Abstract
Pharmacological inhibitors of the transforming growth factor β (TGFβ) type I receptor (ALK5) have shown promise in blocking growth of xenotransplanted cancer cell lines but the effect on a multistage cancer model is not known. To test this, we treated mouse skin with SB431542 (SB), a well-characterized ALK5 inhibitor, during a two-stage skin carcinogenesis assay. Topical SB significantly reduced the total number, incidence and size of papillomas compared with 12-O-tetradecanoylphorbol 13-acetate (TPA) promotion alone, and this was linked to increased epidermal apoptosis, decreased proliferation and decreased cutaneous inflammation during promotion. In contrast, the frequency of conversion to squamous cell carcinoma (SCC) was 2-fold higher in papillomas treated with SB. Although there was no difference in tumor cell proliferation in early premalignant lesions, those that formed after SB treatment exhibited reduced squamous differentiation and an altered inflammatory microenvironment similar to SCC. In an inducible epidermal RAS transgenic model, treatment with SB enhanced proliferation and cutaneous inflammation in skin but decreased expression of keratin 1 and increased expression of simple epithelial keratin 18, markers of premalignant progression. In agreement with increased frequency of progression in the multistage model, SB treatment resulted in increased tumor formation with a more malignant phenotype following long-term RAS induction. In contrast to the current paradigm for TGFβ in carcinogenesis, these results demonstrate that cutaneous TGFβ signaling enables promotion of benign tumors but suppresses premalignant progression through context-dependent regulation of epidermal homeostasis and inflammation.
Introduction
Transforming growth factor β1 (TGFβ1) is a member of a large family of regulatory molecules that play both positive and negative roles in epithelial cancer. Most epithelial cells produce TGFβ1 and respond to it through a heterodimeric receptor complex composed of the TGFβ type II and TGFβ type I (ALK5) receptor (1). Small molecule inhibitors of ALK5 have been developed for therapeutic use in cancer and other diseases where TGFβ1 overexpression is linked to disease phenotype. A commercially available small molecule inhibitor of the TGFβ type I receptor, SB431542 (SB) (4-[4-(3,4-Methylenedioxyphenyl)-5-(2-pyridyl)-1H-imidazol-2-yl]-benzamide), shows selectivity for the kinase activity of ALK5 and to a lesser extent other members of the TGFβ1 superfamily, including the activin signaling receptor ALK4 and the nodal receptor ALK7. It acts as a competitive adenosine triphosphate-binding site kinase inhibitor and has been shown to inhibit the in vitro phosphorylation of Smad3 (2) and Smad2 (3). This inhibitor has no direct effect on Smad-independent pathways, such as extracellular signal-regulated kinase, c-jun N-terminal kinase or p38 mitogen-activated protein kinase pathways (4).
Recent in vitro and in vivo studies with ALK5 inhibitors have shown promise in preclinical models for inhibition of malignant tumor growth (5–10) and suppression of fibrotic diseases (11–14), but studies in genetically altered mouse models indicate that disruption of this pathway could enhance malignancy (15–18). Furthermore, the complex role of TGFβ1 signaling in epithelial carcinogenesis suggests that ALK5 inhibitors could have both inhibitory and promoting effects on cancer, but this has not been tested in a long-term multistage cancer model, which could be informative to clinical studies. The mouse two-stage chemical carcinogenesis model in which the epidermis is treated once with the carcinogen 7, 12-dimethylbenz(a)anthracene (DMBA) followed by repeated application of the tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA) has been extensively used to determine how the TGFβ signaling pathway affects cancer development. In this model, nearly all tumors that form have activating mutations in codon 61 of the HRas gene, but only a small fraction of benign lesions progress to squamous cell carcinoma (SCC) (19,20). Both overexpression of TGFβ1 (21) or TGFβ superfamily members (22) in the epidermis or reduction of TGFβ1 expression (23) and genetic deletion of Smad3 (24) suppress formation of benign tumors. In contrast, reduction of TGFβ1 levels or inactivation of TGFβ1 receptor signaling in the epidermis enhances conversion to SCC (23,25).
Here, we have determined how topical SB treatment of DMBA-initiated skin during the promotion phase affects squamous tumor formation and progression. In contrast to the generally accepted paradigm of TGFβ1 as a tumor suppressor during early stages of tumor formation and a promoter of malignant progression and metastasis, our results show that pharmacological inhibition of cutaneous TGFβ signaling blocks benign tumor formation. However, the tumors that form have an enhanced frequency of conversion to SCC. This differential effect on benign and premalignant lesions is associated with potent effects of ALK5 inhibition on the squamous epithelial phenotype and the inflammatory microenvironment associated with tumor formation and progression.
Materials and methods
Animal studies
To induce skin tumors, FVB/n mice were dosed once topically with 50 μg of DMBA (Sigma, St Louis, MO) and then treated with 5 μg of TPA (Calbiochem, La Jolla, CA) in 200 μl of acetone twice a week and/or 200 μl of 10.0 μM SB (Sigma) for 25 weeks. An additional SB treatment was given in between TPA doses. After 25 weeks, the tumors were allowed to develop up to 52 weeks. The number of papillomas per mouse (>1 mm in diameter) was calculated by dividing the total number of papillomas at each time point by the maximum number of mice developing tumors. In a second study, early tumors were harvested at 12 weeks of tumor promotion, 4 h after the last TPA/SB treatment. For acute tumor promotion studies, mice were pretreated twice daily with SB and then with TPA and harvested either 4 or 24 h after TPA treatment. For chronic promotion, mice were treated for 2 weeks 2×/week with TPA or TPA + SB as in tumor induction study. To induce oncogenic RAS expression in the epidermis, bitransgenic Involucrin tTA × tetORAS mice were generated from a cross of the Involucrin tTA line (26) and the tetORASV12G line (27). Seven-week-old mice were removed from water containing 10 μg/ml doxycycline (Dox) and treated with 200 μl of acetone and/or 200 μl of 10.0 μM SB every other day for 5 days. Skin was harvested 24 h after the last SB treatment. All animals were kept under a controlled environment of temperature and humidity and a 12 h light/dark cycle. Animal studies were conducted under approved Institutional protocols.
