Abstract
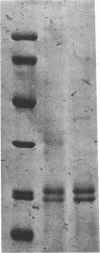
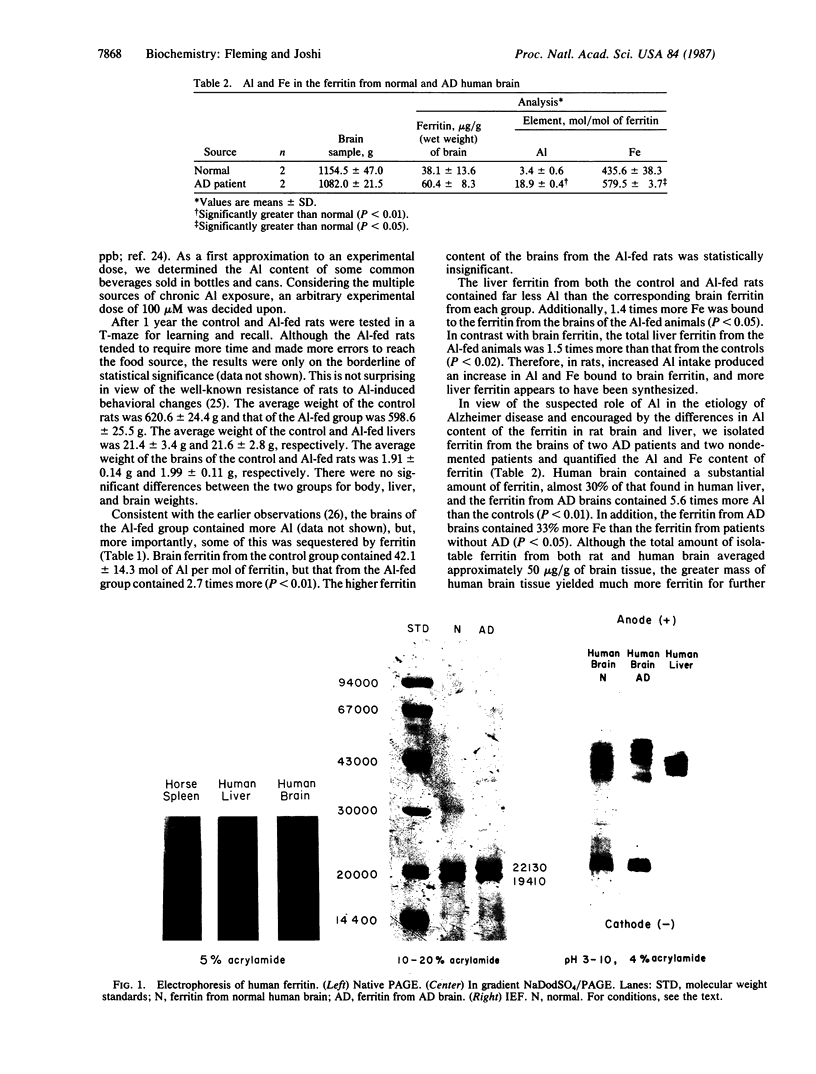
Ferritin was isolated from the livers and brains of two groups of rats, one of which was fed aluminum chloride (100 microM) for 1 year in the drinking water. Brain tissue contained about one-third of the amount of ferritin found in the liver. While brain ferritin from normal rats contained 42.1 +/- 14.3 mol of aluminum, that from the aluminum-fed group contained 115.4 +/- 48.3 mol of aluminum per mol of ferritin. Liver ferritin from both groups contained similar amounts of both aluminum and iron, and the amounts were less than that found associated with brain ferritin. Ferritin isolated from the brains of patients who died of Alzheimer disease contained more aluminum and more iron than that from age-matched controls. Human brain ferritin is composed of two types of subunits--about 70% heavy chain (Mr, 22,000) and 30% light chain (Mr, 19,500). The isoelectric focusing pattern of human brain ferritin was considerably different from that of human liver. Only 5 of the 20 brain ferritin bands migrated similarly to the acidic isoferritins from the liver, and the major component of brain ferritin, representing 30% of the total ferritin, had a pI of 8.0.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman T. G., Arosio P., Drysdale J. W. Multiple subunits in human ferritins: evidence for hybrid molecules. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1056–1062. doi: 10.1016/0006-291x(75)90676-2. [DOI] [PubMed] [Google Scholar]
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Alfrey A. C., LeGendre G. R., Kaehny W. D. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med. 1976 Jan 22;294(4):184–188. doi: 10.1056/NEJM197601222940402. [DOI] [PubMed] [Google Scholar]
- Bothe D., Simonis M., von Döhren H. A sodium dodecyl sulfate-gradient gel electrophoresis system that separates polypeptides in the molecular weight range of 1500 to 100,000. Anal Biochem. 1985 Nov 15;151(1):49–54. doi: 10.1016/0003-2697(85)90051-x. [DOI] [PubMed] [Google Scholar]
- Bowdler N. C., Beasley D. S., Fritze E. C., Goulette A. M., Hatton J. D., Hession J., Ostman D. L., Rugg D. J., Schmittdiel C. J. Behavioral effects of aluminum ingestion on animal and human subjects. Pharmacol Biochem Behav. 1979 Apr;10(4):505–512. doi: 10.1016/0091-3057(79)90225-9. [DOI] [PubMed] [Google Scholar]
