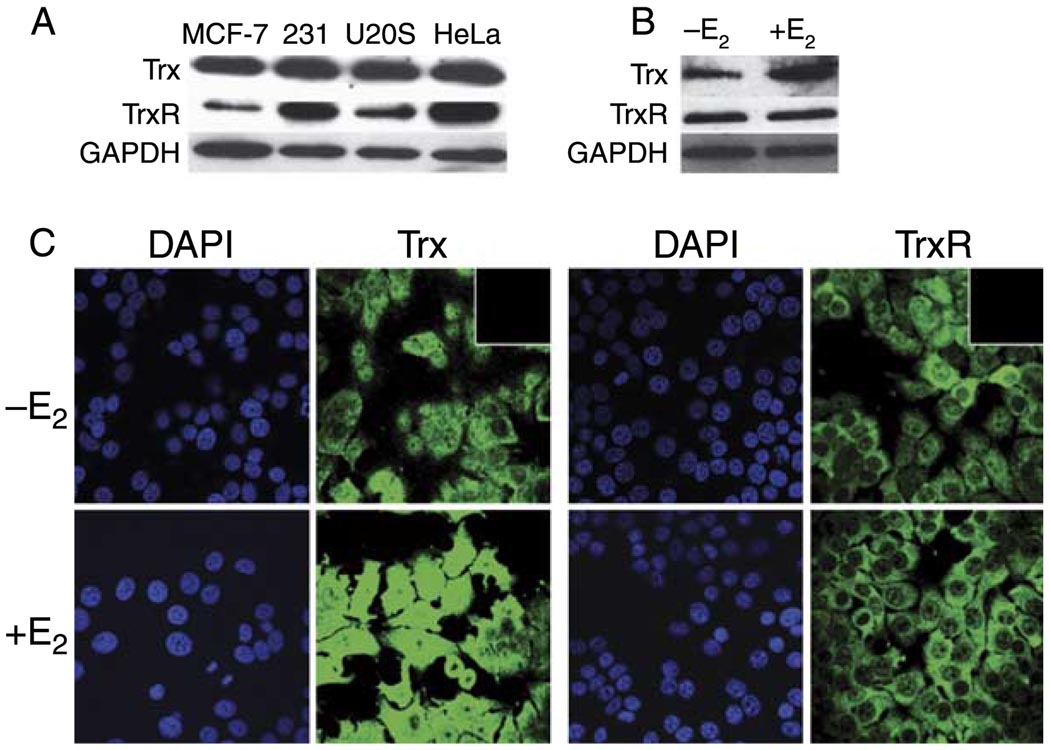
We originally identified Trx and TrxR as proteins associated with the ERE-bound ERα (Schultz-Norton et al. 2008, 2009). Although HeLa nuclear extracts had been utilized in these initial experiments, we examined the expression of these two proteins in various cultured cell lines that have been used to study estrogen responsiveness (Druege et al. 1986, Green et al. 1986, Greene et al. 1986, Katzenellenbogen et al. 1987). Western blot analysis with Trx- and TrxR-specific antibodies demonstrated that both proteins are expressed in ERα-positive (MCF-7) and ERα-negative (MDA-MB-231) human breast cancer cells, U2OS, and, as expected, HeLa cervical cancer cells (Fig. 1A). Interestingly, when MCF-7 cells were treated with 10 nM E2 for 24 h, the expression of Trx, but not TrxR, was enhanced (Fig. 1B). A GAPDH-specific antibody was used to demonstrate that similar amounts of protein were loaded in each lane.
Figure 1.
Trx and TrxR expression and localization. (A) The levels of Trx and TrxR were examined in nuclear extracts (10 µg) from human ERα-positive breast (MCF-7) and ERα-negative breast (MDA-MB-231), U2 osteosarcoma (U2OS), and cervical (HeLa) cancer cells. (B) Trx and TrxR expression was examined in MCF-7 cells that had been treated with ethanol (−E2) or 10 nM E2 (+E2) for 24 h. Samples were fractionated by SDS-PAGE and subjected to western analysis with a Trx- or TrxR-specific antibody. GAPDH levels were monitored to demonstrate that similar amounts of sample were loaded in each lane. (C) MCF-7 cells were treated with ethanol (−E2) or 10 nM E2 (+E2) for 24 h. Expression of Trx and TrxR was monitored using immunocyto-chemistry with Trx- and TrxR-specific antibodies. DAPI counter-staining was used to detect cell nuclei. The insert in the upper right hand corner of the −E2 images demonstrated that when the primary antibody was omitted, the secondary antibodies produced no signal. Full colour version of this figure available via http://dx.doi.org/10.1677/JME-09-0053.
ERα resides in the nuclei of MCF-7 cells (Schultz-Norton et al. 2006). Although Trx and TrxR have been localized in the nuclei of cultured cells, they have more often been described as cytoplasmic proteins (Arner & Holmgren 2000, Nordberg & Arner 2001, Yoshida et al. 2003). To determine whether Trx and TrxR were present in the nuclear compartment of MCF-7 cells where they might be able to interact with ERa and influence gene expression, immunocytochemistry was performed. As seen in Fig. 1C, Trx and TrxR were expressed in the cytoplasm, but were also present in the nuclei of MCF-7 cells. Exposure of MCF-7 cells to E2 for 24 h dramatically increased Trx expression. The increase in Trx expression might be expected since it has been reported that ERa is associated with the Trx gene in MCF-7 cells (Carroll et al. 2005). No changes were observed in TrxR expression or the localization of Trx and TrxR with hormone treatment. Thus, although previous studies have reported the ability of ionizing radiation, nitric oxide, or oxidative stress to induce translocation of Trx to the nucleus (Hirota et al. 1999, Wei et al. 2000, Arai et al. 2006), E2 did not alter the localization of Trx or TrxR in MCF-7 cells. These findings are consistent with those reported in the Human Protein Atlas (www.proteinatlas.org).
Endogenously expressed Trx, TrxR, and ERα interact
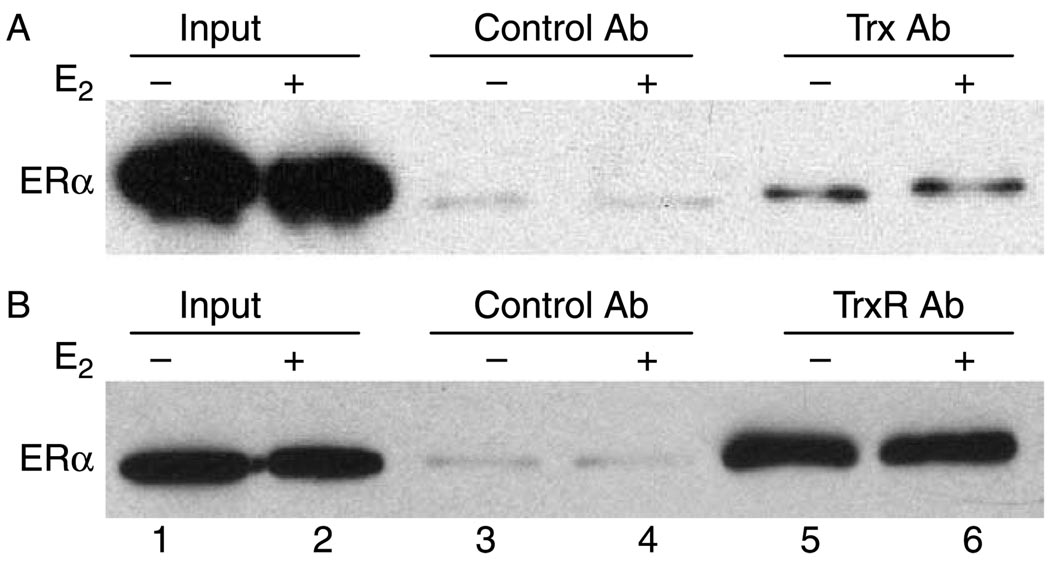
Because Trx and TrxR were originally isolated in a large complex associated with the DNA-bound ERα using HeLa nuclear extracts (Schultz-Norton et al. 2008, 2009), we determined whether endogenously expressed Trx, TrxR, and ERα from MCF-7 cells could interact. Trx- and TrxR-specific antibodies were used to immunoprecipitate the proteins from MCF-7 extracts, and then western analysis was performed with an ERα-specific antibody. ERa was immunoprecipitated with Trx- (Fig. 2A, lanes 5 and 6) and TrxR- (Fig. 2B, lanes 5 and 6) specific antibodies. In contrast, a control antibody directed against fluorescein was unable to immunoprecipitate ERα regardless of whether cells had or had not been exposed to E2 (lanes 3 and 4). These studies demonstrate that endogenously expressed Trx and TrxR associate with ERα in the absence and in the presence of hormone.