Tissue analysis
Histopathological analysis of tumors was done blindly on hematoxylin and eosin stained sections by a board certified veterinary pathologist (M.J.K.). To determine epidermal thickness, the number of cell layers in the epidermis was counted every 20 basal cells per formalin-fixed section. Anti-bromodeoxyuridine immunohistochemistry to measure cell proliferation, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling assay staining to measure apoptosis and anti-myeloperoxidase (MPO) immunohistochemistry was done as described (23). Immunohistochemistry for keratins 1, 13 (28) and 8 (29) was done using ethanol-fixed sections of skin. Photomicrographs of tissue sections were made using an Olympus BX61Epi-Fluorescence Microscope, Olympus DP71 camera, UPlan Fl objectives and DP-BSW Basic Software. Rabbit anti-phospho-Smad2 (Millipore, Billerica, MA) (23) and anti-F4/80 (Serotec, Raleigh, NC) (30) antibodies were used for indirect immunofluorescence in ethanol-fixed sections and were imaged using an Olympus FV300 Laser Scanning Confocal Microscope, Inverted Olympus IX-70 microscope, using Fluoview 300 Version 4.3b software. Quantitation of epidermal layers and immunostaining was done blindly.
Analysis of protein and RNA
Whole skin was homogenized using a Qiagen Tissuelyzer (Qiagen, Valencia, CA) in 0.1% NP-40 lysis buffer with protease and phosphatase inhibitors. Specific proteins were detected by immunoblotting and ECL (Pierce, Rockford, IL) using antibodies directed against Smad 2/3, p-Smad2, GAPDH (Cell Signaling Technology, Inc., Danvers, MA) and β-actin (Millipore). Total RNA was isolated from whole skin using Trizol (Invitrogen, Carlsbad, CA) and quantitative reverse transcription–polymerase chain reaction done for the indicated genes using the MyIQ system (Bio-Rad Laboratories, Hercules, CA). All values were normalized to GAPDH. Primer sequences were obtained from published studies or using Primer 3 (31) software with Genebank sequence information.
Statistical analysis
Values are expressed as the mean ± SD. Student’s t-test was used to compare the indicated groups and the significance of the difference was described. P-values of <0.05 were regarded as indicating a significant difference. Fischer’s exact test was used to determine significance of malignant conversion data.
Results
Topical SB inhibits TGFβ signaling in the mouse epidermis
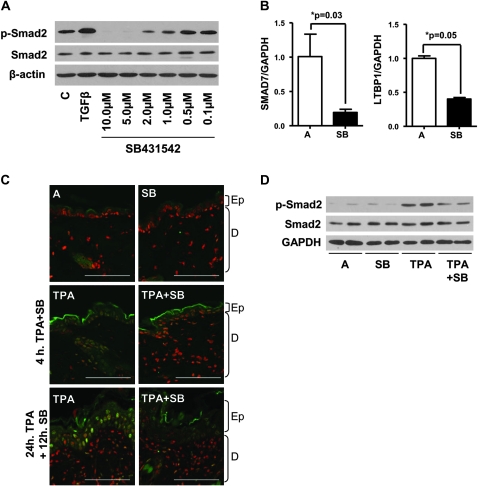
We treated primary mouse keratinocytes with SB to determine a useful dose range for inhibition of basal Smad2 phosphorylation. Figure 1A and supplementary Figure S1A (available at Carcinogenesis Online) show that as expected, SB significantly inhibited baseline Smad2 phosphorylation between 1 and 10 μM. Since detection of baseline p-Smad2 in the normal epidermis was difficult, we analyzed expression of TGFβ1 target genes by quantitative reverse transcription–polymerase chain reaction following topical SB treatment and found that expression of both Smad7, which is induced by TGFβ1 (32,33), and latent TGFβ-binding protein 1 (LTBP1) was significantly reduced within 4 h of SB treatment (Figure 1B). Since TPA induces TGFβ1 expression (34) and Smad2 phosphorylation (23) in the epidermis, we used indirect immunofluorescence to determine the effect of topical SB on TPA-induced TGFβ1 signaling. Basal levels of p-Smad2 were undetectable in acetone or SB-treated skin sections (Figure 1C, top), but 4 and 24 h after TPA treatment, the increase in nuclear p-Smad2 localization in keratinocytes was blocked by a pre- and post-TPA treatment with SB (Figure 1C, middle and bottom and Figure S2 is available at Carcinogenesis Online). Immunoblotting verified the reduced levels of TPA-induced p-Smad2 (Figure 1D and supplementary Figure S1B is available at Carcinogenesis Online), but no change was found in TPA-induced extracellular signal-regulated kinase phosphorylation following SB treatment (supplementary Figure S3 is available at Carcinogenesis Online). These results indicate that the effects of SB are specific for TPA-induced TGFβ1 signaling and sustained for at least 12 h after dosing.
Fig. 1.
