Abstract
Tamoxifen is the most commonly prescribed therapy for patients with estrogen receptor (ER)α-positive breast tumors. Tumor resistance to tamoxifen remains a serious clinical problem especially in patients with tumors that also overexpress human epidermal growth factor receptor 2 (HER2). Current preclinical models of HER2 overexpression fail to recapitulate the clinical spectrum of endocrine resistance associated with HER2/ER-positive tumors. Here, we show that ectopic expression of a clinically important oncogenic isoform of HER2, HER2Δ16, which is expressed in >30% of ER-positive breast tumors, promotes tamoxifen resistance and estrogen independence of MCF-7 xenografts. MCF-7/HER2Δ16 cells evade tamoxifen through upregulation of BCL-2, whereas mediated suppression of BCL-2 expression or treatment of MCF-7/HER2Δ16 cells with the BCL-2 family pharmacological inhibitor ABT-737 restores tamoxifen sensitivity. Tamoxifen-resistant MCF-7/HER2Δ16 cells upregulate BCL-2 protein levels in response to suppressed ERα signaling mediated by estrogen withdrawal, tamoxifen treatment or fulvestrant treatment. In addition, HER2Δ16 expression results in suppression of BCL-2-targeting microRNAs miR-15a and miR-16. Reintroduction of miR-15a/16 reduced tamoxifen-induced BCL-2 expression and sensitized MCF-7/HER2Δ16 to tamoxifen. Conversely, inhibition of miR-15a/16 in tamoxifen-sensitive cells activated BCL-2 expression and promoted tamoxifen resistance. Our results suggest that HER2Δ16 expression promotes endocrine-resistant HER2/ERα-positive breast tumors and in contrast to wild-type HER2, preclinical models of HER2Δ16 overexpression recapitulate multiple phenotypes of endocrine-resistant human breast tumors. The mechanism of HER2Δ16 therapeutic evasion, involving tamoxifen-induced upregulation of BCL-2 and suppression of miR-15a/16, provides a template for unique therapeutic interventions combining tamoxifen with modulation of microRNAs and/or ABT-737-mediated BCL-2 inhibition and apoptosis.
Introduction
MicroRNAs (miRNAs) are small non-coding single-stranded RNA regulatory molecules of ∼22 bp that suppress gene expression by binding the 3′-untranslated regions of target gene messenger RNAs (mRNAs). Once bound to their target mRNA, miRNAs may repress gene expression through enhanced degradation of the mRNA or more commonly by inhibiting target gene translation (1). Temporal and spatial regulations of miR expression have profound effects on normal cellular processes, including proliferation, differentiation and apoptosis. In addition, profiling experiments have identified aberrant miR regulation during tumorigenesis suggesting that miRNAs may play a role in cancer as well (2). Indeed, miRNAs influence multiple stages of breast cancer, including tumor development (3), metastasis (4) and therapeutic evasion (5,6).
Breast cancer is the most commonly diagnosed cancer in women and an estimated one in eight women will develop breast caner in their lifetimes. Nearly, 70% of breast cancer patients develop tumors expressing the estrogen receptor (ER)α and are therefore candidates for endocrine therapy. The selective ERα modulator tamoxifen is the most commonly prescribed endocrine therapy in the breast cancer clinic and has recently been recommended as a preventative in individuals at high risk of developing breast cancer (7). Nevertheless, 30–40% of breast cancer patients fail adjuvant tamoxifen therapy and nearly all patients with metastatic disease develop tamoxifen resistance (8–10). Unfortunately, de novo and acquired tumor resistance to tamoxifen therapy remains a poorly understood and serious clinical problem.
Several clinical studies implicate tumor expression of the human epidermal growth factor receptor 2 (HER2) receptor tyrosine kinase as a significant risk for tamoxifen failure. Patients with HER2-expressing breast tumors account for approximately half of the ERα-positive population and >70% of these patients may exhibit de novo tamoxifen resistance (10). Furthermore, a large percentage of HER2/ERα-positive tumors are estrogen independent and therefore continue to grow when patients are estrogen depleted (11). Preclinical models of HER2 overexpression have provided insights into possible mechanisms underlying tamoxifen resistance; however, only the occasional HER2-overexpressing ERα-positive cell line exhibits at best partial tamoxifen resistance, and in contrast to a significant percentage of primary breast tumors, HER2-overexpressing ERα-positive breast tumor cell lines remain estrogen dependent (12–17).
We have recently shown that an oncogenic isoform of HER2 (18,19), HER2Δ16, is clinically important and commonly coexpressed with HER2 in ERα-positive primary breast tumors (20). HER2Δ16 harbors an in-frame deletion, which promotes constitutive dimerization of the receptor, thereby coupling HER2Δ16 to unique oncogenic signaling pathways. We have shown previously that, in contrast to wild-type HER2, HER2Δ16 expression is associated with node-positive breast cancer and trastuzumab resistance (20). Here, we demonstrate that expression of HER2Δ16, but not wild-type HER2, in ERα-positive breast tumor cells promotes estrogen-independent growth and de novo resistance to tamoxifen therapy. We further demonstrate that HER2Δ16 evades tamoxifen therapy through a novel mechanism involving altered regulation of BCL-2, in part by modulating expression of BCL-2 targeting miRNAs.
Materials and methods
Cell lines
The MCF-7 and MDA-MB-231 cell lines were purchased from American Type Culture Collection (Manassas, VA) and cultured according to their instructions. Stable MCF-7 cell lines expressing pcDNA3, pcDNA3-HER2 or pcDNA3-HER2Δ16 and referred to here as MCF-7/Vector, MCF-7/HER2 and MCF-7/HER2Δ16, respectively, have been described elsewhere (20,21).