Figure 2.
Endogenously expressed Trx, TrxR, and ERα interact. MCF-7 cells were treated with ethanol (−E2) or 10 nM E2 (+E2) for 0.75 h and lysed. Cell extracts were incubated with (A) Trx- or (B) TrxR- specific antibody. Specifically bound proteins were eluted and subjected to western analysis with an ERα-specific antibody. MCF-7 extracts (10% input) were included in each experiment for comparison. Data shown are representative of three (A) or six (B) independent experiments.
TrxR associates with endogenous estrogen-responsive genes
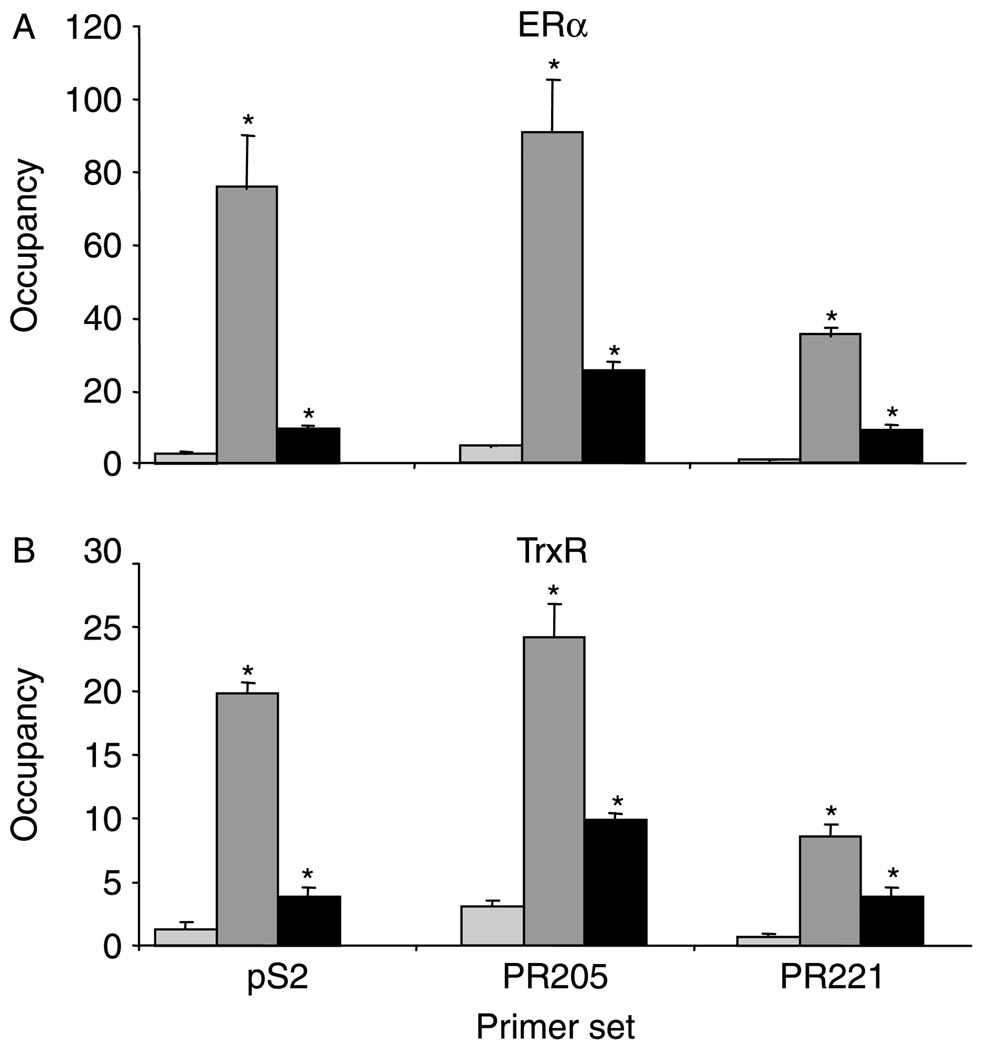
The interaction of endogenously expressed ERα, Trx, and TrxR (Fig. 2) and the association of Trx and TrxR with the DNA-bound ERα in vitro (Schultz-Norton et al. 2008, 2009) led us to investigate whether these proteins associate with native estrogen-responsive genes in MCF-7 cells. Using ChIP assays, we previously demonstrated that ERα and other coregulatory proteins associate with an ERE-containing region of the pS2 gene and with two regions of the PR gene located 205 kb (PR205) and 221 kb (PR221) upstream of the PR-B transcription start site (JL Boney-Montoya, YS Ziegler, CD Curtis, JA Montoya & AM Nardulli, unpublished observations, Schultz-Norton et al. 2006, 2007, Curtis et al. 2007, Creekmore et al. 2008, Rao et al. 2008). PR205 and PR221 contain one and two imperfect EREs respectively.
Significantly more ERa (Fig. 3A) and TrxR (Fig. 3B) were associated with the ERE-containing regions of the pS2 and PR genes in the presence than in the absence of E2, suggesting that TrxR may influence estrogen responsiveness of these genes by associating with the DNA-bound ERα in native chromatin. However, we were unable to detect any significant change in the association of Trx with the pS2 and PR genes in the absence and in the presence of E2 using three different Trx-specific antibodies (data not shown). This could result from the inability of the Trx-specific antibodies to effectively immunoprecipitate Trx (as suggested in Fig. 2A), a transient association of Trx with these gene regions, and/or the relatively small size of Trx (12 kDa), which could make it less accessible to antibody and more susceptible to epitope masking. No changes were observed in the association of ERα or TrxR with the internal control gene, 36B4, which was used for normalization of each sample.
Figure 3.