SB reduces TPA-induced Smad2 phosphorylation in vivo. (A) Reduction in Smad2 phosphorylation following treatment with SB in keratinocytes. Full-length blots presented in supplementary Figure S1A (available at Carcinogenesis Online). (B) Quantitative reverse transcription–polymerase chain reaction analysis of Smad7 and LTBP-1 gene expression in acetone or SB-treated whole skin. Normalized to acetone only samples (n = 4). (C) Indirect immunofluorescence showing a reduction in TPA-induced p-Smad2 by SB at 4 and 24 h TPA treatment. Magnification ×800, bar represents 15 μm. P-Smad2 was detected with Alexa-fluor-488 (green), and nuclei counterstained with TO-PRO3 (red), (representative of n = 5). Ep, epidermis and D, dermis. Single color images presented in supplementary Figure S2 (available at Carcinogenesis Online). (D) Western blot of whole skin treated 24 h with TPA and 12 h SB, showing reduced TPA-induced Smad2 phosphorylation with SB treatment (representative of n = 5). Full-length blots presented in supplementary Figure S1B (available at Carcinogenesis Online). C, control and A, acetone.
SB inhibits papilloma formation but enhances malignant progression
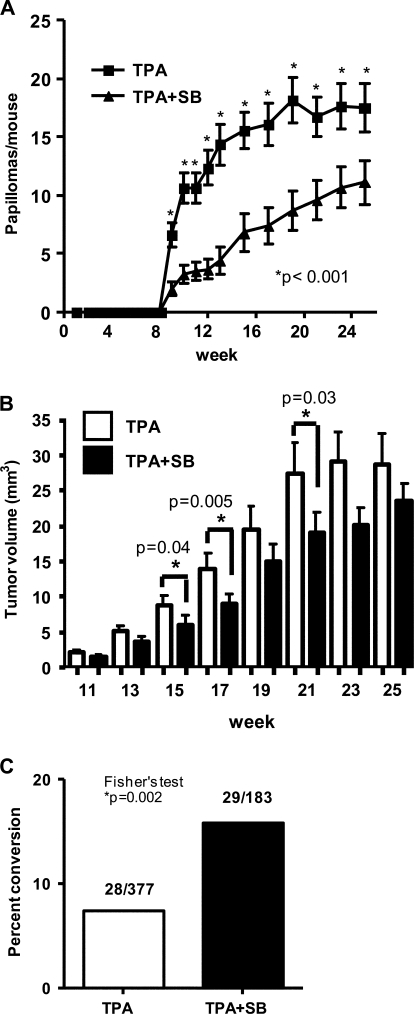
To determine the effect of TGFβ type I receptor pharmacological inhibition on cutaneous tumor formation and progression, we treated FVB/n mice with DMBA and then promoted with TPA twice a week with and without 10 μM SB for 25 weeks. In this protocol, mice were treated with SB just prior to TPA and an additional time in between each TPA treatment to maintain suppression of TGFβ1 signaling. By itself, SB treatment for 25 weeks did not induce tumor development in DMBA-initiated skin (data not shown). Surprisingly, topical SB treatment significantly inhibited TPA-induced papilloma formation at all time points (Figure 2A). In mice treated with TPA alone, the peak number of papillomas per mouse was 18.2 ± 8.2 at week 19 but only 11.1 ± 7.8 at week 25 in the TPA + SB-treated mice. In addition, the papillomas that formed with SB treatment were smaller than those that arose with TPA promotion alone between weeks 15 and 21 although this difference was not significant after week 23 (Figure 2B). Despite the reduced number of benign tumors with SB treatment, the total number and frequency of SCC that formed (28 total, 1.47 ± 0.70 SCC per mouse in the TPA and 29 total, 1.93 ± 1.7 SCC per mouse in the SB + TPA group) as well as the latency of SCC formation (supplementary Figure S4 is available at Carcinogenesis Online) was the same between the two treatment groups. Thus, papillomas that arose with SB treatment had a 2-fold increase (15.8%) in the frequency of malignant conversion compared with TPA alone (7.4%) (Figure 2C). Five of five SCCs in each group had a codon 61 mutation in HRas, indicating that SB was not causing outgrowth of a population of epidermal keratinocytes with a distinct initiating mutation (supplementary Figure S5 is available at Carcinogenesis Online). Since most SCC appeared after TPA + SB treatments were stopped at 25 weeks, these data suggest that the increased risk for malignant progression was established during the initial stages of promotion with SB and TPA.
Fig. 2.
ALK5 inhibition reduces tumor formation but enhances malignant conversion. (A) A significant reduction in tumor formation was observed at weeks 9–25 in mice treated with TPA + SB compared with TPA alone. (B) A significant decrease in tumor volume with SB treatment was observed at weeks 15, 17 and 21. Average volumes were determined from measurements of length × width × height using a digital micrometer. (C) Increased frequency of malignant conversion with SB treatment. Percent conversion was determined by dividing the total number of SCC that formed during the course of the study by the maximum number of papillomas.
Suppression of papilloma formation linked to altered epidermal homeostasis and reduced cutaneous inflammation
Since SB blocked TPA-mediated activation of TGFβ1 signaling in the skin, we tested if the reduction in papilloma formation was linked to inhibition of the tumor-promoting effects of TPA on normal skin. We analyzed epidermal hyperplasia, proliferation and apoptosis following 2 weeks of repeated TPA treatment with or without SB. Figure 3A shows that SB treatment caused a slight inhibition of TPA-induced epidermal hyperplasia (top) associated with a 10% decrease in keratinocyte proliferation (middle) and an increase in terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling assay + keratinocytes (bottom). Similar to that seen with a TGFβ type II receptor knockout (18), SB by itself caused an increase in apoptosis in the epidermis suggesting that the effects on apoptosis were due to direct inhibition of TGFβ signaling. In addition to inducing epidermal hyperplasia, TPA caused significant cutaneous inflammation, which is critical for tumor promotion. Figure 3B shows that in mice treated topically with SB, there was a sustained inhibition of both acute and chronic TPA-induced cutaneous inflammation. Twenty-four hours after treatment with TPA and SB, there was a 4-fold reduction in MPO + cell infiltrate into the skin compared with TPA alone, and this inhibition was maintained throughout a 2 week chronic treatment with TPA and SB (Figure 3B and supplementary Figure S6 is available at Carcinogenesis Online). Based on low frequency of F4/80+ cells in the skin after TPA treatment (9%), the majority of these MPO+ cells are probably neutrophils (data not shown). Consistent with the inhibition of neutrophil infiltration, SB suppressed acute (Figure 3C, top and bottom) and chronic (Figure 3C, middle and bottom) TPA-mediated induction of the neutrophil chemokine keratinocyte chemokine and macrophage-inflammatory protein 2 messenger RNA. Taken together, these results suggest that the combined inhibition of TPA-induced changes in normal epidermal homeostasis and cutaneous inflammation underlie the significant reduction in papilloma number.