Tumor formation in nude mice
Tumor xenografts were developed by injecting 5 × 106 cells in a Matrigel Basement Membrane Matrix (BD Biosciences, Bedford, MA) into 4- to 5-week-old NU/NU immune compromised female mice (Charles River Laboratories, Wilmington, MA) and analyzed as described elsewhere (21) with the following modifications. The estradiol groups were implanted subcutaneous in the scapular region with a 60 day release 0.72 mg estradiol pellet (Innovative Research of America, Sarasota, FL) and the minus estradiol groups were implanted with placebo pellets during the entire experiment. Tumors were allowed to develop for 21 days at which time mice were randomized and implanted with a 60 day release 5 mg tamoxifen pellet (Innovative Research of America) or a 60 day release 5 mg placebo (Innovative Research of America). Tumor volume was calculated every 3 days.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Cell proliferation was measured as a function of metabolism by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (Sigma, St Louis, MO) assay exactly as described elsewhere (22) with the modification that 3000 cells per well in a 96-well plate were preincubated in phenol red-free modified Eagle’s medium supplemented with 5% charcoal-stripped fetal bovine serum [charcoal stripped minimal essential media (CS-MEM)] for 48 h and were treated with 100 pM 17-β-estradiol (Sigma) alone or in combination with 1.0 μM 4-hydroxytamoxifen (TAM; Sigma) for 5 days. Each sample was prepared in triplicate and the data represent the mean and standard error (SE) of at least three independent experiments. Statistically significant differences between data sets were determined using paired Student’s t-test.
Western blot analysis of cell lysates
Total cell lysates were prepared from ∼5 × 106 cells in a 100 mm tissue culture dish and analyzed by western blot exactly as described elsewhere (23). Primary antibodies used for western blot analysis included ER Ab-1 (Neomarkers, Fremont, CA), HER2 #RB103 (Neomarkers), α-tubulin #05829 (Upstate Biotechnology, Lake Placid, NY) and BCL-2 (Cell Signaling Technology, Danvers, MA). Secondary antibodies were Alexa-fluor 680 Conjugated Affinity Purified Anti-Rabbit or Anti-Mouse IgG (Invitrogen, Carlsbad, CA) detected using an Odyssey Infrared Imaging System (Licor Biosciences, Lincoln, NE). Western blot images were quantitated using the Odyssey Infrared Imaging System software. The integrated intensity (a measure of the magnitude of the signal and the area over which is distributed) was measured for each band. The ‘average or median background’ methods were used to correct for noise signals.
Luciferase reporter assay
Luciferase reporter assay to measure ERα transcriptional activity in MCF-7/Vector, MCF-7/HER2 and MCF-7/HER2Δ16 stable cell lines was performed by transfecting 2 × 105 cells in a six-well plate with 1 μg of an estrogen-response-element-luciferase reporter. The estrogen-response-element-luciferase reporter was stimulated with 100 pM 17-β-estradiol alone or in combination with 1 μM 4-hydroxytamoxifen or 100 nM ICI 182780 (ICI; Tocris, Ellisville, MO) for 48 h. Cell lysates were prepared in 500 μl of cell culture lysis reagent (Promega, Madison, WI) and luciferase assay was performed on 30 μl of lysate using the luciferase assay system (Promega) in a Berthold AutoLumat Plus luminometer. Each sample was prepared in triplicate and the data represent the mean of the triplicate samples.
Analysis of BCL-2 mRNA by quantitative reverse transcription–polymerase chain reaction
Each stable MCF-7 cell line was cultured in CS-MEM for 24 h and then treated with 100 pM 17-β-estradiol alone or in combination with 1 μM 4-hydroxytamoxifen for 1, 4, 8, 12, 24 or 48 h. Total RNA was extracted from ∼5 × 106 cells in a 100 mm tissue culture dish using PureLink Micro to Midi Total RNA Purification System (Invitrogen) according to manufacturer’s instructions. First-strand complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the Superscript III First-Strand Synthesis System for reverse transcription–polymerase chain reaction (Invitrogen) exactly as described by the manufacturer. Quantitative reverse transcription–polymerase chain reaction (qRT–PCR) was performed with 0.2 μl of cDNA in 1× SYBR-Green Master Mix (IQ SYBR, Bio-Rad, Hercules, CA) with 400 nM of each oligonucleotide primer for BCL-2 (forward 5′-TGGGATGCCTTTGTGGAACT-3′ and reverse 5′-GAGACAGCCAGGAGAAATCAAAC-3′). The β-actin internal control was analyzed by qRT–PCR as above using 400 nM of RNA β-actin Internal Standards (Ambion, Austin, TX). The qRT–PCR reaction was performed in an iQ5Cycler (Bio-Rad) using the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The Ct analysis for each reaction was performed using the iQ5Cycler software and standard curves were generated to determine qRT–PCR efficiency. BCL-2 mRNA levels were normalized to β-actin mRNA levels using iQ5Cycler software and the 2−ΔΔCt method. Each sample was prepared in triplicate and the data represent the mean and SE of at least three independent RNA extractions. Statistically significant differences between data sets were determined using paired Student’s t-test.
Suppression of BCL-2 expression
Cells were transfected with BCL-2 small-interference RNA SMARTpool or Nonspecific Negative Control Pool (Upstate Biotechnology, Boston, MA) exactly as described elsewhere (24).
Apoptosis assay
Cell death as a result of apoptosis was quantitated by measuring mononucleosomes and oligonucleosomes release using the Cell Death Detection ELISA PLUS Kit (Roche, Indianapolis, IN) following the manufacturer’s instructions using 3000 cells per well cultured in a 96-well tissue culture plate. Each sample was prepared in triplicate and the data represent the mean and SE of at least three independent experiments. Statistically significant differences between data sets were determined using paired Student’s t-test.
Quantification of miR-15a and miR-16
Total RNA was isolated from ∼5 × 106 cells in a 100 mm tissue culture dish using the MirVana PARIS RNA isolation system (Ambion) according to the manufacturer’s instructions. cDNA from mature miR-15a and miR-16 was synthesized from 30 ng of total RNA using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) as described by the manufacturer. qRT–PCR was performed on 1.0 μl of cDNA using TaqMan MicroRNA Assay hsa-miR-15a or hsa-miR-16 probe sets (Applied Biosystems) and the TaqMan Universal PCR Master Mix, No Amperase UNG (Applied Biosystems) exactly as described by the manufacturer. For normalization, a β-actin qRT–PCR reaction was performed as described above.