E2 increases association of TrxR with endogenous, estrogen-responsive genes. MCF-7 cells were treated with ethanol (light gray bars) or 10 nM E2 for 0.75 (dark gray bars) or 24 h (black bars) and subjected to ChIP analysis with an (A) ERα-or (B) TrxR-specific antibody. Quantitative real-time PCR was used to examine the association of ERα and TrxR with the ERE-containing regions of the pS2 and PR (PR205 and PR221) genes. Data are presented as the number of copies of each estrogen-responsive gene region pulled down relative to the number of copies of 36B4 gene region pulled down (occupancy). A significant increase in the association of ERα and TrxR with these gene regions in the presence of E2 is indicated by an. asterisk (*p≤0.05).
Trx and TrxR influence endogenous estrogen-responsive gene expression
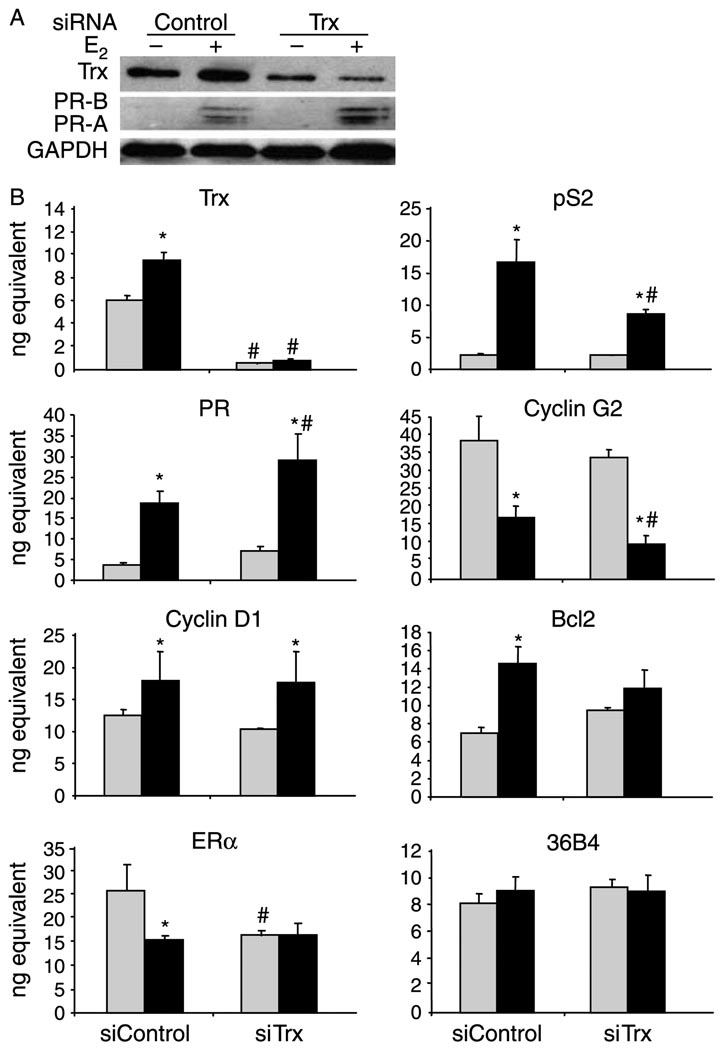
To determine more directly whether Trx and TrxR influence the expression of endogenous, estrogen-responsive genes, RNA interference assays were performed to individually knock down Trx and TrxR expression. MCF-7 cells were transfected with siRNA directed against endogenously expressed Trx or TrxR mRNA. In addition, a control siRNA directed against Renilla luciferase, which is not expressed in these cells, was utilized. Trx and TrxR siRNA successfully reduced the protein and mRNA levels of Trx (Fig. 4) and TrxR (Fig. 5) respectively. When control siRNA was used, pS2, PR, cyclin D1, and Bcl2 mRNA and PR protein levels were increased and cyclin G2 mRNA levels were decreased in the presence of E2 (Figs 4 and 5). These findings are consistent with earlier studies from our laboratory and others (Westley & May 1987, Nardulli et al. 1988, Altucci et al. 1996, Kim et al. 2000, Stossi et al. 2006, Curtis et al. 2007, Creekmore et al. 2008, Rao et al. 2008).
Figure 4.
Knocking down Trx influences estrogen responsiveness. MCF-7 cells were transfected with 50 pmol control or Trx siRNA, treated with ethanol (−E2 and light gray bars) or 10 nM E2 (+E2 and black bars) for 24 h, and processed for protein or mRNA analysis. (A) Proteins were subjected to western analysis with an antibody that recognizes Trx, PR-A and PR-B, or GAPDH. (B) RNA was isolated and cDNA was synthesized for quantitative RT-PCR analysis with primers specific to Trx, pS2, PR, cyclin G2, cyclin D1, Bcl2, ERα, and 36B4 (internal control) mRNA sequences. Data from three independent experiments, which had been performed in triplicate, were combined and are presented as the mean ±s.e.m. ANOVA was used to detect significant differences in mRNA levels in response to E2 (*P≤0.05) or in response to Trx siRNA (#P≤0.05).
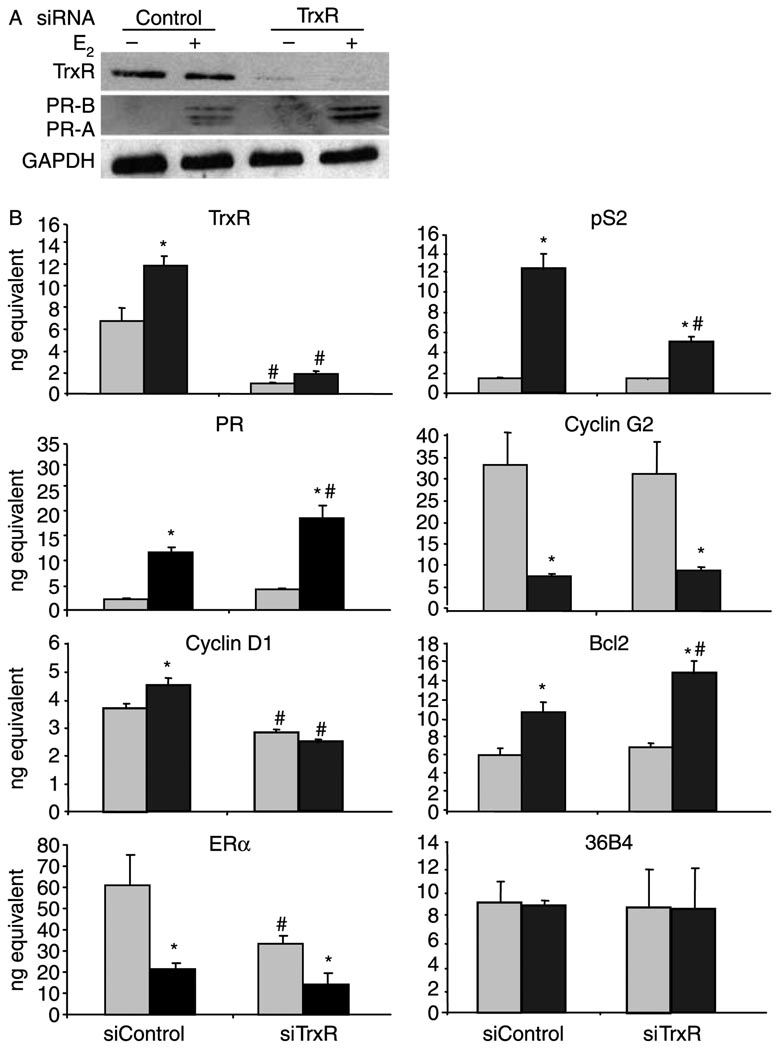
Figure 5.