Fig. 3.
SB suppresses TPA-induced inflammation. (A) Effect of SB on chronic TPA-induced hyperplasia (top) proliferation (middle) and apoptosis (bottom) (B) Decreased cutaneous neutrophils (MPO+ cells) after acute and chronic TPA + SB treatment. (C) Decreased keratinocyte chemokine (KC) and macrophage-inflammatory protein 2 (MIP2) gene expression in acute and chronically SB- and TPA-treated whole skin. Results normalized to TPA only samples. Reverse transcription–polymerase chain reaction showing induction of these chemokines by TPA (bottom). n = 4–6 mice per treatment group. A, acetone.
Early papillomas that develop following TPA and SB exhibit a progressed tumor phenotype
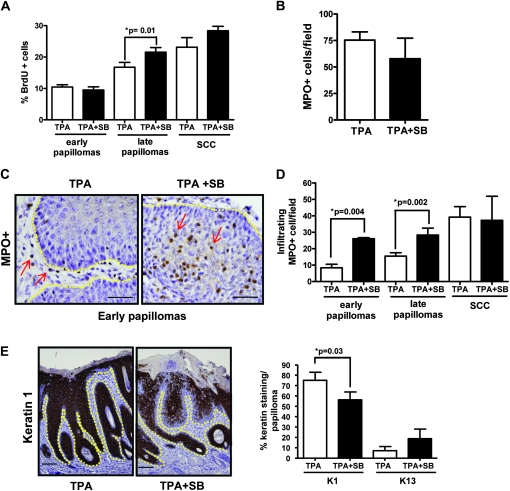
To identify potential mechanisms underlying enhanced malignant conversion, we analyzed early tumors that arose following 12 weeks of promotion with TPA alone or TPA + SB since any differences would reflect direct effects of SB rather than secondary changes due to progression. Figure 4A shows that tumor cell proliferation was not significantly different between early papillomas from either treatment group although at later time points after SB treatment had been discontinued tumor cell proliferation was greater in these tumors. In addition, there was no difference in tumor cell apoptosis as measured by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling assay staining in these early papillomas (supplementary Figure S7 is available at Carcinogenesis Online). However, although the total tumor-associated neutrophils in these early premalignant lesions were not significantly different (Figure 4B), their localization within the tumor was distinct. In papillomas that arose during TPA promotion, neutrophils were located primarily in the tumor stroma (Figure 4C, left) but in tumors promoted with TPA + SB, there was a significant increase in MPO+ cells within the epithelial component of the tumor (Figure 4C, right). In TPA-promoted early tumors, the number of tumor infiltrating neutrophils was <10 per field and this increased to an average of 40 in the SCC. In contrast, the average number of infiltrating neutrophils per field was 26 in early TPA + SB-promoted tumors and not significantly different from that found in papillomas harvested at much later time points or in SCC that developed in either treatment protocol suggesting that SB promoted outgrowth of papillomas with a progressed inflammatory microenvironment (Figure 4D).
Fig. 4.
Early papillomas from SB-treated mice have a progressed phenotype. (A) Tumor cell proliferation in early (n = 6) and late papillomas (n = 10) and SCC (n = 5) measured by bromodeoxyuridine incorporation counted at magnification ×400. (B) No significant change in total neutrophils (MPO+ cells) per field, counted at magnification ×400 (n = 6). (C) Localization of neutrophils in early papillomas from TPA-and SB/TPA-treated mice at magnification ×400 with scale bar representing 20 μm. Arrows indicate neutrophils. Yellow line shows epidermal/dermal junction. (D) Effect of SB on tumor infiltrating neutrophils in early (n = 6) and late (n = 18) papillomas and SCC (n = 5). (E) Reduced keratin 1 levels in early SB-treated papillomas (left). Representative micrographs, magnification ×100, scale bar representing 50 μm. Yellow line shows epidermal/dermal junction. Decreased expression of keratin 1 and increased expression of keratin 13 messenger RNA in early papillomas treated with SB (n = 12) (right). Percent keratin staining in the skin was scored blindly by two individuals.
To further examine the possibility that early tumors arising with SB treatment exhibited a more progressed phenotype, we analyzed expression of specific keratins that reflect tumor progression. Keratin 1 is a suprabasal differentiation marker in the epidermis that is highly expressed in early benign lesions but lost during tumor progression (35). In the majority of TPA only treated early papillomas, keratin 1 was strongly expressed throughout all the suprabasal layers, whereas expression was reduced in tumors that arose during SB treatment (Figure 4E). In contrast, TPA + SB-promoted papillomas had an increase in percentage of keratin 13-positive cells compared with TPA only tumors. Since keratin 13 is not normally expressed in the epidermis or in early papillomas but is increased during tumor progression (36), these data suggest that at early time points, SB-treated tumors represent a more progressed stage of tumor development.