Suppression of BCL-2 expression with pre-miR-15a and pre-miR-16
Each cell line plated at 3000 cells per well in a 96-well tissue culture plate was cultured for 24 h in CS-MEM and then transfected with 30 nM of the miRNA Precursor Molecules (pre-miR) non-specific control #2, pre-miR hsa-miR-15a or pre-miR hsa-miR-16 (Qiagen, Valencia, CA) using Hyperfect Reagent (Qiagen) as described by the manufacturer. At 1 day posttransfection, cells were treated with 100 pM 17-β-estradiol alone or in combination with 1.0 μM 4-hydroxytamoxifen. After 48 h, cell lysates were analyzed for BCL-2 expression by western blot or if growth assays were performed, cells were transfected a second time with pre-miR non-specific control 2, pre-miR hsa-miR-15a or pre-miR hsa-miR-16. The 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide growth assay was performed after an additional 72 h. Each sample was prepared in triplicate and the data represent the mean and SE of at least three independent experiments. Statistically significant differences between data sets were determined using paired Student’s t-test.
Inhibition of miR-15a and miR-16
Each cell line plated at 3000 cells per well in a 96-well tissue culture plate was cultured for 24 h in CS-MEM and then transfected with 50 or 100 nM of miRIDIAN miRNA inhibitor non-specific control 1, miRIDIAN miRNA inhibitor hsa-miR-15a or miRIDIAN miRNA inhibitor hsa-miR-16 (Dharmacon, Lafayette, CO) using Hyperfect Reagent (Qiagen) according to the manufacturer’s instructions. At 1 day posttransfection, cells were treated with 100 pM 17-β-estradiol alone or in combination with 1.0 μM 4-hydroxytamoxifen and a 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide growth assay was performed at 5 days posttransfection. Each sample was prepared in triplicate and the data represent the mean and SE of at least three independent experiments. Statistically significant differences between data sets were determined using paired Student’s t-test.
Results
HER2Δ16 expression promotes estrogen independence and tamoxifen resistance
We have shown that >50% of HER2-positive breast tumors coexpress the oncogenic HER2 isoform HER2Δ16. We further demonstrated that HER2Δ16 expression is strongly associated with metastatic breast tumors and tumor cell resistance to the HER2-targeted therapy trastuzumab (20). These findings implicated HER2Δ16 as a clinically important oncogenic event driving aggressive and therapy refractory HER2-positive breast cancer. In the same study, we found that 26% of HER2Δ16-expressing breast tumors were also ERα positive (20). Importantly, current clinical assays that detect tumor amplification or overexpression of HER2 cannot discriminate between HER2Δ16 and wild-type HER2 expression. The covert influence of HER2Δ16 oncogenic activity on ERα function and tumor cell response to endocrine therapy may explain the inability of preclinical models of wild-type HER2 overexpression to fully recapitulate the aggressive and variable clinical nature of HER2/ERα-positive tumors.
To determine the impact of HER2Δ16 expression on the biology of ERα-positive breast tumor cells, we compared the activities of HER2Δ16 and wild-type HER2 in the ERα-positive MCF-7 breast tumor cell line. Stable expression of HER2Δ16 resulted in reduced ERα levels when compared with the MCF-7/Vector and MCF-7/HER2 cell lines (supplementary Figure S1a is available at Carcinogenesis Online). However, equivalent levels of ERα transcriptional activity was observed in each cell line and ERα activity was abolished by treatment with tamoxifen or fulvestrant (supplementary Figure S1b is available at Carcinogenesis Online). Each cell line therefore appears to retain normal regulation of ERα function by estrogen and the two endocrine therapies tested.
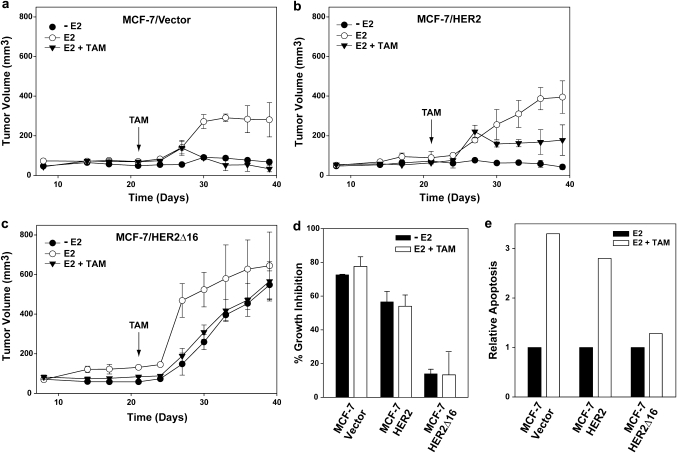
We first compared the ability of each cell line to form xenograft tumors under different growth conditions. As expected, MCF-7/Vector xenografts were estrogen dependent, failing to become established in the absence of exogenous estrogen. In addition, MCF-7/Vector tumors established in the presence of estrogen rapidly regressed when mice were treated with tamoxifen (Figure 1a). Consistent with other reports, we found that MCF-7/HER2 xenografts were also estrogen dependent (17). Established MCF-7/HER2 xenografts initially regressed in response to tamoxifen but then continued to slowly expand. However, in concordance with other studies using similar HER2-overexpressing cell lines, the final MCF-7/HER2 tumor volume was less than half of estrogen control xenografts (13,17) (Figure 1b). MCF-7/HER2Δ16 xenografts were estrogen responsive forming rapidly expanding large tumors in the presence of estrogen. In contrast to the other cell lines, MCF-7/HER2Δ16 tumors were estrogen independent and in the absence of estrogen formed tumors larger than estrogen-treated MCF-7/Vector and MCF-7/HER2 xenografts. Furthermore, MCF-7/HER2Δ16 xenografts exhibited robust tamoxifen resistance with only a 13% reduction in final tumor volume when compared with estrogen-treated MCF-7/HER2Δ16 xenografts (Figure 1c). Interestingly, the growth kinetics of tamoxifen-treated MCF-7/HER2Δ16 xenografts were nearly identical to MCF-7/HER2Δ16 xenografts grown in the absence of estrogen, suggesting that ERα signaling has minimal impact on MCF-7/HER2Δ16 tumor growth.
Fig. 1.