Knocking down TrxR influences estrogen responsiveness. MCF-7 cells were transfected with 50 pmol control or TrxR siRNA, treated with ethanol (–E2 and light gray bars) or 10 nM E2 (+E2 and black bars) for 24 h, and processed for protein or mRNA analysis. (A) Proteins were subjected to western analysis with an antibody that recognizes TrxR, PR-A and PR-B, or GAPDH. (B) RNA was isolated and cDNA was synthesized for quantitative RT-PCR analysis with primers specific to TrxR, pS2, PR, cyclin G2, cyclin D1, Bcl2, ERα, and 36B4 (internal control) mRNA sequences. Data from three independent experiments, which had been performed in triplicate, were combined and are presented as the mean±S.E.M. ANOVA was used to detect significant differences in mRNA levels in response to E2 (*P≤0.05) or in response to TrxR siRNA (#P≤0.05).
While the E2-induced increase in pS2 mRNA expression was reduced when Trx siRNA was included, PR mRNA and protein levels were further enhanced (Fig. 4). The E2-induced repression in cyclin G2 was enhanced, resulting in further reduction in cyclin G2 mRNA expression. In contrast, cyclin D1, Bcl2, and ERα mRNA levels were unaltered when Trx expression was reduced. The internal control gene, 36B4, which contains no apparent ERα-binding sites, was unaffected by E2 or the Trx siRNA (Fig. 4B).
When TrxR was knocked down, pS2 and cyclin D1 mRNA levels decreased and PR and Bcl2 mRNA levels as well as PR protein increased in the presence of hormone (Fig. 5). Cyclin G2 mRNA levels were not significantly altered when TrxR expression was reduced and ERα mRNA levels were decreased in the absence, but not in the presence of E2. Again, 36B4 mRNA levels were unaffected by E2 or TrxR siRNA (Fig. 5B). The Trx and TrxR siRNAs were protein specific. Decreasing the level of one protein did not significantly affect the expression of the other (data not shown). Furthermore, two different Trx and TrxR siRNAs produced similar effects on estrogen-responsive gene expression. Taken together, these studies indicate that Trx and TrxR have gene-specific, rather than global effects, on estrogen-responsive gene expression.
Trx and TrxR alter H2O2 levels in MCF-7 cells
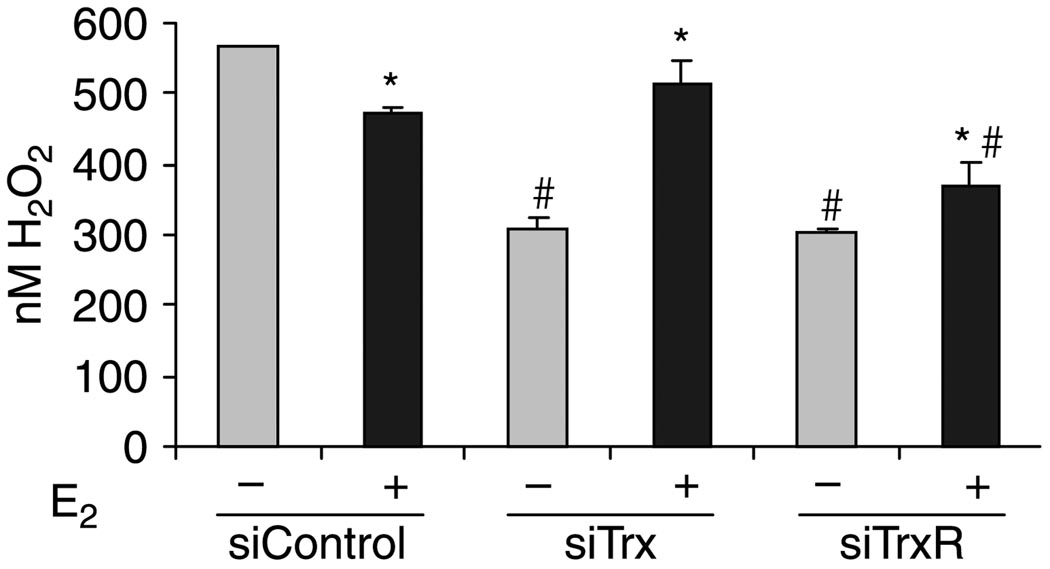
It is well established that Trx influences ROS distribution by activating antioxidant enzymes that convert H2O2 to H2O (Tam et al. 2003, Arner & Holmgren 2006). Since E2 increases Trx expression (Figs 1 and 4), we hypothesized that E2 treatment might affect H2O2 levels in MCF-7 cells. Furthermore, since TrxR is required to activate Trx, it seemed possible that TrxR might also play a role in regulating H2O2 levels. To test these hypotheses, MCF-7 cells that were exposed to control, Trx, or TrxR siRNA in the absence and in the presence of E2 and H2O2 levels were measured. When control siRNA was used, H2O2 levels were higher in the absence than in the presence of E2 (Fig. 6). This decreased level in H2O2 after treatment of MCF-7 cells with E2 for 24 h most likely results from the dissipation of ROS by the E2-mediated increase in antioxidant proteins (Mobley & Brueggemeier 2004, Rao et al. 2008).
Figure 6.
Trx, TrxR, and E2 modulate H2O2 levels. MCF-7 cells were transfected with 50 pmol control, Trx, or TrxR siRNA and treated with ethanol (−E2) or 10 nM E2 (+E2) for 24 h. Cell extracts were prepared and incubated with Amplex Red to detect the levels of H2O2. Data from three independent experiments were combined and are expressed as the mean±S.E.M. ANOVA was used to detect significant differences in the levels of H2O2 in the presence of E2 (*P≤0.05) or in response to Trx or TrxR siRNA (#P≤0.05).
When Trx or TrxR expression was knocked down and cells were treated with ethanol, H2O2 levels were significantly reduced. However, when cells were treated with E2 and Trx or TrxR siRNA, H2O2 levels were enhanced. These studies demonstrate that individually and collectively Trx, TrxR, and E2 alter H2O2 levels in MCF-7 cells.
Discussion
Previous studies have shown that Trx and TrxR help to dissipate ROS, maintain a reduced intracellular environment, and protect cellular macromolecules from oxidative damage (Holmgren 1985, Holmgren & Bjornstedt 1995, Osborne et al. 2001, Lincoln et al. 2003, Smart et al. 2004). We now demonstrate that endogenously expressed Trx and TrxR interact with ERα and alter estrogen-responsive gene expression and that together, Trx, TrxR, and E2 influence H2O2 levels in MCF-7 human breast cancer cells.
Effect of Trx and TrxR on H2O2 levels
Although a previous study reported that ROS levels increase when MCF-7 cells are treated with 100 mM E2 for 15 min (Felty et al. 2005), we, in fact, observed an E2-dependent reduction in H2O2 levels when MCF-7 cells had been exposed to 10 nM E2 for 24 h. While the 1000-fold difference in E2 concentrations used in these two studies might account for some of the difference observed, we believe that the diminished H2O2 levels observed after 24 h of E2 treatment are primarily due to the increased expression of oxidative stress proteins such as SOD1 (Rao et al. 2008) and Trx (Figs 1 and 4), increases that would not be observed after a 15 min exposure to E2. In addition, although an earlier study monitored H2O2 levels after the addition of exogenous H2O2 to the culture media (Mobley & Brueggemeier 2004), these experiments are distinctly different from our studies in which cells were exposed to vehicle or hormone, and endogenous production of H2O2 was measured. Overall, our findings support the idea that E2 plays an important role in regulating H2O2 levels in MCF-7 cells by modulating the expression of oxidative stress proteins.
