Abstract
BACKGROUND
Peripartum administration of single-dose nevirapine reduces mother-to-child transmission of human immunodeficiency virus type 1 (HIV-1) but selects for nevirapine-resistant virus.
METHODS
In seven African countries, women infected with HIV-1 whose CD4+ T-cell counts were below 200 per cubic millimeter and who either had or had not taken single-dose nevirapine at least 6 months before enrollment were randomly assigned to receive antiretroviral therapy with tenofovir–emtricitabine plus nevirapine or tenofovir-emtricitabine plus lopinavir boosted by a low dose of ritonavir. The primary end point was the time to confirmed virologic failure or death.
RESULTS
A total of 241 women who had been exposed to single-dose nevirapine began the study treatments (121 received nevirapine and 120 received ritonavir-boosted lopinavir). Significantly more women in the nevirapine group reached the primary end point than in the ritonavir-boosted lopinavir group (26% vs. 8%) (adjusted P = 0.001). Virologic failure occurred in 37 (28 in the nevirapine group and 9 in the ritonavir-boosted lopinavir group), and 5 died without prior virologic failure (4 in the nevirapine group and 1 in the ritonavir-boosted lopinavir group). The group differences appeared to decrease as the interval between single-dose nevirapine exposure and the start of antiretroviral therapy increased. Retrospective bulk sequencing of baseline plasma samples showed nevirapine resistance in 33 of 239 women tested (14%). Among 500 women without prior exposure to single-dose nevirapine, 34 of 249 in the nevirapine group (14%) and 36 of 251 in the ritonavir-boosted lopinavir group (14%) had virologic failure or died.
CONCLUSIONS
In women with prior exposure to peripartum single-dose nevirapine (but not in those without prior exposure), ritonavir-boosted lopinavir plus tenofovir–emtricitabine was superior to nevirapine plus tenofovir–emtricitabine for initial antiretroviral therapy. (Funded by the National Institute of Allergy and Infectious Diseases and the National Research Center; ClinicalTrials.gov number, NCT00089505.)
In 2008, approximately 430,000 infants were newly infected with human immunodeficiency virus type 1 (HIV-1).1 In 2007, only one in three pregnant women infected with HIV received antiretroviral drugs to prevent mother-to-child transmission. The most frequently used drug for this purpose was single-dose nevirapine,2 a component of the regimen recommended by the World Health Organization (WHO) for pregnant women who are not receiving three-drug anti-retroviral therapy.3 Nevirapine-resistant virus is detected in a large proportion of women after they have received single-dose nevirapine.4–9 Combining other antiretroviral drugs with single-dose nevirapine reduces but does not eliminate the risk that nevirapine–resistant viral strains will emerge.10–13
Nevirapine resistance declines over time after exposure to single-dose nevirapine5,14,15; however, low-level resistance persists8,9,16,17 and may compromise the subsequent response to a regimen that includes nevirapine,18,19 particularly when antiretroviral therapy is initiated within 6 to 18 months after single-dose nevirapine exposure.19–22
Previous studies have not determined whether women exposed to single-dose nevirapine who later require antiretroviral treatment should receive a non–nevirapine-containing regimen. This is a critical question, since hundreds of thousands of women have received and continue to take single-dose nevirapine to prevent mother-to-child transmission of HIV infection.2,3 In addition, nevirapine is a component of first-line antiretroviral therapy in most resource-limited settings.23 Hence, we compared initial antiretroviral treatment with nevirapine (a nonnucleoside reverse-transcriptase inhibitor) plus tenofovir–emtricitabine and ritonavir-boosted lopinavir (a protease inhibitor) plus tenofovir–emtricitabine among women with and those without prior exposure to single-dose nevirapine.
METHODS
STUDY DESIGN AND PARTICIPANTS
The Optimal Combination Therapy after Nevirapine Exposure (OCTANE) study comprised two concurrent, randomized, open-label trials of antiretroviral therapy. Trial 1 included 243 women who had previously received single-dose nevirapine, and trial 2 included 502 women without prior exposure to single-dose nevirapine (with target enrollments of 240 and 500 women, respectively). This report describes the full results of trial 1, as well as the results for the primary end point from trial 2 (to inform the interpretation of trial 1 results), including data from both trials through October 6, 2008.
The participants were women infected with HIV-1 who were not pregnant or breast-feeding, at 10 African sites (3 in South Africa; 2 in Kenya; and 1 each in Zimbabwe, Botswana, Zambia, Malawi, and Uganda) (see the Supplementary Appendix, available with the full text of this article at NEJM.org). Their screening CD4+ T-cell counts were less than 200 per cubic millimeter, and they had not previously received antiretroviral therapy, with two exceptions: trial 1 participants had received single-dose nevirapine on one or more occasions (not as part of this study) 6 or more months before enrollment, and participants in both trials were permitted to have received up to 10 weeks (cumulative) of treatment with zidovudine, with the last dose taken at least 6 months before study entry. Participants were followed for at least 48 weeks after the last patient was enrolled.
The women were randomly assigned either to open-label ritonavir-boosted lopinavir (400 mg of lopinavir with 100 mg of ritonavir) twice a day plus tenofovir–emtricitabine (300 mg of tenofovir and 200 mg of emtricitabine) daily, or to nevirapine (200 mg twice a day after an initial period of 200 mg daily) plus tenofovir–emtricitabine (300 mg and 200 mg, respectively) daily. Tenofovir–emtricitabine was supplied as coformulated Truvada (Gilead). Ritonavir-boosted lopinavir was initially supplied as Kaletra capsules (Abbott) but was changed to Aluvia tablets (Abbott) in April 2007. Randomization was stratified according to the CD4+ count (<50 or ≥50 cells per cubic millimeter), with balancing carried out within each site. Participants who did not tolerate or respond to one regimen could switch to the other regimen. Zidovudine, didanosine, and efavirenz were provided as needed.