SB rapidly alters phenotype of RAS-expressing epidermis
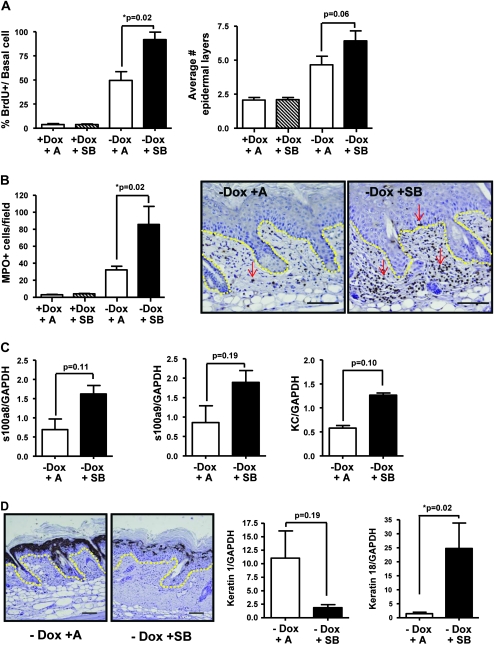
Since papillomas arising in SB-treated skin had a phenotype distinct from that of TPA-promoted papillomas and from TPA + SB-treated normal skin, we tested if oncogenic Ras altered the response to ALK5 inhibition using a bitransgenic model in which human RASV12G (27) is conditionally overexpressed in the epidermis with an Involucrin tTA driver line (26). When adult bitransgenic mice are removed from 10 ug/ml Dox in the drinking water RAS expression is induced within 1–2 days. Seven-week-old InvtTA/tetORAS mice were taken off Dox and treated with SB or acetone for 5 days. Overexpression of RASV12G caused epidermal hyperproliferation and this was enhanced by topical treatment with SB, although there was only a modest increase in epidermal thickness (Figure 5A) and no difference in apoptosis (data not shown). In contrast to normal skin, RAS-induced neutrophil infiltration into the dermis was increased by treatment with SB from 40 MPO+ cells per field to 80 MPO+ cells per field (Figure 5B), and there was a 3-fold increase in expression of the proinflammatory chemokines S100A8, S100A9 and keratinocyte chemokine (Figure 5C). Similar to the papillomas that arose during SB treatment in the two-stage carcinogenesis study, SB treatment of RAS-expressing epidermis downregulated keratin 1 expression at both the RNA and the protein level (Figure 5D). Although we did not detect increased expression of keratin 13, SB treatment caused a significant increase in messenger RNA level expression of keratin 18, a marker of simple epithelia and malignant conversion of squamous epithelia (37,38) (Figure 5D, right). When InvtTA/tetORAS mice are treated with a reduced Dox concentration (250 ng/ml) in drinking water, focal lesions form after 2–3 weeks. Following 5 weeks expression of RAS and treatment with SB, a significant increase in tumor formation was found compared with acetone alone (Figure 6A). SB-treated tumors appeared more disorganized with an appearance that closely resembled chemically induced SCCs (supplementary Figure S8A is available at Carcinogenesis Online) and had significantly reduced keratin 1 expression similar to short-term SB-treated InvtTA/tetORAS skin and SB-treated tumors from the two-stage carcinogenesis study (supplementary Figure S8B is available at Carcinogenesis Online).
Fig. 5.
SB rapidly induces progressed phenotype in epidermis-expressing human C-Ha RASV12G oncogene. (A) Significant increase in proliferation with SB treatment in RAS-expressing skin (left) and slight increase in epidermal thickness (right). (B) An increase in MPO+ cells in the dermis of SB-treated mice expressing the InvtTA × tetORASV12G transgenes. Arrows indicate neutrophils. Yellow line shows epidermal/dermal junction. Representative micrographs (right), magnification, ×100. Scale bar represents 50 μm. (C) Increased messenger RNA expression of neutrophil chemokines s100a8, s100a9 and keratinocyte chemokine with treatment of SB. (D) SB causes significant decrease in keratin 1 staining (left) and messenger RNA expression (middle). Representative micrographs (left), magnification ×100. Scale bar represents 50 μm. Yellow dotted line shows epidermal/dermal junction. Significant increase in keratin 18 messenger RNA expression by quantitative reverse transcription–polymerase chain reaction in RAS-expressing skin following SB (right). n = 4–6 mice per treatment group.
Fig. 6.
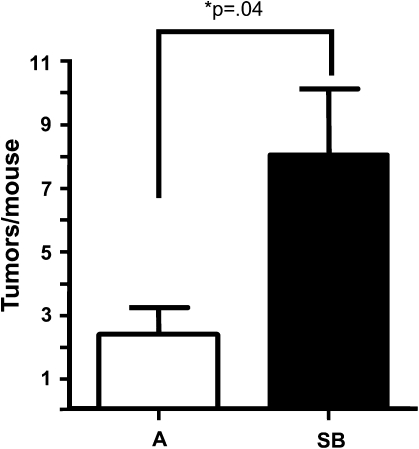
ALK5 inhibition increases tumor formation in Involucrin/tetORAS mice. (A) A significant increase in tumor formation following treatment with SB compared with acetone alone. Tumors were counted at 5 weeks. n = 5 mice.
Discussion
TGFβ1 can act as both a tumor suppressor for early stages of cancer and a cytokine that enhances the malignant and metastatic phenotype (25). Recent studies using small molecule inhibitors of the TGFβ1 type I receptor kinase show that this class of compounds can alter the growth of malignant cancers in vivo and the malignant phenotype in vitro. Thus, the growth of xenotransplanted syngeneic mouse mammary carcinoma (8,10) and mesothelioma (9) and human and mouse glioma cells (7) are suppressed in vivo by ALK5 inhibitors. Despite these promising results, the finding that treatment of Eker rats with an ALK5 inhibitor enhances development of renal cell carcinoma, whereas suppressing incidence and volume of mesenchymal tumors (39) suggests that long-term treatment with these agents could provoke cancer development in epithelial tissues. Here, we have tested the effect of a prototypical ALK5 inhibitor, SB, on tumor formation in the mouse skin chemical carcinogenesis model, a well-characterized model of multistage squamous tumor development for which TGFβ signaling plays an important role.