Effects of Trx and TrxR on estrogen-responsive gene expression
The capacity of Trx to reduce peroxiredoxins, which convert H2O2 to H2O (Arner & Holmgren 2006), helps to maintain a reduced intracellular environment and ensure that transcription factors are in a reduced, active state. Thus, Trx along with its activator, TrxR, help in the overall maintenance of transcription factorbinding activity.
The zinc fingers of ERα provide the specificity required for recognizing and interacting with ERE-containing DNA, but are sensitive to oxidative stress (Webster et al. 2001). Although oxidation of ERα by ROS or the oxidizing agent diamide inhibits the ability of the receptor to interact with ERE-containing DNA, its DNA-binding capacity can be restored by the reducing agent dithiothreitol or Trx (Hayashi et al. 1997). The ability of Trx and TrxR to help maintain ERα structure and function is evident in the altered estrogen-responsive gene expression when either protein is knocked down (Figs 4 and 5). Trx also plays an active role in maintaining the DNA-binding activity of other transcription factors including NF-кB, cAMP response element binding protein, p53, Sp1, AP-1 proteins, and the glucocorticoid receptor (Matthews et al. 1992, Wu et al. 1996, Hayashi et al. 1997, Hirota et al. 1997, Liang et al. 1998, Ueno et al. 1999, Webster et al. 2001).
Although we were unable to detect a difference in the association of Trx with the pS2 and PR genes in the absence and in the presence of hormone, the isolation of Trx in a complex with the DNA-bound ERα (Schultz-Norton et al. 2008, 2009), the immunoprecipitation of ERα with a Trx-specific antibody in the absence and in the presence of hormone (Fig. 2A), and the ability of Trx to reduce ERα and enhance its binding to DNA (Hayashi et al. 1997) suggest that Trx associates with ERα at target genes. The E2-induced increase in the association of TrxR with the pS2 and PR genes in the presence of hormone (Fig. 3B) could help to ensure that any Trx associated with these gene regions is active and capable of reducing ERα and its associated coregulatory proteins and influencing transcription. Thus, TrxR, through its modulation of Trx activity, may play a role in reducing proteins and maintaining a reduced environment (Arner & Holmgren 2000, Nordberg & Arner 2001). In addition, TrxR has been referred to as a ‘redox sensor’ (Sun et al. 1999) and may serve in this capacity to help modulate estrogen-responsive gene expression.
Because of their interdependent nature, it was somewhat surprising that Trx and TrxR would have different effects on cyclin G2, cyclin D1, and Bcl2 gene expression (Table 1). However, it is important to remember that estrogen-responsive genes are regulated not simply by ERα alone, but by a complex array of transcription factors and coregulatory proteins bound to multiple cis elements in extended gene regions, and that the association of a single transcription factor with a single gene region cannot necessarily be used to predict the transcriptional response.
Table 1.
Regulation of endogenous, estrogen-responsive genes in MCF-7 cells
| Trx |
TrxR |
|
|---|---|---|
| Gene | ||
| pS2 | ↑ | ↑ |
| PR | ↓ | ↓ |
| Cyclin G2 | ↑ | – |
| Cyclin D1 | – | ↑ |
| Bcl2 | – | ↓ |
| ERα | – | – |
| 36B4 | – | – |
RNA interference experiments demonstrate the gene-specific effects of endogenously expressed Trx and TrxR on estrogen responsiveness.
Biological roles of Trx and TrxR
Given the role of Trx and TrxR in influencing estrogen-responsive gene expression, it is not surprising that these two proteins would influence reproductive function. An earlier study suggested that Trx and TrxR were part of a uterine antioxidant system required for maintaining estrogen responsiveness of the uterus (Deroo et al. 2004). These and other studies have shown that E2 increases uterine expression of Trx and TrxR in rodents and humans (Maruyama et al. 1997, 1999, Osborne et al. 2001, Deroo et al. 2004). In addition to their roles in reproduction, Trx and TrxR have been implicated in cancer prevention (Urig & Becker 2006) and progression (Turunen et al. 2004, Biaglow & Miller 2005, Arner & Holmgren 2006, Fujino et al. 2006). The ability of Trx and TrxR to alter H2O2 levels (Fig. 6) could help to maintain gene expression by regulating the redox state of critical transcription factors. Furthermore, they could be essential in avoiding oxidative stress and limiting the damage to cellular macromolecules, which has been associated with aging and age-related disease (Harman 1956, 2001, Finkel & Holbrook 2000). In fact, the role of Trx in regulating oxidative stress and influencing the aging process has been previously reported (reviewed in Yoshida et al. (2003)). The overall biological importance of these two proteins is evident in the early embryonic lethality of Trx- and TrxR-null mice (Matsui et al. 1996, Jakupoglu et al. 2005).
Effect of oxidative stress proteins on estrogen responsiveness
Trx and TrxR cooperate with other enzymes to dissipate intracellular ROS and maintain the capacity of transcription factors to bind to DNA (Fig. 7A, reviewed in Webster et al. (2001)). The conversion of superoxide to H2O2 by SOD1 is the first line of defense against ROS. TrxR reduces Trx, which in turn activates peroxiredoxins to aid in the conversion of H2O2 to H2O and O2, and enhances binding of transcription factors including ERα, AP-1 proteins, NFκB, and p53 to their cognate recognition sequences (Xanthoudakis & Curran 1992, Hayashi et al. 1997, Jayaraman et al. 1997, Webster et al. 2001, Nishi et al. 2002). PDI catalyzes disulfide bond formation, reduction, and isomerization (Turano et al. 2002), and functions as a molecular chaperone for numerous proteins (Lyles & Gilbert 1991, Noiva et al. 1993, Wang & Tsou 1993, Puig et al. 1994, Quan et al. 1995, Schultz-Norton et al. 2006). Ape1 plays a role in DNA repair, redox regulation, and, like Trx, stimulates binding of transcription factors to DNA (Xanthoudakis & Curran 1992, Jayaraman et al. 1997, Webster et al. 2001, Nishi et al. 2002). Each of these proteins, Trx, TrxR, SOD1, PDI, and Ape1, plays a critical role in regulating oxidative stress, and each influences ERα-mediated gene expression in MCF-7 cells (Schultz-Norton et al. 2006, Rao et al. 2008, Curtis et al. 2009). Because of the interdependent nature of these proteins, perturbation in expression of any one protein has the potential to create disequilibrium and alter gene expression and ROS distribution as was observed when Trx or TrxR was knocked down (Figs 4–6).