Antiretroviral therapy was generally withheld in the event of possible treatment-related toxicity of grade 3 or higher.24 However, per protocol, nevirapine and efavirenz were permanently discontinued upon elevations in alanine aminotransferase or aspartate aminotransferase that reached grade 2 or higher, with no other apparent cause, or upon increases in these liver enzymes by one grade or more (or evidence of a rash) along with concomitant signs and symptoms of hepatitis or hypersensitivity. Women who became pregnant after enrollment continued to participate in the study.
The primary study end point was time to virologic failure or death. Virologic failure was defined as a confirmed plasma HIV-1 RNA level less than 1 log10 copy per milliliter below baseline at 12 weeks after treatment initiation or a confirmed HIV-1 RNA level that was 400 log10 copies or more per milliliter 24 weeks or more after treatment was begun.
The study was approved by all relevant local (and U.S.) institutional review boards and ethics committees. Participants provided written informed consent. All the authors vouch for the correctness of the data and results of analyses as presented. Coauthors who were also site investigators (and the study staff at each site) collected the data. All the authors made the decision to submit the manuscript for publication. The pharmaceutical sponsors (Abbott, Boehringer Ingelheim, Gilead, Bristol-Myers Squibb, and GlaxoSmithKline) provided the study drugs and participated as members of the study team but did not participate in analysis of the data. This study was conducted in accord with the amended protocol.
STUDY MONITORING
On October 6, 2008, the data safety and monitoring board recommended release of the results from trial 1 (but not trial 2), since the P value (unadjusted for interim analyses) for the comparison of the primary end point between the randomized groups in trial 1 was 0.0005, which met the prespecified criterion of P<0.001 for considering stopping the trial. Target enrollment had been completed and follow-up continued in both trials, but trial 1 results were shared with all participants, and the women who had been taking first-line nevirapine in trial 1 were offered ritonavir-boosted lopinavir instead.
DATA COLLECTION, FOLLOW-UP, AND LABORATORY STUDIES
Study visits (which included laboratory and clinical safety assessments) occurred six times during the first 24 weeks and every 12 weeks thereafter. Measurements of HIV-1 RNA (Roche Amplicor Monitor version 1.5) and CD4+ cell counts were obtained at study entry and every 12 weeks thereafter. Lipid levels (fasting or nonfasting) were evaluated at entry, at 24 and 48 weeks, and then every 48 weeks.
Plasma samples obtained at study entry (before treatment) were subjected to drug-resistance testing at a single laboratory with the use of the ViroSeq assay (Celera Diagnostics), which generally detects resistant virus accounting for 20% or more of the virus population. Samples were tested after study entry, since resistance testing was not available at most study sites and would have substantially delayed the start of therapy in these immunosuppressed patients. Results were interpreted with the use of modified International AIDS Society–USA 2008 tables,25 which included mutations associated with resistance to nevirapine, efavirenz, or both (L100I, K103N, V106A/M, V108I, Y181C/I, Y188C/H/L, G190A/S, and P225H) and to delavirdine (P236L).
STATISTICAL ANALYSIS
The primary analysis was based on the intention-to-treat principle, modified to exclude women who failed to start the study treatment. The Kaplan–Meier method was used to describe the cumulative proportion of participants who had virologic failure or who died. For women who did not reach the primary end point, follow-up data were censored at the time of the last available HIV-1 RNA measurement by October 6, 2008. Cox proportional-hazards models, stratified according to screening CD4+ count (<50 vs. ≥50 cells per cubic millimeter), were used to compare the hazard of reaching an end point between the two groups. Prespecified subgroup analyses concerned the presence or absence of written documentation of prior receipt of single-dose nevirapine, the presence or absence of resistance to nonnucleoside reverse-transcriptase inhibitors (NNRTIs) at study entry (before the study treatment was begun), and the length of time since the participants’ last exposure to single-dose nevirapine. Analyses of adverse events were restricted to the period during which participants received nevirapine or ritonavir-boosted lopinavir, according to the randomization scheme. The Wilcoxon rank-sum test was used to compare the duration of time since the last dose of single-dose nevirapine between women with and those without baseline resistance to NNRTIs, and a linear regression model was used to compare changes in CD4+ cell counts and lipid levels, adjusted for baseline measurements. All reported P values are two-sided. For the primary end point, the hazard ratio, P value, and confidence interval have been adjusted for the multiple interim analyses26; all other values are unadjusted.
RESULTS
ENROLLMENT AND FOLLOW-UP FOR TRIALS 1 AND 2
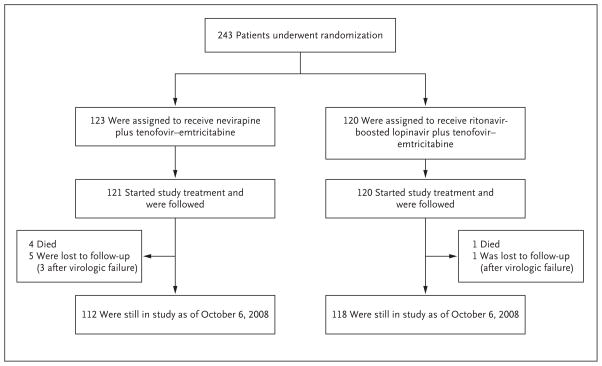
A total of 243 women (17 to 36 per site) were randomly assigned to a study group in trial 1 between November 2005 and February 2008 (Fig. 1). Two women in the nevirapine group did not start the study treatment; therefore, 241 women were included in all analyses: 121 women in the nevirapine group and 120 in the ritonavir-boosted lopinavir group. Six women (2.5%) in trial 1 were lost to follow-up (5 in the nevirapine group and 1 in the ritonavir-boosted lopinavir group), 2 of whom (0.8% of the total) had not yet reached a primary end point. The median duration of follow-up was 74 weeks (range, 2 to 153). In trial 2, a total of 502 women were randomly assigned to a study treatment, 500 of whom started the assigned treatment (249 received nevirapine and 251 received ritonavir-boosted lopinavir).