HER2Δ16 expression promotes estrogen independent and tamoxifen-resistant growth. (a–c) Graphs representing xenograft tumor kinetics of at least five nude mice per group injected with (a) MCF-7/Vector, (b) MCF-7/HER2 or (c) MCF-7/HER2Δ16 cells. With the exception of the E2 treatments, all mice were primed with E2 pellets and after 21 days, mice with established tumors were left untreated or implanted with TAM pellets. (d and e) MCF-7/HER2Δ16 cells are E2 independent and TAM resistant in vitro. (d and e) MCF-7/HER2Δ16 cells are estrogen independent and tamoxifen resistant in vitro. (d) Each cell line was untreated or treated for 5 days with 100 pM E2 alone or in combination with 1.0 μM TAM. 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide assay was used to quantitate cell growth. Results represent mean ± SE percent growth inhibition relative to 100 pM E2 alone. (e) Each cell line was cultured for 24 h in CS-MEM and treated for 72 h with 100 pM E2 alone or in combination with 1.0 μM TAM. Apoptosis was quantitated using a Cell Death Detection ELISA. Results represent apoptosis relative to cells treated with 100 pM E2 alone.
Similar results were observed in an in vitro cell proliferation assay where estrogen withdrawal or tamoxifen treatment significantly reduced MCF-7/Vector and MCF-7/HER2 cell growth (Figure 1d) with a 3-fold increase in cell apoptosis (Figure 1e). In contrast, tamoxifen only marginally inhibited MCF-7/HER2Δ16 cells (Figure 1d) and failed to induce apoptosis (Figure 1e). Taken together, our results demonstrate that expression of HER2Δ16, but not wild-type HER2, renders ERα-positive MCF-7 breast tumor cells estrogen independent and tamoxifen resistant. The initial tamoxifen response and subsequent impaired growth of MCF-7/HER2 xenografts reported here and elsewhere (15) suggest that wild-type HER2 has a greater impact on acquired tamoxifen resistance than de novo tamoxifen resistance. HER2Δ16-expressing MCF-7 xenografts, on the other hand, more closely resemble the estrogen independent and de novo tamoxifen-resistant phenotype of aggressive HER2/ERα-positive tumors commonly observed in the clinic (10).
Upregulation of BCL-2 in MCF-7/HER2Δ16 cells promotes tamoxifen resistance
Tumor cell apoptosis plays an important role in both preclinical and clinical responses to tamoxifen (25). The BCL-2 proto-oncogene is a potent inhibitor of the intrinsic or mitochondrial cell death pathway and several lines of experimental and clinical evidence indicate that activation of the intrinsic apoptotic pathway is an important mechanism of tamoxifen action (21,25). Indeed, we have shown that overexpression of the antiapoptotic BCL-2 proto-oncogene converts tamoxifen-sensitive breast tumor cells to a tamoxifen-resistant phenotype (21). Interestingly, BCL-2 is an estrogen-responsive gene and tamoxifen suppresses BCL-2 expression in tamoxifen-sensitive breast tumor cells (25).
We determined the influence of endogenous BCL-2 expression on the response of tamoxifen-resistant and tamoxifen-sensitive MCF-7 cell lines. Consistent with previous observations, estrogen stimulated BCL-2 mRNA expression by several fold in each MCF-7 cell line tested (26) and as expected, the addition of tamoxifen suppressed BCL-2 expression to below basal levels (Figure 2a). Despite tamoxifen-induced suppression of BCL-2 mRNA in each cell line tested, the tamoxifen-resistant MCF-7/HER2Δ16 cell line accumulated significant levels BCL-2 protein during the 72 h tamoxifen treatment (Figure 2b). To our knowledge, this is the first demonstration of tamoxifen-induced upregulation of BCL-2 protein in breast tumor cells. Interestingly, a similar upregulation of BCL-2 protein was observed in MCF-7/HER2Δ16 cells treated with the pure antiestrogen fulvestrant (ICI 182780) and during estrogen withdrawal (Figure 2c). These results suggest that HER2Δ16-specific upregulation of BCL-2 may occur in response to suppressed ERα activity.
Fig. 2.
BCL-2 upregulation mediates tamoxifen resistance of MCF-7/HER2Δ16 cells. (a) Quantitation of estrogen-induced BCL-2 mRNA expression. Each cell line was treated the indicated time with 100 pM E2 alone or in combination with 1.0 μM TAM. Each RNA sample was analyzed in triplicate, normalized to a β-actin internal control and BCL-2 mRNA expression is represented relative to the untreated MCF-7/Vector. (b) BCL-2 protein is upregulated in tamoxifen-treated MCF-7/HER2Δ16 cells. Each cell line was cultured for 48 h in phenol red-free modified Eagle's medium containing 5% charcoal-stripped fetal bovine serum and then treated as above and cell lysates were analyzed by western blot for BCL-2 expression 24, 48 and 72 h after treatment. (c) Inactivation of ERα signaling induces BCL-2 protein upregulation in MCF-7/HER2Δ16 cells. The MCF-7/HER2Δ16 cell line was cultured for 48 h in and left untreated (-E2) or treated with 1.0 μM TAM or 100 nM ICI 18278 (ICI) for the indicated time. Cell lysates were prepared and analyzed by western blot for BCL-2 expression. In all cases, western blot analysis of α-tubulin was included as a loading control and images were quantitated using the Odyssey Infrared Imaging System software.
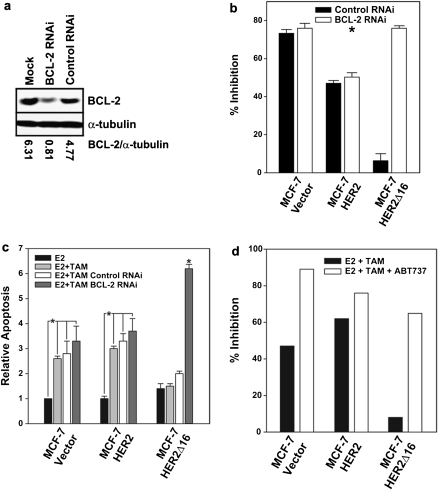
We next determined if BCL-2 activity promotes tamoxifen resistance of MCF-7/HER2Δ16 cells using an RNAi approach and pharmacological inhibition. Significantly, treatment of MCF-7/HER2Δ16 cells with BCL-2 targeting small-interference RNA reduced tamoxifen-induced BCL-2 expression (Figure 3a) and sensitized MCF-7/HER2Δ16 cells to tamoxifen (Figure 3b) with increased cellular apoptosis (Figure 3c). As expected, BCL-2 RNAi failed to significantly increase growth inhibition or apoptosis in the tamoxifen-sensitive MCF-7/Vector and MCF-7/HER2 cell lines (Figure 3b and c).