We and others have shown that treatment of mouse skin with the tumor promoter TPA causes rapid induction of TGFβ1 expression in the epidermis and phosphorylation of Smad2 (23,34). We determined that 10 μM SB suppressed expression of baseline levels of two TGFβ1 target genes in the epidermis as well as the induction of Smad2 phosphorylation by topical treatment with the tumor promoter TPA and that inhibition was apparent for up to 12 h post-SB treatment similar to previous in vivo studies with ALK5 inhibitors like SB (8,9). Although we cannot rule out off target effects on unknown pathways, the lack of effect of SB on TPA-induced mitogen-activated protein kinase pathway activation as well as previous studies on the specificity of SB strongly suggest that the observed effects on tumor formation are due to inhibition of TGFβ signaling.
The carcinogen DMBA causes initiating mutations in the HRas gene in epidermal keratinocytes but repeated application of tumor promoters are required for clonal expansion and formation of premalignant lesions (35). Although transgenic or knockout models with blocked TGFβ1 receptor signaling in cutaneous or oral keratinocytes exhibit a dependence on Ras activation for tumor formation (15,18,40,41), we found that repeated application of SB for 25 weeks did not cause tumor formation in DMBA-initiated epidermis, suggesting either that by itself pharmacological inhibition of TGFβ1 signaling is not sufficient to promote squamous tumors or that inhibition of TGFβ1 signaling in other resident or infiltrating skin cells suppresses any promoting effects on keratinocytes with an activated Ras mutation. In support of the latter concept, our results show that SB suppressed the number and size of TPA-induced premalignant squamous papillomas relative to TPA alone. These results are consistent with our recent study showing that TGFβ1+/− mice developed fewer and smaller papillomas in a similar skin carcinogenesis protocol (23). This reduction in tumor number and size may be accounted for by the decrease in epidermal proliferation and increase in apoptosis observed in SB-treated normal epidermis with chronic TPA promotion. It is significant that both pharmacological inhibition and genetic ablation of the TGFβ type I receptor (18) in the epidermis results in elevated apoptosis. However, this difference appears to be lost in early papillomas and Ras-expressing epidermis, supporting the idea that Ras activation suppresses proapoptotic effects of inactivated TGFβ signaling (18).
An additional mechanism for suppression of epidermal proliferation, papilloma number and size is the inhibition of TPA-induced cutaneous inflammation, which has repeatedly been shown to be a critical component of tumor promotion in the skin (42,43). Our previous study showed that TPA-induced cutaneous inflammation was exaggerated in TGFβ1+/− mice (23) suggesting an important role for TPA-induced TGFβ1 in limiting the inflammatory response. In contrast, SB significantly suppressed acute or chronic TPA-induced infiltration of MPO+ cells into the skin, and this was associated with reduced expression of two proinflammatory cytokines keratinocyte chemokine and macrophage-inflammatory protein 2. However, we could not detect an effect of SB on TPA-induced expression of these or other proinflammatory molecules in primary mouse keratinocytes or fibroblasts (data not shown), indicating that SB is not directly blocking TPA-induced signaling pathways that activate these genes. Interestingly, it has been shown that TGFβ1 is a potent chemoattractant for neutrophils (44) and the TGFβ type I receptor on neutrophils mediates this response (45), suggesting either that inhibition of TGFβ signaling in a skin resident immune cell was critical for blocking the inflammatory response or that inhibition occurred in neutrophils directly. Taken together, these results suggest that the response to TGFβ in an as yet unidentified cutaneous cell type is critical for amplification of TPA-induced inflammation and enhanced outgrowth of benign lesions.
Similar to transgenic and knockout models with blocked TGFβ receptor signaling (16–18,46,47), our results show that chronic treatment with SB increases the conversion to SCC. However, our data reveal new insights into the role of TGFβ signaling in malignant progression of squamous tumors. We have shown previously that in the two-stage chemical carcinogenesis model, SCC arise from a small subpopulation of benign lesions with biochemically distinct properties at an early stage of tumor development, whereas the majority of papillomas do not have the capacity to progress to SCC or do so at a much slower rate (35). Microarray analysis of these different premalignant tumor subclasses showed that early non-progressing papillomas or low-risk lesions had an elevated inflammatory gene signature (48) suggesting that these tumors are dependent on inflammation for outgrowth. Our results suggest that SB blocks a TGFβ-dependent pathway of cutaneous inflammation that is critical for outgrowth of low-risk benign lesions but at the same time promotes outgrowth of lesions with a rapid progression phenotype. Thus, unlike TPA only treated papillomas, early lesions in SB-treated mice have an inflammatory microenvironment similar to SCC, with intraepithelial infiltration of MPO+ cells, reduced expression of normal squamous differentiation markers and expression of keratin markers of progression. The rapid induction of this phenotype in RAS-expressing transgenic epidermis and tumors provides strong evidence that pharmacological inhibition of TGFβ signaling during DMBA–TPA-induced tumor formation directly provokes this response. The observation that keratin 8, a marker of simple epithelium and malignant conversion in squamous epithelium is also induced in pancreas expressing a dominant negative TβRII transgene (49) and keratinocytes by a dominant negative TβRII adenovirus (50) indicates that the induction of these simple epithelial markers in the transgenic RAS epidermis by SB is the result of blocked TGFβ signaling rather than off target effects and directly implicates TGFβ signaling in the maintenance of the squamous differentiation phenotype in the epidermis. It is clear that subsets of keratinocytes with an activated HRas oncogene respond differently to inhibition of ALK5 in the tissue microenvironment. Our results show that a large number of initiated keratinocytes are dependent on ALK5 signaling for inflammatory pathways that enhance tumor outgrowth. A second subset can form tumors despite inhibition of cutaneous inflammation, in part through generation of an altered inflammatory microenvironment and through altered squamous differentiation induced by inhibition of ALK5. This latter group has a higher frequency of malignant conversion. The basis of this differential response to ALK5 inhibition is unknown but could reflect different roles of TGFβ signaling in epidermal keratinocytes with distinct developmental fates or capacity.