Figure 1.
Randomization and Follow-up in Trial 1.
CHARACTERISTICS OF PARTICIPANTS IN TRIAL 1
Baseline characteristics of the participants in trial 1 were similar between the two treatment groups (Table 1). The median baseline CD4+ count was 139 cells per cubic millimeter, the median HIV-1 RNA level was 5.15 log10 copies per milliliter, and the median time since the last exposure to single-dose nevirapine was 17 months (range, 7 to 45). All participants reported prior treatment with single-dose nevirapine, and 73% had written documentation of such treatment.
Table 1.
Selected Baseline Characteristics of the Participants in Trial 1.*
| Characteristic | Nevirapine (N = 121) | Ritonavir-Boosted Lopinavir (N = 120) | Total (N = 241) |
|---|---|---|---|
| Age at randomization — yr | |||
| Median | 30 | 31 | 31 |
| 10th–90th percentile | 24–37 | 24–38 | 24–37 |
| Black race — no. (%)† | 121 (100) | 120 (100) | 241 (100) |
| CD4+ count at baseline | |||
| Median — cells/mm3 | 141 | 138 | 139 |
| 10th–90th percentile — cells/mm3 | 47–202 | 55–212 | 48–208 |
| <50 cells/mm3 — no. (%) | 14 (12) | 11 (9) | 25 (10) |
| HIV-1 RNA | |||
| Median — log10 copies/ml | 5.20 | 5.14 | 5.15 |
| 10th–90th percentile — log10 copies/ml | 4.26–5.84 | 4.19–5.88 | 4.24–5.88 |
| ≥750,000 copies/ml — no. (%) | 11 (9) | 15 (12) | 26 (11) |
| WHO HIV clinical stage — no. (%) | |||
| I | 44 (36) | 56 (47) | 100 (41) |
| II | 42 (35) | 38 (32) | 80 (33) |
| III | 30 (25) | 25 (21) | 55 (23) |
| IV | 5 (4) | 1 (1) | 6 (2) |
| Hepatitis B surface antigen | |||
| Positive — no. (%) | 9 (8) | 5 (4) | 14 (6) |
| Not determined — no. | 2 | 0 | 2 |
| Interval since most recent exposure to single-dose NVP | |||
| Median — mo | 16 | 17 | 17 |
| 10th–90th percentile — mo | 7–49 | 7–41 | 7–45 |
| ≥6 to <12 mo — no. (%) | 41 (34) | 37 (31) | 78 (32) |
| ≥12 to <24 mo — no. (%) | 46 (38) | 52 (43) | 98 (41) |
| ≥24 mo — no. (%) | 34 (28) | 31 (26) | 65 (27) |
| No. of previous exposures to single-dose NVP — no. (%) | |||
| 1 | 115 (95) | 114 (95) | 229 (95) |
| 2 | 6 (5) | 4 (3) | 10 (4) |
| 3 | 0 | 2 (2) | 2 (1) |
| Self-reported prior receipt of single-dose NVP — no. (%) | 121 (100) | 120 (100) | 241 (100) |
| Written documentation of prior nevirapine exposure — no. (%) | 86 (71) | 90 (75) | 176 (73) |
| Previous zidovudine exposure — no. (%) | 13 (11) | 12 (10) | 25 (10) |
| HIV subtype — no. (%) | |||
| A1 | 18 (15) | 15 (13) | 33 (14) |
| C | 87 (73) | 86 (72) | 173 (72) |
| D | 6 (5) | 9 (8) | 15 (6) |
| A2D | 1 (1) | 0 | 1 (0.4) |
| Complex recombination | 8 (7) | 9 (8) | 17 (7) |
| Test result not available — no. | 1 | 1 | 2 |
HIV denotes human immunodeficiency virus, NVP nevirapine, and WHO World Health Organization.
Race was reported by the investigators.