Fig. 3.
Suppression of BCL-2 restores tamoxifen sensitivity. (a) RNAi-mediated suppression of BCL-2 expression. The MCF-7/HER2Δ16 cell line was cultured for 24 h in phenol red-free modified Eagle's medium containing 5% charcoal-stripped fetal bovine serum and treated with non-specific control or BCL-2-specific RNAi. Immediately after transfection, each sample was treated with 1.0 μM 4-hydroxytamoxifen for an additional 48 h. Cell lysates were analyzed for BCL-2 expression by western blot. Western blot analysis of α-tubulin was included as a loading control and images were quantitated using the Odyssey Infrared Imaging System software. (b) Suppression of BCL-2 restores tamoxifen sensitivity to MCF-7/HER2Δ16 cells. Each cell line was treated with non-specific control or BCL-2 RNAi. Each sample was treated with 100 pM E2 alone or in combination with 1.0 μM TAM for 72 h. 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide assay was used to quantitate cell growth. Results represent mean ± SE percent growth inhibition relative to 100 pM E2 alone. Asterisks indicate significant difference by paired Student’s t-test (P = 0.007). (c) Suppression of BCL-2 sensitizes MCF-7/HER2Δ16 cells to tamoxifen-induced apoptosis. Each cell line was treated as above and apoptosis was quantitated using a Cell Death Detection ELISA. Results represent mean ± SE apoptosis relative to MCF-7/Vector treated with 100 pM E2 alone of three independent experiments of samples prepared in triplicate. Asterisks indicate samples with significant differences by paired Student’s t-test (P < 0.001). (d) The BCL-2 inhibitor ABT-737 sensitizes MCF-7/HER2Δ16 cells to tamoxifen. Each cell line was cultured for 48 h in CS-MEM and then treated with 100 pM E2, 100 pM E2 and 1.0 μM TAM alone or in combination with 5 μM of the BCL-2 family inhibitor ABT-737 for 5 days. Results represent mean percent growth inhibition of triplicate samples relative to cells treated with 100 pM E2 alone.
ABT-737 (Abbott Laboratories, Abbott Park, IL) is a small molecule BH3 mimetic, which binds to and inhibits antiapoptotic members of the BCL-2 family, including BCL-2 itself (27). An oral version of ABT-737 is currently in clinical trials for the treatment of a broad spectrum of malignancies (28); however, the impact of ABT-737 on endocrine resistance of breast tumors has not been investigated. Treatment of cell lines with physiological levels of ABT-737 increased tamoxifen activity in MCF-7/Vector cells and sensitized MCF-7/HER2Δ16 cells to tamoxifen with a dramatic increase in growth inhibition (Figure 3d). Taken together, these results indicate that HER2Δ16 promotes tamoxifen resistance through a unique mechanism involving tamoxifen-induced upregulation of BCL-2 protein expression. Our findings have important clinical implications and may explain why quantitation of pretreatment or basal levels of tumor BCL-2 expression fails to predict tamoxifen response (25).
HER2Δ16 suppresses expression of BCL-2 targeting miR-15a and miR-16
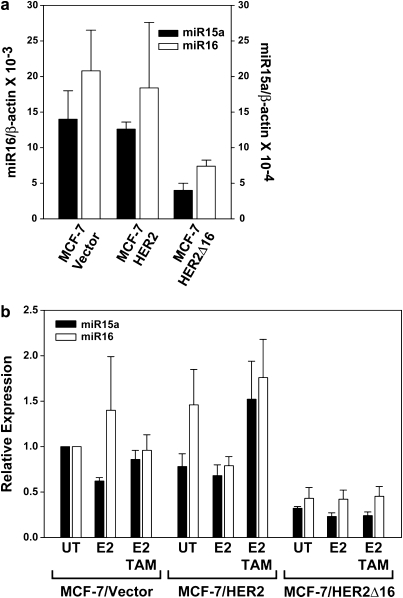
Despite the fact that tamoxifen suppresses BCL-2 mRNA expression in each cell line examined, a dramatic upregulation of BCL-2 protein in the MCF-7/HER2Δ16 cells was observed and our data indicate that the enhanced BCL-2 expression levels directly contributes to tamoxifen resistance. We next explored the molecular mechanisms underlying altered BCL-2 expression in the tamoxifen-resistant MCF-7/HER2Δ16 cells. Each cell line had similar rates of BCL-2 mRNA decay (supplementary Figure S2a is available at Carcinogenesis Online) ruling out increased BCL-2 mRNA stability as a possible mechanism of BCL-2 upregulation. Another possible mechanism of upregulated BCL-2 expression is the involvement of miRNAs, which have been shown to alter gene expression, independent of mRNA levels, through the suppression of target gene translation (1). We therefore investigated the potential role of BCL-2 targeting miR-15a and miR-16 (29) in the suppression of BCL-2 translation. Significantly, basal levels of both miR-15a and miR-16 were similar in the tamoxifen-sensitive MCF-7/Vector and MCF-7/HER2 cells (Figure 4a) and tamoxifen failed to enhance BCL-2 expression in these cell lines. In contrast, the levels of miR-15a and miR-16 in the tamoxifen resistant and BCL-2-expressing MCF-7/HER2Δ16 cell line were reduced by 71 and 64%, respectively (Figure 4a). A reduction in miR-15a and miR-16 levels by only 40% was sufficient to relieve repression of BCL-2 protein expression (29). Expression of miR-15a/16 was not significantly altered when cells were treated with estrogen or tamoxifen (Figure 4b). In addition, HER2Δ16 expression failed to impact miR-15a/16 in the ERα-negative MDA-MB-231 cell line, which expressed lower levels of miR-15a/16 when compared with MCF-7 cells and lacked tamoxifen-induced expression of BCL-2 (supplementary Figure S2b and c is available at Carcinogenesis Online).
Fig. 4.