Previous in vivo studies with different small molecule inhibitors of the TGFβ type I receptor kinase suggest that these compounds could have potential use as anticancer and antifibrotic therapeutics and point to immune mechanisms as key to their ability to block growth of syngeneic tumors in mice. Thus, in keeping with the well-documented role of TGFβ1 as an immunosuppressive cytokine in the tumor microenvironment, this class of inhibitors has been shown to increase the T-cell antitumor response (8–10) and natural killer cells and macrophages (7). In a model of squamous cancer driven by chronic inflammation and tissue remodeling, our results show that pharmacological inhibition of TGFβ signaling also blocks tumor outgrowth in part through inhibition of TGFβ-dependent epidermal inflammation. However, the differential outgrowth of premalignant lesions with an inflammatory phenotype similar to SCC following long-term treatment with SB and at high risk for malignant conversion suggests that effects of these inhibitors on the tumor immune microenvironment during tumor formation and premalignant progression may be different than on malignant tumors. Thus, these data open the possibility for use of these inhibitors in settings where TGFβ signaling is a component of disease processes associated with chronic inflammation but also suggests that long-term use of this inhibitor class should be approached with caution.
Supplementary material
Supplementary Figures S1–8 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (RO1 CA117957, RO1 CA122109 to A.B.G); the authors would also like to thank the Dermal Toxicology Specialty Section of the National Society of Toxicology for partial funding of this project through the Battelle Student Research Award.
Supplementary Material
Acknowledgments
All confocal and digital microscopy was done at the Cytometry Facility at the Huck Institutes of the Life Sciences, Penn State University.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- DMBA
7, 12-dimethylbenz(a)anthracene
- Dox
doxycycline
- MPO
myeloperoxidase
- SB
SB431542
- SCC
squamous cell carcinoma
- TGFβ1
transforming growth factor β1
- TPA
12-O-tetradecanoylphorbol 13-acetate
References
- 1.Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 2.Callahan JF, et al. Identification of novel inhibitors of the transforming growth factor beta1 (TGF-beta1) type 1 receptor (ALK5) J. Med. Chem. 2002;45:999–1001. doi: 10.1021/jm010493y. [DOI] [PubMed] [Google Scholar]
- 3.Inman GJ, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Laping NJ, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: sB-431542. Mol. Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 5.Halder SK, et al. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–521. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tojo M, et al. The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci. 2005;96:791–800. doi: 10.1111/j.1349-7006.2005.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhl M, et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 8.Ge R, et al. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin. Cancer Res. 2006;12:4315–4330. doi: 10.1158/1078-0432.CCR-06-0162. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki E, et al. A novel small-molecule inhibitor of transforming growth factor beta type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res. 2007;67:2351–2359. doi: 10.1158/0008-5472.CAN-06-2389. [DOI] [PubMed] [Google Scholar]
- 10.Rausch MP, et al. An orally active small molecule TGF-beta receptor I antagonist inhibits the growth of metastatic murine breast cancer. Anticancer Res. 2009;29:2099–2109. [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa T, et al. SB-431542 inhibits TGF-beta-induced contraction of collagen gel by normal and keloid fibroblasts. J. Dermatol. Sci. 2005;39:33–38. doi: 10.1016/j.jdermsci.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, et al. Contribution of activin receptor-like kinase 5 (transforming growth factor beta receptor type I) signaling to the fibrotic phenotype of scleroderma fibroblasts. Arthritis Rheum. 2006;54:1309–1316. doi: 10.1002/art.21725. [DOI] [PubMed] [Google Scholar]
- 13.Grygielko ET, et al. Inhibition of gene markers of fibrosis with a novel inhibitor of TGF{beta}-type I receptor kinase in puromycin-induced nephritis. J. Pharmacol. Exp. Ther. 2005;313:943–951. doi: 10.1124/jpet.104.082099. [DOI] [PubMed] [Google Scholar]
- 14.Petersen M, et al. Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008;73:705–715. doi: 10.1038/sj.ki.5002717. [DOI] [PubMed] [Google Scholar]
- 15.Bian Y, et al. Progressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res. 2009;69:5918–5926. doi: 10.1158/0008-5472.CAN-08-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mincione G, et al. Loss of expression of TGF-beta1, TbetaRI, and TbetaRII correlates with differentiation in human oral squamous cell carcinomas. Int. J. Oncol. 2008;32:323–331. [PubMed] [Google Scholar]
- 17.Honjo Y, et al. TGF-beta receptor I conditional knockout mice develop spontaneous squamous cell carcinoma. Cell Cycle. 2007;6:1360–1366. doi: 10.4161/cc.6.11.4268. [DOI] [PubMed] [Google Scholar]
- 18.Guasch G, et al. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–327. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis—Thirty-third G.H.A. Clowes Memorial Award Lecture. Cancer Res. 1994;54:1178–1189. [PubMed] [Google Scholar]