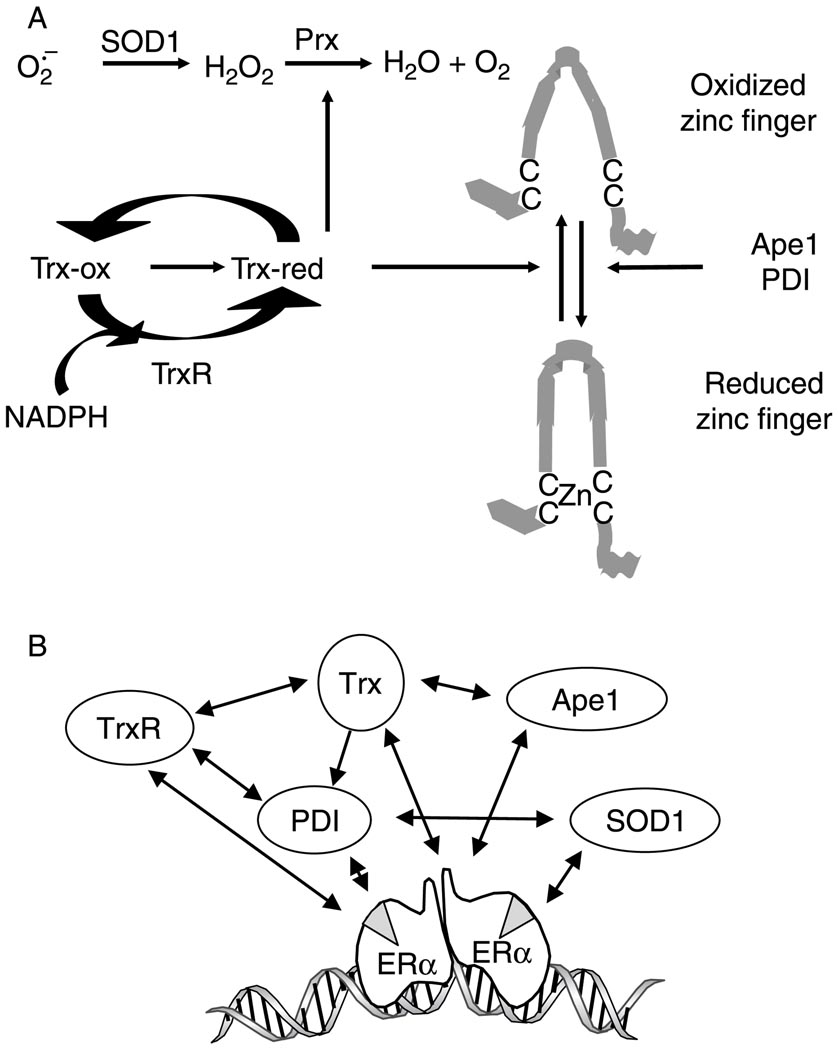
Figure 7.
Oxidative stress response protein forms an interconnected network that alters ROS distribution and influences estrogen responsiveness. (A) Oxidized Trx (Trx-ox) is reduced (Trx-red) by TrxR using NADPH as a cofactor. SOD1 dismutates superoxide to form H2O2 and reduced Trx activates peroxiredoxins (Prx) to help eliminate H2O2. Trx, Ape1, and PDI reduce zinc finger proteins, enhance interaction with their cognate-binding sites in DNA, and alter transcription. Adapted from Webster et al. 2001. (B) Trx, TrxR, SOD1, PDI, and Ape1 form an interconnected network of proteins (Lundstrom & Holmgren 1990, Cheung et al. 1999, Wei et al. 2000, Webster et al. 2001, Atkin et al. 2006, Schultz-Norton et al. 2006, 2008, Ando et al. 2008, Rao et al. 2008, Curtis et al. 2009) that are recruited to the DNA-bound ERα (Schultz-Norton et al. 2008) and influence ERα-mediated gene expression (Schultz-Norton et al. 2006, Rao et al. 2008, Curtis et al. 2009).
The current study combined with our previous work (Schultz-Norton et al. 2006, 2008, Rao et al. 2008, Curtis et al. 2009) supports the idea that Trx and TrxR are members of an interconnected network of proteins (Lundstrom & Holmgren 1990, Cheung et al. 1999, Wei et al. 2000, Webster et al. 2001, Atkin et al. 2006, Schultz-Norton et al. 2006, 2008, Ando et al. 2008, Rao et al. 2008, Curtis et al. 2009), which collectively help to maintain the structural integrity and activity of ERα, its associated coregulatory proteins, and other complex members (Fig. 7B). TrxR is required to maintain Trx in an active, reduced state (Webster et al. 2001); Trx reduces Ape1 to bring about changes in transcription factor activity (Wei et al. 2000); Ape1 reduces Trx to influence gene expression (Ando et al. 2008); together, Trx and TrxR reduce PDI (Lundstrom & Holmgren 1990); PDI prevents misfolding of many proteins including TrxR and SOD1 (Cheung et al. 1999, Atkin et al. 2006) and acts as a molecular chaperone for ERα (Schultz-Norton et al. 2006); and ERα associates with TrxR, PDI, SOD1, and Ape1 at endogenous estrogen-responsive genes (Fig. 3, Rao et al. 2008, Schultz-Norton et al. 2008, Curtis et al. 2009)). Taken together, our studies suggest that ERα serves as a nucleating factor to recruit proteins involved in regulating oxidative stress to estrogen-responsive genes, and that oxidative stress proteins are, in turn, instrumental in altering estrogen-responsive gene expression and redox regulation.
Acknowledgements
We thank Carol Curtis for helpful discussions.
Funding
This work was supported by NIH Grants R01 DK 53884 and R56 DK 53884 (to A M Nardulli) and P41 RR11823-10 (to J R Yates).
Footnotes
Declaration of interest
None of the authors has a conflict of interest.