HER2Δ16 expression suppresses miR-15a and miR-16. (a) Expression of miR-15a and miR-16 is suppressed in MCF-7/HER2Δ16 cells. Total RNA was extracted and analyzed for miR-15a or miR-16 expression by qRT–PCR. Results from three independent RNA extractions are represented as mean ± SE expression relative to β-actin. The lower levels of miR-15a and miR-16 expression in the MCF-7/HER2Δ16 cells failed to reach significance (paired Student’s t-test; P = 0.07 and P = 0.08, respectively). (b) Expression of miR-15a and miR-16 is not altered by estrogen or tamoxifen. Each cell line was cultured for 48 h in phenol red-free modified Eagle;s medium containing 5% charcoal-stripped fetal bovine serum and then left untreated or treated for 16 h with 100 pM E2 alone or in combination with 1.0 μM TAM. Three independent total RNA extractions from each cell line were analyzed in triplicate for miR-15a and miR-16 expression by qRT–PCR. Results were normalized to β-actin and represented as mean ± SE expression relative to untreated MCF-7/Vector cells. Differences failed to obtain significance as determined by paired Student’s t-test.
Reestablished miR-15a/16 expression sensitizes MCF-7/HER2Δ16 cells to tamoxifen
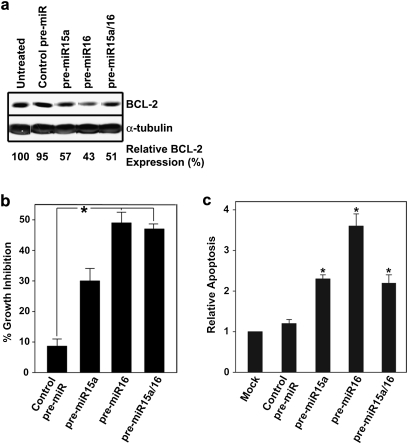
We next determined if reintroduction of miR-15a and/or miR-16 was sufficient to suppress BCL-2 expression and sensitize MCF-7/HER2Δ16 cells to tamoxifen treatment. Transfection of MCF-7/HER2Δ16 cells with pre-miR-15a, pre-miR-16 or the combination of both resulted in suppression of BCL-2 expression by 43, 57 and 49%, respectively (Figure 5a). The miR-15a and/or miR-16-treated MCF-7/HER2Δ16 cells were sensitized to tamoxifen with a significant increase in growth inhibition observed in cells treated with pre-miR-16 and the pre-miR-15a/16 combination (Figure 5b). The levels of growth inhibition were in concordance with the levels of BCL-2 suppression induced by the different pre-miR-15a and pre-miR-16 combinations (Figure 5a and b). A significant increase in MCF-7/HER2Δ16 cell apoptosis in response to tamoxifen was also observed when cells were treated with the different pre-miR combinations (Figure 5c). These results suggest that HER2Δ16 employs a novel mechanism of tamoxifen resistance by suppressing expression of BCL-2 regulating miRNAs.
Fig. 5.
Expression of pre-miR-15a/16 sensitizes MCF-7/HER2Δ16 cells to tamoxifen. (a) Pre-miR-15a and/or pre-miR-16 suppresses BCL-2 expression. The MCF-7/HER2Δ16 cell line was untreated or treated with 30 nM of the indicated pre-miR and treated with 100 pM E2 and 1.0 μM TAM for 48 h. Cell lysates were analyzed by western blot and BCL-2 expression relative to the untreated control was quantitated by densitometry. (b and c) Pre-miR-15a and/or pre-miR-16 sensitizes MCF-7/HER2Δ16 cells to tamoxifen. The MCF-7/HER2Δ16 cell line was transfected with pre-miRs as above, treated with 100 pM E2 alone or in combination with 1.0 μM TAM for 72 h. (b) 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide assay was used to quantitate cell growth or (c) apoptosis was quantitated using a Cell Death Detection ELISA. Data represents mean ± SE of three independent experiments performed with triplicate samples relative to mock and E2/TAM-treated cells. Asterisks indicate samples with significant differences as determined by paired Student’s t-test (b, P < 0.001; c, pre-miR-15a, P = 0.029; pre-miR16, P = 0.004; pre-miR15a/16, P = 0.017).
Suppressed miR-15a/16 expression promotes tamoxifen resistance
Based upon the ability of reestablished miR-15a and miR-16 expression to suppress BCL-2 expression and sensitize MCF-7/HER2Δ16 cells to tamoxifen, we next performed the converse experiment and determined if suppression of miR-15a or miR-16 expression would convert tamoxifen-sensitive MCF-7/Vector and MCF-7/HER2 cell lines to a tamoxifen-resistant phenotype. Pretreatment of MCF-7/Vector and MCF-7/HER2 cells with inhibitors of miR-15a or miR-16 enhanced BCL-2 expression in the MCF-7/Vector and MCF-7/HER2 cell lines (Figure 6a). Although antisense inhibition of miR expression is an inefficient process, introduction of anti-miR-15a or anti-miR-16 in the MCF-7/Vector and MCF-7/HER2 cell lines resulted in a significant increase in tamoxifen resistance (Figure 6b). The increase in tamoxifen resistance was accompanied by a significant reduction in apoptosis in MCF-7/Vector and MCF-7/HER2 cells treated with anti-miR-15a or anti-miR-16 (Figure 6c). These results further implicate the BCL-2 targeting miR-15a/16 as important regulators of BCL-2 expression and tamoxifen response and suggest that oncogene suppression of miR-15a and/or miR-16 may represent an important mechanism of tamoxifen resistance.
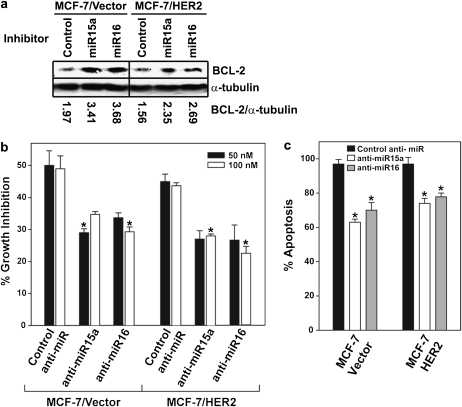
Fig. 6.