PRIMARY END POINT IN TRIAL 1
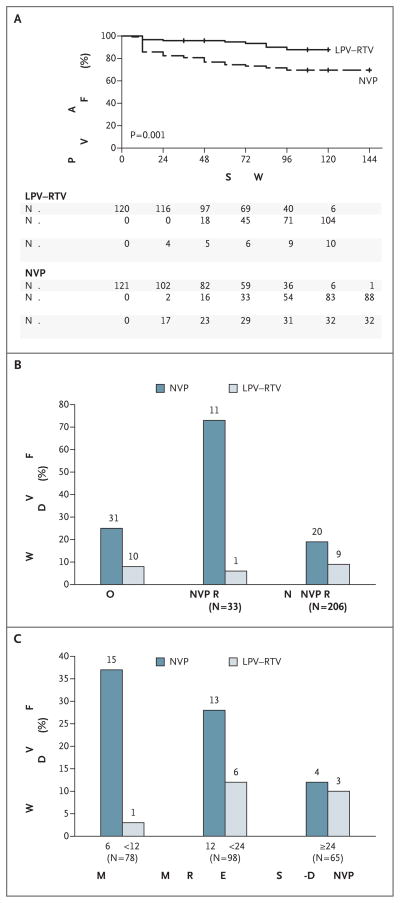
In the primary, modified intention-to treat analysis, 42 women reached an end point: 32 (26%) in the nevirapine group and 10 (8%) in the ritonavir-boosted lopinavir group (adjusted hazard ratio for the nevirapine group, 3.6; adjusted 95% confidence interval [CI], 1.7 to 7.5; adjusted P = 0.001) (Fig. 2A). These numbers included 28 women (23%) and 9 women (8%), respectively, who had virologic failure and 4 women (3%) and 1 woman (1%) who died without virologic failure. The difference favoring ritonavir-boosted lopinavir was evident early: by week 12, the primary end point was reached in 17 of the women in the nevirapine group and 4 of those in the ritonavir-boosted lopinavir group, although the hazard ratio did not decline significantly with increasing follow-up time (P = 0.22). The proportion of women who reached a primary end point in the nevirapine group equaled or exceeded that in the ritonavir-boosted lopinavir group at each study site. The difference between treatment groups did not vary according to whether written evidence of prior exposure to single-dose nevirapine was available (P = 0.63 for interaction).
Figure 2. Primary End Point According to Treatment Group in Trial 1.
The Kaplan–Meier plot in Panel A shows the proportions of participants in the ritonavir-boosted lopinavir (LPV–RTV) group and the nevirapine (NVP) group who were alive and free of virologic failure at the end of the study. Panel B shows the proportions of women for whom resistance results were available (239) who reached a primary end point in the overall study population and according to the presence or absence of baseline nevirapine resistance. Panel C shows the proportions of women who reached a primary end point according to the time since the most recent exposure to single-dose NVP. Numbers above bars indicate numbers of women.
Death was attributed to tuberculosis in two women (one of whom was in the ritonavir-boosted lopinavir group) and to suspected bacterial sepsis, acute leukemia, or multiple organ failure in the other three women. No death was considered to be related to the study treatments.
The results of a prespecified as-treated analysis in trial 1 (in which follow-up results and end points were included only if they occurred while the participant was taking nevirapine or ritonavir-boosted lopinavir for the initial study regimen) were similar to those of the modified intention-to-treat analysis. In the as-treated analysis, 29 women in the nevirapine group (24%) reached an end point, as compared with 10 women in the ritonavir-boosted lopinavir group (8%) (hazard ratio, 3.8; 95% CI, 1.8 to 7.9; P<0.001).
We assessed rates of subsequent virologic suppression among the 17 participants in trial 1 (14 in the nevirapine group and 3 in the ritonavir-boosted lopinavir group) who had early, confirmed virologic failure (i.e., a plasma HIV-1 RNA level that was less than 1 log10 copies per milliliter below baseline 12 weeks after treatment was begun). Among the 14 women in the nevirapine group, 6 had a subsequent HIV-1 RNA level of at least 400 copies per milliliter while taking nevirapine, and 8 discontinued nevirapine without undergoing further HIV-1 RNA measurements while taking nevirapine. In the ritonavir-boosted lopinavir group, 1 woman had a subsequent HIV-1 RNA measurement that confirmed failure, and 2 women had HIV-1 RNA levels below 400 copies per milliliter by 36 weeks while taking ritonavir-boosted lopinavir.
Baseline Nevirapine Resistance
For 2 women, samples were tested but no resistance results were obtained. Thirty-three (14%) of the 239 women with resistance results had baseline nevirapine-resistance mutations — 15 (13%) in the nevirapine group and 18 (15%) in the ritonavir-boosted lopinavir group (Table 2). The median time since the last exposure to single-dose nevirapine was shorter among the 33 women with nevirapine resistance (11 months [range, 6 to 50]) than among the 206 women without resistance (17 months [range, 6 to 74]) (P = 0.02). Among women with nevirapine resistance at baseline, 11 (73%) of 15 in the nevirapine group versus 1 (6%) of 18 in the ritonavir-boosted lopinavir group reached a primary end point (P = 0.006) (Fig. 2B). Among women without baseline nevirapine resistance, 20 (19%) of 105 in the nevirapine group versus 9 (9%) of 101 in the ritonavir-boosted lopinavir group reached a primary end point (P = 0.04). There was evidence that the between-group difference in the primary end point was greater among women with detectable nevirapine resistance than among those without detectable resistance (P = 0.046 for interaction).
Table 2.
Mutations Causing Nevirapine Resistance at Baseline in Trial 1.*
| Mutation | Nevirapine (N = 121) | Ritonavir-Boosted Lopinavir (N = 120) | Total (N = 241) |
|---|---|---|---|
| number (percent) | |||
| Present | |||
| No | 105 (88) | 101 (85) | 206 (86) |
| Yes | 15 (13) | 18 (15) | 33 (14) |
| K103N | 11 (9) | 12 (10) | 23 (10) |
| K103N, G190A | 0 | 1 (1) | 1 (0.4) |
| K103N, Y181C | 1 (1) | 2 (2) | 3 (1) |
| K103N, V108I, G190A | 0 | 1 (1) | 1 (0.4) |
| G190A | 1 (1) | 0 | 1 (0.4) |
| V108I | 0 | 1 (1) | 1 (0.4) |
| Y181C | 1 (1) | 1 (1) | 2 (1) |
| Y181I | 1 (1) | 0 | 1 (0.4) |
| Test result not available | 1 | 1 | 2 |
Percentages shown are for participants with available test results.