- Burks J. S., Alfrey A. C., Huddlestone J., Norenberg M. D., Lewin E. A fatal encephalopathy in chronic haemodialysis patients. Lancet. 1976 Apr 10;1(7963):764–768. doi: 10.1016/s0140-6736(76)91608-1. [DOI] [PubMed] [Google Scholar]
- Crapper D. R., Krishnan S. S., Quittkat S. Aluminium, neurofibrillary degeneration and Alzheimer's disease. Brain. 1976 Mar;99(1):67–80. doi: 10.1093/brain/99.1.67. [DOI] [PubMed] [Google Scholar]
- Deshpande V. V., Joshi J. G. Vit C.Fe(III) induced loss of the covalently bound phosphate and enzyme activity of phosphoglucomutase. J Biol Chem. 1985 Jan 25;260(2):757–764. [PubMed] [Google Scholar]
- Hurlbut B. J., Lubar J. F., Switzer R., Dougherty J., Eisenstadt M. L. Basal forebrain infusion of HC-3 in rats: maze learning deficits and neuropathology. Physiol Behav. 1987;39(3):381–393. doi: 10.1016/0031-9384(87)90239-3. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- King S. W., Savory J., Wills M. R. The clinical biochemistry of aluminum. Crit Rev Clin Lab Sci. 1981;14(1):1–20. doi: 10.3109/10408368109105861. [DOI] [PubMed] [Google Scholar]
- Lindenschmidt R. C., Sendelbach L. E., Witschi H. P., Price D. J., Fleming J., Joshi J. G. Ferritin and in vivo beryllium toxicity. Toxicol Appl Pharmacol. 1986 Feb;82(2):344–350. doi: 10.1016/0041-008x(86)90211-5. [DOI] [PubMed] [Google Scholar]
- Price D. J., Joshi J. G. Ferritin. Binding of beryllium and other divalent metal ions. J Biol Chem. 1983 Sep 25;258(18):10873–10880. [PubMed] [Google Scholar]
- Price D., Joshi J. G. Ferritin: a zinc detoxicant and a zinc ion donor. Proc Natl Acad Sci U S A. 1982 May;79(10):3116–3119. doi: 10.1073/pnas.79.10.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Harrison P. M., Shinjo S. Discrete bands in ferritin electrofocussing patterns. Br J Haematol. 1978 Feb;38(2):296–298. doi: 10.1111/j.1365-2141.1978.tb01047.x. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Translational control of ferritin synthesis by iron in embryonic reticulocytes of the bullfrog. J Biol Chem. 1982 Dec 10;257(23):14187–14191. [PubMed] [Google Scholar]
- Thomas C. E., Aust S. D. Reductive release of iron from ferritin by cation free radicals of paraquat and other bipyridyls. J Biol Chem. 1986 Oct 5;261(28):13064–13070. [PubMed] [Google Scholar]