Suppressed miR-15a/16 expression promotes tamoxifen resistance. (a) Inhibition of miR-15a/16 results in enhanced BCL-2 expression. Each cell line was treated with 50 nM of the indicated miR inhibitor for 48 h and BCL-2 expression was analyzed by western blot. Analysis of α-tubulin was included as a loading control and images were quantitated using the Odyssey Infrared Imaging System software. (b and c) Suppression of miR-15a/16 promotes tamoxifen resistance. Each cell line was untreated or treated with the indicated anti-miR and treated with 100 pM E2 alone or in combination with 1.0 μM TAM for five days. (b) 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide assay was used to quantitate cell growth and (c) apoptosis was quantitated using a Cell Death Detection ELISA. Data are represented as mean ± SE of three independent experiments performed with triplicate samples relative to mock anti-miR and E2/TAM-treated MCF-7/Vector cells. Asterisks indicate samples with significant differences as determined by paired Student’s t-test (P < 0.008).
Discussion
Deciphering the mechanistic basis of tumor resistance to tamoxifen therapy continues to pose a significant challenge to both researchers and clinicians. Clinically, HER2 expression has been implicated as a possible mechanism of tamoxifen resistance (10); however, preclinical models of HER2 overexpression fail to fully recapitulate the phenotypes of refractory HER2- and ERα-positive tumors. We recently identified an oncogenic isoform of HER2, HER2Δ16, coexpressed in a significant percentage of HER2- and ERα-positive breast tumors (20). Here, we show that similar to clinical observations, HER2Δ16-expressing xenografts are both tamoxifen resistant and estrogen independent, whereas consistent with other reports, HER2-expressing xenografts display only partial acquired tamoxifen resistance and remain estrogen dependent (12–17). Our data indicates that HER2Δ16 xenografts phenocopy tamoxifen resistance observed clinically; therefore, this preclinical model may provide unique insights into the molecular complexity of endocrine-resistant HER2- and ERα-positive tumors.
Although tamoxifen induces growth arrest of sensitive tumor cells, apoptosis has emerged as an important mechanism of tamoxifen action (21,25) and tumor cell evasion of apoptosis contributes to tamoxifen resistance (21). In this communication and elsewhere, we have shown that tamoxifen-sensitive xenograft tumors decrease in size following tamoxifen treatment further supporting cell death as an important mechanism of tamoxifen action (21). In contrast, tamoxifen-resistant HER2Δ16-expressing cells evade apoptosis in part through upregulation of antiapoptotic BCL-2. Indeed, suppression of BCL-2 expression by RNAi or treatment with the pharmacological inhibitor of antiapoptotic BCL-2 family members, ABT-737, sensitized HER2Δ16-expressing cells to tamoxifen with increased apoptosis.
Importantly, HER2Δ16 employs a novel mechanism to upregulate BCL-2 protein levels in response to suppression of ERα activity. Consistent with other reports, we found that BCL-2 transcription is suppressed in response to tamoxifen (30,31). However, when ERα activity is disengaged by tamoxifen or fulvestrant treatment or estrogen withdrawal, we observe a dramatic upregulation of BCL-2 protein in HER2Δ16-expressing MCF-7 cells. Our preclinical results may explain the absence of clinical evidence implicating tumor expression of BCL-2 in tamoxifen resistance. Similar to our preclinical models, pretreatment levels of BCL-2 are similar in both tamoxifen-sensitive and tamoxifen-resistant tumors (32,33). However, also in concordance with our findings, high levels of BCL-2 expression are observed in tamoxifen refractory tumors (34) raising the possibility that BCL-2 expression is treatment induced in this clinical setting. Thus, preclinical and clinical evidence suggests that measuring tumor cell BCL-2 levels during tamoxifen treatment, especially in HER2- and ERα-positive tumors, may strengthen the clinical significance of BCL-2 as a marker of resistance.
We investigated the molecular basis of enhanced BCL-2 protein expression in HER2Δ16-expressing cells and found a potential role for miRNAs. BCL-2 translation is repressed by binding of miR-15a or miR-16 to a seed sequence in BCL-2 mRNA 3′-untranslated regions, and loss of miR-15a/16 in several cancer cell lines and tumors is associated with BCL-2 upregulation (29,35,36) and resistance to therapy (36). We found that miR-15a/16 modulate BCL-2 expression in breast tumor cells and contribute to tamoxifen resistance. For example, BCL-2 is upregulated in HER2Δ16 expressing tamoxifen-resistant cells where levels of miR-15a/16 were reduced compared with tamoxifen-sensitive cell lines. Reintroduction of miR-15a/16 suppressed BCL-2 expression and sensitized resistant cells to tamoxifen. Conversely, suppression of miR-15a/16 resulted in upregulation of BCL-2 and converted sensitive cells to a tamoxifen-resistant phenotype.
We failed to detect altered miR-15a/16 expression in response to disrupted ERα signaling suggesting that additional mechanisms may contribute to the increased expression of BCL-2 in HER2Δ16 expressing cells. Bioinformatic algorithms predict that the 3′-untranslated regions of BCL-2 is targeted by >40 miRNAs broadly conserved among vertebrates. Of particular interest, for our studies, is the BCL-2 targeting miR-21 (37,38), which appears to be upregulated in response to endocrine therapy, thereby suppressing BCL-2 expression in therapeutic-sensitive MCF-7 cells (38). Similar to our observations of miR-15a/16, however, expression of miR-21 is suppressed in tamoxifen-resistant MCF-7 cells (5) and, although not formally investigated, one would predict that BCL-2 expression would be upregulated in these resistant cells. We could not confirm a direct role for miR-21 in HER2Δ16-induced endocrine resistance (D.Cittelly and F.Jones, unpublished data). Nevertheless, altered regulation of BCL-2-targeting miRNAs has emerged as a potential mechanism of endocrine resistance worthy of clinical validation. Additional miRNA pathways involving targets other than BCL-2 have also been shown to influence tamoxifen response of breast tumor cells (5,6) further underscoring the potential complexity of miRNA regulation of multiple non-overlapping endocrine resistance pathways.