Time since Last Exposure to Single-Dose Nevirapine
Among the 78 women who started antiretroviral therapy from 6 to less than 12 months after the last exposure to single-dose nevirapine, 37% of the women in the nevirapine group (15 of 41) as compared with 3% of those in the ritonavir-boosted lopinavir group (1 of 37) reached a primary end point (hazard ratio, 15.8; 95% CI, 2.1 to 121). The respective percentages were 28% (13 of 46 women) and 12% (6 of 52) among the 98 women who started antiretroviral therapy from 12 to less than 24 months after the last exposure (hazard ratio, 3.0; 95% CI, 1.1 to 8.0) and 12% (4 of 34) and 10% (3 of 31) among the 65 women who started antiretroviral therapy 24 months or longer after exposure to single-dose nevirapine (hazard ratio, 1.3; 95% CI, 0.3 to 6.1) (Fig. 2C). This decreasing difference in efficacy between regimens with increasing time since the last exposure to single-dose nevirapine was not significant in the prespecified analysis, in which the time since the last exposure to single-dose nevirapine was included as a continuous variable (P = 0.21 for interaction). A post hoc analysis for a trend for the difference between treatment groups across the three categories of time since the last exposure to single-dose nevirapine yielded a P value of 0.051 (on a test of interaction).
RISK FACTORS FOR VIROLOGIC FAILURE OR DEATH IN THE TRIAL 1 NEVIRAPINE GROUP
Univariate models showed that among women in the nevirapine group, the baseline presence of nevirapine resistance, a lower CD4+ cell count, a higher plasma HIV-1 RNA level, and a shorter interval since the last exposure to single-dose nevirapine were each significantly associated with an increased risk of reaching a primary end point (P<0.05 for all comparisons). The following factors were not associated with the risk of reaching a primary end point: the presence or absence of written documentation of prior exposure to single-dose nevirapine, number of prior exposures, HIV-1 subtype (C vs. non-C), and baseline HIV disease stage (WHO classification). In multivariate analyses, baseline nevirapine resistance continued to be associated with an increased risk of virologic failure or death (hazard ratio, 8.7; 95% CI, 3.5 to 21.6), whereas a higher baseline CD4+ cell count was associated with a decreased risk (hazard ratio per increase of 100 cells per cubic millimeter, 0.2; 95% CI, 0.1 to 0.4).
CD4+ RESPONSE IN TRIAL 1
At 48 weeks, the mean change from baseline in the CD4+ count was an increase of 205 cells per cubic millimeter in the nevirapine group and an increase of 221 cells per cubic millimeter in the ritonavir-boosted lopinavir group; at 96 weeks, the mean increases from baseline were 298 and 279 cells per cubic millimeter, respectively. The differences between the two groups in the change in the CD4+ count from baseline were not significant.
ADHERENCE TO THERAPY IN TRIAL 1
During follow-up, for all visits, 83% of women in the nevirapine group and 81% in the ritonavir-boosted lopinavir group had a high level of adherence to therapy (at least 95% of expected doses were taken), according to pill counts; 89% of the women in each group reported that they had not missed a dose of either of the assigned drugs during the previous month.
TREATMENT DISCONTINUATION, ADVERSE EVENTS, AND NEW DIAGNOSES IN TRIAL 1
Significantly more women discontinued treatment in the nevirapine group than in the ritonavir-boosted lopinavir group (38 [31%] vs. 6 [5%]; hazard ratio, 7.4; P<0.001). Reasons for treatment discontinuation were as follows: death (in 3% of women in the nevirapine group vs. 1% in the ritonavir-boosted lopinavir group), adverse events (12% vs. 1%), virologic failure (12% vs. 2%), and other reasons (4% vs. 2%).
Nevirapine was discontinued in 13 women because of elevations in liver-enzyme levels (2 of the women also had a rash), in 1 woman because of a rash, and in another woman because of acute pancreatitis and renal failure. One participant in the ritonavir-boosted lopinavir group discontinued treatment owing to an elevated serum creatinine level.
Similar proportions of women in the nevirapine and ritonavir-boosted lopinavir groups had a grade 3 or higher sign or symptom (14% and 11%, respectively) or a grade 3 or higher laboratory abnormality (27% and 22%, respectively) (Table 3). No grade 3 or higher rashes were reported, but 11% of the women in the nevirapine group versus 3% of those in the ritonavir-boosted lopinavir group had a grade 3 or higher abnormality on liver-function testing.
Table 3.