- 20.Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. J. Dermatol. Sci. 1998;17:1–7. doi: 10.1016/s0923-1811(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 21.Cui W, et al. TGFβ1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 22.Blessing M, et al. Chemical skin carcinogenesis is prevented in mice by the induced expression of a TGF-β related transgene. Teratog. Carcinog. Mutagen. 1995;15:11–21. doi: 10.1002/tcm.1770150103. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Lorenzo R, et al. Transforming growth factor {beta}1 enhances tumor promotion in mouse skin carcinogenesis. Carcinogenesis. 2010;31:1116–1123. doi: 10.1093/carcin/bgq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li AG, et al. Smad3 knockout mice exhibit a resistance to skin chemical carcinogenesis. Cancer Res. 2004;64:7836–7845. doi: 10.1158/0008-5472.CAN-04-1331. [DOI] [PubMed] [Google Scholar]
- 25.Glick AB. TGFbeta1, back to the future: revisiting its role as a transforming growth factor. Cancer Biol. Ther. 2004;3:276–283. doi: 10.4161/cbt.3.3.849. [DOI] [PubMed] [Google Scholar]
- 26.Jaubert J, et al. Tetracycline-regulated transactivators driven by the involucrin promoter to achieve epidermal conditional gene expression. J. Invest. Dermatol. 2004;123:313–318. doi: 10.1111/j.0022-202X.2004.23203.x. [DOI] [PubMed] [Google Scholar]
- 27.Chin L, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 28.Roop DR, et al. Transcriptional control of high molecular weight keratin gene expression in multistage mouse skin carcinogenesis. Cancer Res. 1988;48:3245–3252. [PubMed] [Google Scholar]
- 29.Cheng C, et al. The v-ras oncogene inhibits the expression of differentiation markers and facilitates expression of cytokeratins 8 and 18 in mouse keratinocytes. Mol. Carcinog. 1990;3:363–373. doi: 10.1002/mc.2940030608. [DOI] [PubMed] [Google Scholar]
- 30.Pizza FX, et al. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J. Physiol. 2005;562:899–913. doi: 10.1113/jphysiol.2004.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozen S, et al. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 32.Afrakhte M, et al. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. Biochem. Biophys. Res. Commun. 1998;249:505–511. doi: 10.1006/bbrc.1998.9170. [DOI] [PubMed] [Google Scholar]
- 33.Brodin G, et al. Efficient TGF-beta induction of the Smad7 gene requires cooperation between AP-1, Sp1, and Smad proteins on the mouse Smad7 promoter. J. Biol. Chem. 2000;275:29023–29030. doi: 10.1074/jbc.M002815200. [DOI] [PubMed] [Google Scholar]
- 34.Akhurst RJ, et al. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988;331:363–365. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- 35.Glick A, et al. The high-risk benign tumor: evidence from the two-stage skin cancer model and relevance for human cancer. Mol. Carcinog. 2007;46:605–610. doi: 10.1002/mc.20345. [DOI] [PubMed] [Google Scholar]
- 36.Nischt R, et al. Aberrant expression during two-stage mouse skin carcinogenesis of type I 47-kDa Keratin, K13, normally associated with terminal differentiation of internal stratified epithelia. Mol. Carcinog. 1988;1:96–108. doi: 10.1002/mc.2940010205. [DOI] [PubMed] [Google Scholar]
- 37.Larcher F, et al. Aberrant expression of the simple epithelial type II keratin 8 by mouse skin carcinomas but not papillomas. Mol. Carcinog. 1992;6:112–121. doi: 10.1002/mc.2940060206. [DOI] [PubMed] [Google Scholar]
- 38.Fillies T, et al. Cytokeratin 8/18 expression indicates a poor prognosis in squamous cell carcinomas of the oral cavity. BMC Cancer. 2006;6:10. doi: 10.1186/1471-2407-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laping NJ, et al. Tumor-specific efficacy of transforming growth factor-beta RI inhibition in Eker rats. Clin. Cancer Res. 2007;13:3087–3099. doi: 10.1158/1078-0432.CCR-06-1811. [DOI] [PubMed] [Google Scholar]
- 40.Go C, et al. Blocking transforming growth factor β signaling in transgenic epidermis accelerates chemical carcinogenesis: a mechanism associated with increased angiogenesis. Cancer Res. 1999;59:2861–2868. [PubMed] [Google Scholar]
- 41.Lu SL, et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Visser KE, et al. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Balkwill F, et al. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 44.Reibman J, et al. Transforming growth factor beta 1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proc. Natl Acad. Sci. USA. 1991;88:6805–6809. doi: 10.1073/pnas.88.15.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandes ME, et al. Type I transforming growth factor-beta receptors on neutrophils mediate chemotaxis to transforming growth factor-beta. J. Immunol. 1991;147:1600–1606. [PubMed] [Google Scholar]
- 46.Amendt C, et al. Expression of a dominant negative type II TGF-β receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene. 1998;17:25–34. doi: 10.1038/sj.onc.1202161. [DOI] [PubMed] [Google Scholar]
- 47.Go C, et al. Aberrant cell cycle progression contributes to the early-stage accelerated carcinogenesis in transgenic epidermis expressing the dominant negative TGFbetaRII. Oncogene. 2000;19:3623–3631. doi: 10.1038/sj.onc.1203701. [DOI] [PubMed] [Google Scholar]
- 48.Darwiche N, et al. Expression profile of skin papillomas with high cancer risk displays a unique genetic signature that clusters with squamous cell carcinomas and predicts risk for malignant conversion. Oncogene. 2007;26:6885–6895. doi: 10.1038/sj.onc.1210491. [DOI] [PubMed] [Google Scholar]
- 49.Casanova ML, et al. Exocrine pancreatic disorders in transsgenic mice expressing human keratin 8. J. Clin. Invest. 1999;103:1587–1595. doi: 10.1172/JCI5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shukla A, et al. Cripto-1 alters keratinocyte differentiation via blockade of transforming growth factor-beta1 signaling: role in skin carcinogenesis. Mol. Cancer Res. 2008;6:509–516. doi: 10.1158/1541-7786.MCR-07-0396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.