In summary, we show that the clinically important HER2 isoform, HER2Δ16, promotes estrogen independent growth of ERα-positive breast tumor cells and cooperates with BCL-2 to evade tamoxifen therapy. We further show that HER2Δ16-expressing cells upregulate BCL-2 expression in response to tamoxifen, in part, through a unique mechanism involving suppression of the BCL-2 targeting miR-15a/16. The covert clinical influence of HER2Δ16 expression in HER2/ERα-positive tumors may explain the inability of wild-type HER2 preclinical models to fully recapitulate the aggressive and variable clinical nature of HER2/ERα-positive breast tumors (12,13,15–17). Breast tumor expression analysis of both HER2Δ16 and miR-15a/16 may provide improved markers of tamoxifen resistance and novel targets for therapeutic intervention. One intriguing possibility based upon our preclinical data involves combining endocrine therapy with the BCL-2 family pharmacological inhibitor ABT-737 for the treatment of women presenting with HER2Δ16/ERα-positive tumors and therefore predicted to be at increased risk of endocrine therapy failure.
Supplementary material
Supplementary Figures S1 and S2 can be found at http://carcin.oxfordjournals.org/
Funding
US AMRMC (W81XWH-08-1-0458 to D.M.C. and F.E.J.).
Supplementary Material
Acknowledgments
We thank members of the Jones laboratory for helpful discussions.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- cDNA
complementary DNA
- CS-MEM
charcoal stripped minimal essential media
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- mRNA
messenger RNA
- qRT–PCR
quantitative reverse transcription–polymerase chain reaction
- SE
standard error
References
- 1.Fabbri M, et al. MicroRNAs. Cancer J. 2008;14:1–6. doi: 10.1097/PPO.0b013e318164145e. [DOI] [PubMed] [Google Scholar]
- 2.Esquela-Kerscher A, et al. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 3.Verghese ET, et al. Small is beautiful: microRNAs and breast cancer-where are we now? J. Pathol. 2008;215:214–221. doi: 10.1002/path.2359. [DOI] [PubMed] [Google Scholar]
- 4.Tavazoie SF, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller TE, et al. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27(Kip1) J. Biol. Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao JJ, et al. MicroRNA-221/222 negatively regulates ERalpha and associates with tamoxifen resistance in breast cancer. J. Biol. Chem. 2008;283:31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Fisher B, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 9.Normanno N, et al. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr. Relat. Cancer. 2005;12:721–747. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- 10.Osborne CK, et al. Estrogen-receptor biology: continuing progress and therapeutic implications. J. Clin. Oncol. 2005;23:1616–1622. doi: 10.1200/JCO.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 11.Osborne CK, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 12.Benz CC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res. Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 13.Kurokawa H, et al. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 14.Liu B, et al. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int. J. Cancer. 2007;120:1874–1882. doi: 10.1002/ijc.22423. [DOI] [PubMed] [Google Scholar]
- 15.Massarweh S, et al. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006;66:8266–8273. doi: 10.1158/0008-5472.CAN-05-4045. [DOI] [PubMed] [Google Scholar]
- 16.Wang LH, et al. Disruption of estrogen receptor DNA-binding domain and related intramolecular communication restores tamoxifen sensitivity in resistant breast cancer. Cancer Cell. 2006;10:487–499. doi: 10.1016/j.ccr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Massarweh S, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 18.Kwong KY, et al. A novel splice variant of HER2 with increased transformation activity. Mol. Carcinog. 1998;23:62–68. doi: 10.1002/(sici)1098-2744(199810)23:2<62::aid-mc2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Siegel PM, et al. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitra D, et al. An oncogenic isoform of HER2 promotes metastatic breast cancer and trastuzumab resistance. Mol. Cancer Ther. 2009;8:2152–2162. doi: 10.1158/1535-7163.MCT-09-0295. [DOI] [PubMed] [Google Scholar]
- 21.Naresh A, et al. The HER4/4ICD estrogen receptor coactivator and BH3-only protein is an effector of tamoxifen-induced apoptosis. Cancer Res. 2008;68:6387–6395. doi: 10.1158/0008-5472.CAN-08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, et al. Coregulation of estrogen receptor by estrogen-inducible ERBB4/HER4 establishes a growth promoting autocrine signal in breast cancer. Cancer Res. 2006;66:7991–7998. doi: 10.1158/0008-5472.CAN-05-4397. [DOI] [PubMed] [Google Scholar]
- 23.Jones FE, et al. ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J. Cell. Biol. 1999;147:77–87. doi: 10.1083/jcb.147.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naresh A, et al. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66:6412–6420. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- 25.Mandlekar S, et al. Mechanisms of tamoxifen-induced apoptosis. Apoptosis. 2001;6:469–477. doi: 10.1023/a:1012437607881. [DOI] [PubMed] [Google Scholar]
- 26.Wang TT, et al. Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res. 1995;55:2487–2489. [PubMed] [Google Scholar]
- 27.Deng J, et al. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Kang MH, et al. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cimmino A, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl Acad. Sci. USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiantanawat A, et al. Signaling pathways of apoptosis activated by aromatase inhibitors and antiestrogens. Cancer Res. 2003;63:8037–8050. [PubMed] [Google Scholar]
- 31.Zhang GJ, et al. Tamoxifen-induced apoptosis in breast cancer cells relates to down-regulation of bcl-2, but not bax and bcl-X(L), without alteration of p53 protein levels. Clin. Cancer Res. 1999;5:2971–2977. [PubMed] [Google Scholar]
- 32.Cannings E, et al. Bad expression predicts outcome in patients treated with tamoxifen. Breast Cancer Res. Treat. 2007;102:173–179. doi: 10.1007/s10549-006-9323-8. [DOI] [PubMed] [Google Scholar]
- 33.Henriksen KL, et al. An ER activity profile including ER, PR, Bcl-2 and IGF-IR may have potential as selection criterion for letrozole or tamoxifen treatment of patients with advanced breast cancer. Acta Oncol. 2009;48:522–531. doi: 10.1080/02841860802676383. [DOI] [PubMed] [Google Scholar]
- 34.Planas-Silva MD, et al. Overexpression of c-Myc and Bcl-2 during progression and distant metastasis of hormone-treated breast cancer. Exp. Mol. Pathol. 2007;82:85–90. doi: 10.1016/j.yexmp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Bonci D, et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 2008 doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 36.Xia L, et al. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int. J. Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 37.Si ML, et al. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 38.Wickramasinghe NS, et al. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.