Signs and Symptoms, Laboratory Abnormalities, New Diagnoses, and Lipid Results in Trial 1.*
| Variable | Nevirapine (N = 121) | Ritonavir-Boosted Lopinavir (N = 120) | Total (N = 241) | Unadjusted P Value‡ |
|---|---|---|---|---|
| Grade 3 or higher sign or symptom — no. (%)† | 17 (14) | 13 (11) | 30 (12) | 0.56 |
| General (pain, fatigue, weight loss, fever) — no. | 9 | 7 | 16 | |
| Gastrointestinal (diarrhea, nausea, or vomiting) — no. | 5 | 3 | 8 | |
| Cough or dyspnea — no. | 2 | 3 | 5 | |
| Cardiovascular dysfunction — no. | 2 | 1 | 3 | |
| Hepatic — no. | 1 | 0 | 1 | |
| Grade 3 or higher laboratory abnormality — no. (%)† | 32 (26) | 26 (22) | 58 (24) | 0.45 |
| Creatinine >1.8 times upper limit of normal | 1 | 2 | 3 | |
| Sodium >154 or <125 mmol/liter | 3 | 5 | 8 | |
| Potassium >6.5 or <2.5 mmol/liter | 1 | 2 | 3 | |
| Liver enzymes | 13 | 4 | 17 | |
| Aspartate aminotransferase >5 times upper limit of normal | 6 | 3 | 9 | |
| Alanine aminotransferase >5 times upper limit of normal | 10 | 2 | 12 | |
| Total bilirubin >2.5 times upper limit of normal | 2 | 0 | 2 | |
| Hematologic abnormality | 12 | 16 | 28 | |
| Platelets count <50,000/mm3 | 0 | 1 | 1 | |
| Hemoglobin <7.5 g/dl | 3 | 5 | 8 | |
| Absolute neutrophil count <750/mm3 | 10 | 11 | 21 | |
| No. of participants with new diagnoses | 73 | 75 | 148 | 0.79 |
| Change in lipids from baseline to 48 wk (mg/dl) | ||||
| Total cholesterol | 21.0±3.3 | 25.1±3.0 | 0.35 | |
| HDL cholesterol | 17.6±1.7 | 10.7±1.5 | 0.002 | |
| LDL cholesterol | 6.7±2.9 | 13.1±2.6 | 0.10 | |
| Triglycerides | −14.1±5.4 | 16.5±8.6 | 0.006 | |
Plus–minus values are means ±SE. Participants may have had more than one event.
Grading was performed with the use of the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, December 2004.
P values for the comparison between the two treatment groups are shown. Those for the changes in lipids were calculated by means of the linear regression model; all other P values were obtained with the use of Fisher’s exact test.
Similar numbers of women in the nevirapine group (73) and the ritonavir-boosted lopinavir group (75) had new diagnoses. Malaria was the only category in which there was a difference of more than 5 women affected between the two groups (13 in the nevirapine group vs. 20 in the ritonavir-boosted lopinavir group). Five participants in the nevirapine group and 4 in the ritonavir-boosted lopinavir group had tuberculosis, and 6 and 4 women in the respective groups died or had HIV disease progression to a higher WHO stage (hazard ratio with nevirapine, 1.5; 95% CI, 0.4 to 5.4; P = 0.52). Four pregnancies were reported in the nevirapine group and 10 in the ritonavir-boosted lopinavir group.
PRIMARY END POINT IN TRIAL 2
In trial 2, by October 6, 2008, a total of 34 (14%) of 249 women in the nevirapine group and 36 (14%) of 251 women in the ritonavir-boosted lopinavir group reached the primary end point (hazard ratio, 0.97; 95% CI, 0.6 to 1.6). Of the 70 women who reached an end point, 61 had virologic failure (29 in the nevirapine group and 32 in the ritonavir-boosted lopinavir group) and 9 died (5 in the nevirapine group and 4 in the ritonavir-boosted lopinavir group). There was significant evidence that the difference in the efficacy of the randomized treatments varied between trial 1 and trial 2 (P = 0.002 for interaction between study treatment and trial).
DISCUSSION
The regimen of ritonavir-boosted lopinavir plus tenofovir–emtricitabine had superior efficacy to the regimen of nevirapine plus tenofovir–emtricitabine among women with pretreatment CD4+ counts below 200 cells per cubic millimeter who had been exposed to single-dose nevirapine at least 6 months before the initiation of antiretroviral therapy but not among women without previous exposure to nevirapine. These findings were similar in modified intention-to-treat and as-treated analyses, as well as across study sites, and among women with previous exposure to single-dose nevirapine, the results were similar for women with and those without documentation of exposure.
The rate of virologic failure (23%) in the trial 1 nevirapine group is similar to failure rates in other studies of nevirapine-based treatment among women exposed to single-dose nevirapine.19,22,27 The failure rate with nevirapine-based treatment among patients who have not previously taken any antiretroviral medications is lower,28 although in one study, 22% of 35 patients had virologic failure with once-daily nevirapine plus tenofovir–emtricitabine.29 The rate of virologic response to antiretroviral therapy containing ritonavir-boosted lopinavir was slightly lower in other studies (71 to 86%, depending on the definition used) than it was in our study.30–34
The difference in the rate of virologic failure or death between study regimens was greater among women who had pretreatment nevirapine resistance than among those who did not. Nevirapine resistance detected within 1 month after exposure to single-dose nevirapine was not associated with subsequent virologic failure in a multivariate analysis involving women in Thailand who had previously received single-dose nevirapine.18 In South Africa, the presence of low-frequency K103N variants before treatment was associated with virologic failure among women receiving nevirapine-based antiretroviral therapy; however, previous exposure to single-dose nevirapine predicted neither resistance nor treatment success.35 Our data support pretreatment testing for drug resistance among women with prior exposure to single-dose nevirapine when this is feasible and when non–nevirapine-based antiretroviral therapy is available.
Increasing time since exposure to single-dose nevirapine appeared to be associated with a decreasing difference in treatment outcomes, possibly suggesting a nonlinear decline in the difference between the efficacy of the two treatments with increasing time. Other studies have shown that the deleterious effect of prior exposure to single-dose nevirapine on the efficacy of nevirapine-based antiretroviral therapy declines or disappears with increasing time since exposure,18–21 perhaps because of a decrease in the frequency of resistant variants (with low levels of resistant virus incorporated into DNA) in the absence of ongoing nevirapine exposure.