References
- Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker MG, Truss M, Beato M, Sica V, Bresciani F, Weisz A. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene. 1996;12:2315–2324. [PubMed] [Google Scholar]
- Ando K, Hirao S, Kabe Y, Ogura Y, Sato I, Yamaguchi Y, Wada T, Handa H. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Research. 2008;36:4327–4336. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai RJ, Masutani H, Yodoi J, Debbas V, Laurindo FR, Stern A, Monteiro HP. Nitric oxide induces thioredoxin-1 nuclear translocation: possible association with the p21Ras survival pathway. Biochemical and Biophysical Research Communications. 2006;348:1254–1260. doi: 10.1016/j.bbrc.2006.07.178. [DOI] [PubMed] [Google Scholar]
- Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. European Journal of Biochemistry. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Arner ES, Holmgren A. The thioredoxin system in cancer. Seminars in Cancer Biology. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Atkin JD, Farg MA, Turner BJ, Tomas D, Lysaght JA, Nunan J, Rembach A, Nagley P, Beart PM, Cheema SS, et al. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. Journal of Biological Chemistry. 2006;281:30152–30165. doi: 10.1074/jbc.M603393200. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. PNAS. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaglow JE, Miller RA. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biology and Therapy. 2005;4:6–13. doi: 10.4161/cbt.4.1.1434. [DOI] [PubMed] [Google Scholar]
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radical Biology and Medicine. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Cheung PY, Churchich JE, Lee KS. Refolding of thioredoxin reductase assisted by groEL and PDI. Biochemical and Biophysical Research Communications. 1999;255:17–22. doi: 10.1006/bbrc.1998.0135. [DOI] [PubMed] [Google Scholar]
- Creekmore AL, Walt KA, Schultz-Norton JR, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. The role of retinoblastoma associated proteins 46 and 48 in estrogen receptor alpha mediated gene expression. Molecular and Cellular Endocrinology. 2008;291:79–86. doi: 10.1016/j.mce.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CD, Likhite VS, McLeod IX, Yates JR, Nardulli AM. Interaction of the tumor metastasis suppressor nonmetstatic protein 23 homologue H1 and estrogen receptor alpha alters estrogen-responsive gene expression. Cancer Research. 2007;67:10600–10607. doi: 10.1158/0008-5472.CAN-07-0055. [DOI] [PubMed] [Google Scholar]
- Curtis CD, Thorngren DL, Ziegler YS, Sarkeshik A, Yates JR, Nardulli AM. Apurinic/apyrimidinic endonuclease 1 alters estrogen receptor activity and estrogen responsive gene expression. Molecular Endocrinology. 2009;23:1346–1359. doi: 10.1210/me.2009-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KC, Lewis-Molock Y, White CW. Elevation of manganese superoxide dismutase gene expression by thioredoxin. American Journal of Respiratory Cell and Molecular Biology. 1997;17:713–726. doi: 10.1165/ajrcmb.17.6.2809. [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Hewitt SC, Peddada SD, Korach KS. Estradiol regulates the thioredoxin antioxidant system in the mouse uterus. Endocrinology. 2004;145:5485–5492. doi: 10.1210/en.2004-0471. [DOI] [PubMed] [Google Scholar]
- Druege PM, Klein-Hitpass L, Green S, Stack G, Chambon P, Ryffel GU. Introduction of estrogen-responsiveness into mammalian cell lines. Nucleic Acids Research. 1986;14:9329–9337. doi: 10.1093/nar/14.23.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Katzenellenbogen BS. Effects of estrogens and antiestrogens on estrogen receptor dynamics and the induction of progesterone receptor in MCF-7 breast cancer cells. Cancer Research. 1982;42:139–144. [PubMed] [Google Scholar]
- Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. British Journal of Radiology. 2005;78:3–7. doi: 10.1259/bjr/63353075. [DOI] [PubMed] [Google Scholar]
- Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J, Roy D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005;44:6900–6909. doi: 10.1021/bi047629p. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fujino G, Noguchi T, Takeda K, Ichijo H. Thioredoxin and protein kinases in redox signaling. Seminars in Cancer Biology. 2006;16:427–435. doi: 10.1016/j.semcancer.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxidants & Redox Signaling. 2005;7:1021–1031. doi: 10.1089/ars.2005.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert J-M, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. The importance of free radicals and catalytic metal ions in human diseases. Molecular Aspects of Medicine. 1985;8:89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: overview. Annals of the New York Academy of Sciences. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- Hashemy SI, Ungerstedt JS, Zahedi Avval F, Holmgren A. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. Journal of Biological Chemistry. 2006;281:10691–10697. doi: 10.1074/jbc.M511373200. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Hajiro-Nakanishi K, Makino Y, Eguchi H, Yodoi J, Tanaka H. Functional modulation of estrogen receptor by redox state with reference to thioredoxin as a mediator. Nucleic Acids Research. 1997;25:4035–4040. doi: 10.1093/nar/25.20.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. PNAS. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. Journal of Biological Chemistry. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. Journal of Biological Chemistry. 1979;254:9113–9119. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annual Review of Biochemistry. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods in Enzymology. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- Jakupoglu C, Przemeck G, Schneider M, Moreno S, Mayr N, Hatzopoulos A, de Angelis M, Wurst W, Bornkamm G, Brielmeier M, et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Molecular and Cellular Biology. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman L, Murthy KG, Zhu C, Curran T, Xanthoudakis S, Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes and Development. 1997;11:558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Kendra KL, Norman MJ, Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Research. 1987;47:4355–4360. [PubMed] [Google Scholar]
- Kim J, Petz LN, Ziegler YS, Wood JR, Potthoff SJ, Nardulli AM. Regulation of the estrogen-responsive pS2 gene in MCF-7 human breast cancer cells. Journal of Steroid Biochemistry and Molecular Biology. 2000;74:157–168. doi: 10.1016/s0960-0760(00)00119-9. [DOI] [PubMed] [Google Scholar]
- Kirkwood T, Austad S. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Lehnert BE, Iyer R. Exposure to low-level chemicals and ionizing radiation: reactive oxygen species and cellular pathways. Human & Experimental Toxicology. 2002;21:65–69. doi: 10.1191/0960327102ht212oa. [DOI] [PubMed] [Google Scholar]
- Liang X, Lu B, Scott GK, Chang CH, Baldwin MA, Benz CC. Oxidant stress impaired DNA-binding of estrogen receptor from human breast cancer. Molecular and Cellular Endocrinology. 1998;146:151–161. doi: 10.1016/s0303-7207(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Lincoln DT, Ali Emadi EM, Tonissen KF, Clarke FM. The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anticancer Research. 2003;23:2425–2433. [PubMed] [Google Scholar]
- Lundstrom J, Holmgren A. Protein disulfide-isomerase is a substrate for thioredoxin reductase and has thioredoxin-like activity. Journal of Biological Chemistry. 1990;265:9114–9120. [PubMed] [Google Scholar]
- Lyles MM, Gilbert HF. Catalysis of the oxidative folding of ribonuclease A by protein disulfide isomerase: pre-steady-state kinetics and the utilization of the oxidizing equivalents of the isomerase. Biochemistry. 1991;30:619–625. doi: 10.1021/bi00217a005. [DOI] [PubMed] [Google Scholar]
- Makino Y, Okamoto K, Yoshikawa N, Aoshima M, Hirota K, Yodoi J, Umesono K, Makino I, Tanaka H. Thioredoxin: a redox-regulating cellular cofactor for glucocorticoid hormone action. Cross talk between endocrine control of stress response and cellular antioxidant defense system. Journal of Clinical Investigation. 1996;98:2469–2477. doi: 10.1172/JCI119065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kitaoka Y, Sachi Y, Nakanoin K, Hirota K, Shiozawa T, Yoshimura Y, Fujii S, Yodoi J. Thioredoxin expression in the human endometrium during the menstrual cycle. Molecular Human Reproduction. 1997;3:989–993. doi: 10.1093/molehr/3.11.989. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Sachi Y, Furuke K, Kitaoka Y, Kanzaki H, Yoshimura Y, Yodoi J. Induction of thioredoxin, a redox-active protein, by ovarian steroid hormones during growth and differentiation of endometrial stromal cells in vitro. Endocrinology. 1999;140:365–372. doi: 10.1210/endo.140.1.6455. [DOI] [PubMed] [Google Scholar]
- Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Developmental Biology. 1996;178:179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Research. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley JA, Brueggemeier RW. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis. 2004;25:3–9. doi: 10.1093/carcin/bgg175. [DOI] [PubMed] [Google Scholar]
- Mustacich D, Powis G. Thioredoxin reductase. Biochemical Journal. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- Nardulli AM, Greene GL, O’Malley BW, Katzenellenbogen BS. Regulation of progesterone receptor messenger ribonucleic acid and protein levels in MCF-7 cells by estradiol: analysis of estrogen’s effect on progesterone receptor synthesis and degradation. Endocrinology. 1988;122:935–944. doi: 10.1210/endo-122-3-935. [DOI] [PubMed] [Google Scholar]
- Nishi T, Shimizu N, Hiramoto M, Sato I, Yamaguchi Y, Hasegawa M, Aizawa S, Tanaka H, Kataoka K, Watanabe H, et al. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo. Journal of Biological Chemistry. 2002;277:44548–44556. doi: 10.1074/jbc.M202970200. [DOI] [PubMed] [Google Scholar]
- Noiva R, Freedman RB, Lennarz WJ. Peptide binding to protein disulfide isomerase occurs at a site distinct from the active sites. Journal of Biological Chemistry. 1993;268:19210–19217. [PubMed] [Google Scholar]
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biology and Medicine. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Osborne LJ, Tonissen KF, Tang VH, Clarke FM. Expression and localisation of thioredoxin in mouse reproductive tissues during the oestrous cycle. Molecular Reproduction and Development. 2001;58:359–367. doi: 10.1002/1098-2795(20010401)58:4<359::AID-MRD2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Powell C, Swenberg J, Rusyn I. Expression of base excision DNA repair genes as a biomarker of oxidative DNA damage. Cancer Letters. 2005;229:1–11. doi: 10.1016/j.canlet.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Puig A, Lyles MM, Noiva R, Gilbert HF. The role of the thiol/disulfide centers and peptide binding site in the chaperone and anti-chaperone activities of protein disulfide isomerase. Journal of Biological Chemistry. 1994;269:19128–19135. [PubMed] [Google Scholar]
- Quan H, Fan G, Wang CC. Independence of the chaperone activity of protein disulfide isomerase from its thioredoxin-like active site. Journal of Biological Chemistry. 1995;270:17078–17080. doi: 10.1074/jbc.270.29.17078. [DOI] [PubMed] [Google Scholar]
- Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM. Effects of Cu/Zn superoxide dismutase on estrogen responsiveness and oxidative stress in human breast cancer cells. Molecular Endocrinolgy. 2008;22:1113–1124. doi: 10.1210/me.2007-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salganik RI. The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. Journal of the American College of Nutrition. 2001;20:464S–472S. doi: 10.1080/07315724.2001.10719185. discussion 473S–475S. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, McDonald WH, Yates JR, Nardulli AM. Protein disulfide isomerase serves as a molecular chaperone to maintain estrogen receptor {alpha} structure and function. Molecular Endocrinology. 2006;20:1982–1995. doi: 10.1210/me.2006-0006. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, Walt KA, Ziegler YS, McLeod IX, Yates JR, Raetzman LT, Nardulli AM. The deoxyribonucleic acid repair protein flap endonuclease-1 modulates estrogen-responsive gene expression. Molecular Endocrinology. 2007;21:1569–1580. doi: 10.1210/me.2006-0519. [DOI] [PubMed] [Google Scholar]
- Schultz-Norton JR, Ziegler YS, Likhite VS, Yates JR, Nardulli AM. Isolation of novel coregulatory protein networks associated with DNA-bound estrogen receptor alpha. BMC Molecular Biology. 2008;9:97. doi: 10.1186/1471-2199-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Norton JR, Ziegler YS, Likhite VS, Nardulli AM. Isolation of proteins associated with the DNA-bound estrogen receptor α. In: Sarge O-K Park, Curry T., editors. In Molecular Endocrinology: A Comprehensive Guide to Current Methodologies. Totowa, NJ: USA: Humana Press; 2009. [Google Scholar]
- Smart DK, Ortiz KL, Mattson D, Bradbury CM, Bisht KS, Sieck LK, Brechbiel MW, Gius D. Thioredoxin reductase as a potential molecular target for anticancer agents that induce oxidative stress. Cancer Research. 2004;64:6716–6724. doi: 10.1158/0008-5472.CAN-03-3990. [DOI] [PubMed] [Google Scholar]
- Storz G, Tartaglia LA, Farr SB, Ames BN. Bacterial defenses against oxidative stress. Trends in Genetics. 1990;6:363–368. doi: 10.1016/0168-9525(90)90278-e. [DOI] [PubMed] [Google Scholar]
- Stossi F, Likhite VS, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cyclin G2 promoter. Journal of Biological Chemistry. 2006;281:16272–16278. doi: 10.1074/jbc.M513405200. [DOI] [PubMed] [Google Scholar]
- Sun QA, Wu Y, Zappacosta F, Jeang KT, Lee BJ, Hatfield DL, Gladyshev VN. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. Journal of Biological Chemistry. 1999;274:24522–24530. doi: 10.1074/jbc.274.35.24522. [DOI] [PubMed] [Google Scholar]
- Tam NN, Gao Y, Leung YK, Ho SM. Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and antioxidant defense machinery during prostatic involution and regrowth. American Journal of Pathology. 2003;163:2513–2522. doi: 10.1016/S0002-9440(10)63606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint O, Royer V, Salmon M, Remacle J. Stress-induced premature senescence and tissue ageing. Biochemical Pharmacology. 2002;64:1007–1009. doi: 10.1016/s0006-2952(02)01170-x. [DOI] [PubMed] [Google Scholar]
- Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. Journal of Cellular Physiology. 2002;193:154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- Turunen N, Karihtala P, Mantyniemi A, Sormunen R, Holmgren A, Kinnula VL, Soini Y. Thioredoxin is associated with proliferation, p53 expression and negative estrogen and progesterone receptor status in breast carcinoma. Acta Pathologica et Microbiologica Scandinavica. 2004;112:123–132. doi: 10.1111/j.1600-0463.2004.apm1120207.x. [DOI] [PubMed] [Google Scholar]
- Ueno M, Masutani H, Arai RJ, Yamauchi A, Hirota K, Sakai T, Inamoto T, Yamaoka Y, Yodoi J, Nikaido T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. Journal of Biological Chemistry. 1999;274:35809–35815. doi: 10.1074/jbc.274.50.35809. [DOI] [PubMed] [Google Scholar]
- Urig S, Becker K. On the potential of thioredoxin reductase inhibitors for cancer therapy. Seminars in Cancer Biology. 2006;16:452–465. doi: 10.1016/j.semcancer.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Wang CC, Tsou CL. Protein disulfide isomerase is both an enzyme and a chaperone. FASEB Journal. 1993;7:1515–1517. doi: 10.1096/fasebj.7.15.7903263. [DOI] [PubMed] [Google Scholar]
- Webster KA, Prentice H, Bishopric NH. Oxidation of zinc finger transcription factors: physiological consequences. Antioxidants and Redox Signaling. 2001;3:535–548. doi: 10.1089/15230860152542916. [DOI] [PubMed] [Google Scholar]
- Wei SJ, Botero A, Hirota K, Bradbury CM, Markovina S, Laszlo A, Spitz DR, Goswami PC, Yodoi J, Gius D. Thioredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer Research. 2000;60:6688–6695. [PubMed] [Google Scholar]
- Westley BR, May FE. Oestrogen regulates cathepsin D mRNA levels in oestrogen responsive human breast cancer cells. Nucleic Acids Research. 1987;15:3773–3786. doi: 10.1093/nar/15.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JR, Likhite VS, Loven MA, Nardulli AM. Allosteric modulation of estrogen receptor conformation by different estrogen response elements. Molecular Endocrinology. 2001;15:1114–1126. doi: 10.1210/mend.15.7.0671. [DOI] [PubMed] [Google Scholar]
- Wu X, Bishopric NH, Discher DJ, Murphy BJ, Webster KA. Physical and functional sensitivity of zinc finger transcription factors to redox change. Molecular and Cellular Biology. 1996;16:1035–1046. doi: 10.1128/mcb.16.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO Journal. 1992;11:653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Oka S, Masutani H, Nakamura H, Yodoi J. The role of thioredoxin in the aging process: involvement of oxidative stress. Antioxidants and Redox Signaling. 2003;5:563–570. doi: 10.1089/152308603770310211. [DOI] [PubMed] [Google Scholar]