The association between baseline nevirapine resistance and a reduced efficacy of nevirapine-based antiretroviral therapy, the waning advantage of ritonavir-boosted lopinavir over nevirapine as the time since exposure to single-dose nevirapine increases (in trial 1), and the similar rates of virologic failure and death in the two treatment groups in trial 2 all suggest that our findings are largely related to a reduction in the efficacy of nevirapine-based antiretroviral therapy due to nevirapine resistance (including minor resistant variants not detected on standard genotyping) after exposure to single-dose nevirapine rather than to an inherently greater efficacy of ritonavir-boosted lopinavir.
The results of this study cannot necessarily be extrapolated to other populations for several reasons. First, the response to nevirapine-based antiretroviral therapy may be better among women who previously received other peripartum antiretroviral agents along with single-dose nevirapine (because of a lower risk of nevirapine resistance).10–13 (It is important to note that although more extensive regimens are recommended, they remain inaccessible to a large proportion of women globally, including those in most of the OCTANE study sites.) Second, nevirapine resistance after exposure to single-dose nevirapine may emerge more frequently4,36 and fade more rapidly37 with different HIV subtypes. Finally, a longer interval between exposure to single-dose nevirapine and treatment initiation may reflect a higher CD4+ cell count at the time of single-dose nevirapine exposure, which in turn is associated with a lower risk of nevirapine resistance. We cannot determine whether treatment with ritonavir-boosted lopinavir would remain superior to treatment with nevirapine among women with previous exposure to single-dose nevirapine and high CD4+ counts (e.g., ≥350 cells per cubic millimeter).
We conclude that treatment with ritonavir-boosted lopinavir plus tenofovir–emtricitabine is superior to treatment with nevirapine plus tenofovir–emtricitabine among women who previously received single-dose nevirapine, particularly those who had nevirapine resistance at baseline or exposure to single-dose nevirapine within the 24 months before treatment initiation. Regimens based on ritonavir-boosted lopinavir (or other potent regimens based on non-NNRTIs) should be made available to women who require antiretroviral therapy after recent exposure to single-dose nevirapine.
Supplementary Material
Acknowledgments
Supported in part by grants (U01AI068636, AI38838, and SDMC AI68634) from the National Institute of Allergy and Infectious Diseases to the AIDS Clinical Trials Group, by grants from the National Center for Research to the General Clinical Research Center Units, and by grants (K24 AI56933, to Dr. Currier, and 5401A1068636-04, to the Virology Support Laboratory for Adult AIDS Clinical Trials Group) from the National Institutes of Health. Study drugs were provided by Abbott Laboratories, Boehringer Ingelheim Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, and GlaxoSmithKline.
Dr. Hughes reports receiving fees as a member of the data and safety monitoring boards for Boehringer Ingelheim, Medicines Development, Pfizer, Tibotec, and Virionyx and the receipt by his department of financial support from Schering-Plough and Merck for an annual educational workshop; Dr. McIntyre, receiving financial support from the Abbott Speakers Bureau; Dr. Hosseinipour, receiving financial support from Abbott Virology for educational presentations; Dr. Mohapi, receiving reimbursement for travel expenses from Pfizer Laboratories; Dr. Wools-Kaloustian, receiving grant support from Gilead Sciences; Dr. Rooney, being a full-time employee of (and having stock options in) Gilead Sciences; Dr. Rahim, being a full-time employee of (and having stock options in) Abbott Laboratories at the time the study was conducted; Dr. Mellors, serving on the scientific advisory board for Gilead Sciences, receiving consulting fees from Merck, Idenix Pharmaceuticals, Chimerix, RFS Pharmaceuticals, Panacos Pharmaceuticals, and Abbott Laboratories, receiving grant support from Merck, having stock options in RFS Pharmaceuticals, and receiving reimbursement for travel expenses from Gilead Sciences, Merck, Chimerix, Idenix Pharmaceuticals, RFS Pharmaceuticals, and Panacos Pharmaceuticals; Dr. Schooley, receiving consulting fees from Glaxo-SmithKline and Abbott Laboratories and reimbursement for travel expenses from Abbott Laboratories; and Dr. Currier, receiving consulting fees from GlaxoSmithKline and grant support from Merck and Tibotec.
We thank the patients for participating in the study.
This article is dedicated to the memory of Dr. Stephen Lagakos.
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, the Walter Reed Army Institute of Research, the U.S. Army, the U.S. Department of Defense, the Kenya Medical Research Institute, or the Henry M. Jackson Foundation for the Advancement of Military Medicine.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
No other potential conflict of interest relevant to this article was reported.
References
- 1.AIDS epidemic update. Geneva: UNAIDS; Nov, 2009. [Google Scholar]
- 2.Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2008. Geneva: World Health Organization; 2008. [Google Scholar]
- 3.Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants recommendations for a public health approach — 2010 version. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 4.Eshleman SH, Guay LA, Mwatha A, et al. Characterization of nevirapine resistance mutations in women with subtype A vs. D HIV-1 6–8 weeks after single-dose nevirapine (HIVNET 012) J Acquir Immune Defic Syndr. 2004;35:126–30. doi: 10.1097/00126334-200402010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kassaye S, Lee E, Kantor R, et al. Drug resistance in plasma and breast milk after single-dose nevirapine in subtype C HIV type 1: population and clonal sequence analysis. AIDS Res Hum Retroviruses. 2007;23:1055–61. doi: 10.1089/aid.2007.0045. [DOI] [PubMed] [Google Scholar]
- 6.Flys TS, Chen S, Jones DC, et al. Quantitative analysis of HIV-1 variants with the K103N resistance mutation after single-dose nevirapine in women with HIV-1 subtypes A, C, and D. J Acquir Immune Defic Syndr. 2006;42:610–3. doi: 10.1097/01.qai.0000221686.67810.20. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JA, Li JF, Morris L, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192:16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 8.Loubser S, Balfe P, Sherman G, Hammer S, Kuhn L, Morris L. Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission. AIDS. 2006;20:995–1002. doi: 10.1097/01.aids.0000222071.60620.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer S, Boltz V, Martinson N, et al. Persistence of nevirapine-resistant HIV-1 in women after single-dose nevirapine therapy for prevention of maternal-to-fetal HIV-1 transmission. Proc Natl Acad Sci U S A. 2006;103:7094–9. doi: 10.1073/pnas.0602033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaix ML, Ekouevi DK, Rouet F, et al. Low risk of nevirapine resistance mutations in the prevention of mother-to-child transmission of HIV-1: Agence Nationale de Recherches sur le SIDA Ditrame Plus, Abidjan, Cote d’Ivoire. J Infect Dis. 2006;193:482–7. doi: 10.1086/499966. [DOI] [PubMed] [Google Scholar]
- 11.Chi BH, Sinkala M, Mbewe F, et al. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet. 2007;370:1698–705. doi: 10.1016/S0140-6736(07)61605-5. [Erratum, Lancet 2008;371:650.] [DOI] [PubMed] [Google Scholar]
- 12.McIntyre JA, Hopley M, Moodley D, et al. Efficacy of short-course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother-to-child HIV transmission: a randomized clinical trial. PLoS Med. 2009;6(10):e1000172. doi: 10.1371/journal.pmed.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Zyl GU, Claassen M, Engelbrecht S, et al. Zidovudine with nevirapine for the prevention of HIV mother-to-child transmission reduces nevirapine resistance in mothers from the Western Cape, South Africa. J Med Virol. 2008;80:942–6. doi: 10.1002/jmv.21157. [DOI] [PubMed] [Google Scholar]
- 14.Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15:1951–7. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 15.Morris L, Martinson N, Pillay C, et al. Persistence of nevirapine resistance mutations 6 months following single dose nevirapine. Presented at the XV International AIDS Conference; Bangkok, Thailand. July 11–16, 2004; abstract. ( http://gateway.nlm.nih.gov/MeetingAbstracts/ma?f=102280637.html.) [Google Scholar]
- 16.Flys T, Nissley DV, Claasen CW, et al. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J Infect Dis. 2005;192:24–9. doi: 10.1086/430742. [DOI] [PubMed] [Google Scholar]
- 17.Flys TS, Donnell D, Mwatha A, et al. Persistence of K103N-containing HIV-1 variants after single-dose nevirapine for prevention of HIV-1 mother-to-child transmission. J Infect Dis. 2007;195:711–5. doi: 10.1086/511433. [DOI] [PubMed] [Google Scholar]
- 18.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–40. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 19.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 20.Chi BH, Sinkala M, Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;21:957–64. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coovadia A, Hunt G, Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to non-nucleoside reverse-transcriptase inhibitor–based therapy. Clin Infect Dis. 2009;48:462–72. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stringer JS, McConnell MS, Kiarie J, et al. Effectiveness of non-nucleoside reverse-transcriptase inhibitor–based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country, prospective cohort study. PLoS Med. 2010;7(2):e1000233. doi: 10.1371/journal.pmed.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach — 2010 revision. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 24.Division of AIDS. Division of AIDS table for grading the severity of adult and pediatric adverse events. Baltimore: National Institutes of Health; 2004. [Google Scholar]
- 25.Hirsch MS, Günthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47:266–85. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 26.East 5 user manual. Cambridge, MA: Cytel; 2007. [Google Scholar]
- 27.Jourdain G, Ngo-Giang-Huong N, Tungyai P, et al. Exposure to intrapartum single-dose nevirapine and subsequent maternal 6-month response to NNRTI-based regimens. Presented at the 11th Conference on Retroviruses and Opportunistic Infections; San Francisco. February 8–11, 2004; abstract. ( http://www.retroconference.org/2004/cd/Abstract/41LB.htm.) [Google Scholar]
- 28.van Leth F, Phanuphak P, Ruxrungtham K, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–63. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 29.Rey D, Hoen B, Chavanet P, et al. High rate of early virological failure with the once-daily tenofovir/lamivudine/nevirapine combination in naive HIV-1-infected patients. J Antimicrob Chemother. 2009;63:380–8. doi: 10.1093/jac/dkn471. [DOI] [PubMed] [Google Scholar]
- 30.Walmsley S, Bernstein B, King M, et al. Lopinavir–ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–46. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 31.Eron J, Jr, Yeni P, Gathe J, Jr, et al. The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trial. Lancet. 2006;368:476–82. doi: 10.1016/S0140-6736(06)69155-1. [Erratum, Lancet 2006;368:1238.] [DOI] [PubMed] [Google Scholar]
- 32.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–55. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22:1389–97. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- 35.Coovadia A, Hunt G, Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose nevi-rapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48:462–72. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshleman SH, Hoover DR, Chen S, et al. Nevirapine (NVP) resistance in women with HIV-1 subtype C, compared with subtypes A and D, after the administration of single-dose NVP. J Infect Dis. 2005;192:30–6. doi: 10.1086/430764. [DOI] [PubMed] [Google Scholar]
- 37.Eshleman SH, Guay LA, Wang J, et al. Distinct patterns of emergence and fading of K103N and Y181C in women with subtype A vs. D after single-dose nevirapine: HIVNET 012. J Acquir Immune Defic Syndr. 2005;40:24–9. doi: 10.1097/01.qai.0000174656.71276.d6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.