Abstract
Crosses between the North American deer mouse species Peromyscus maniculatus (BW) and P. polionotus (PO) produce dramatic asymmetric developmental effects. BW females mated to PO males (female bw × male po) produce viable growth-retarded offspring. In contrast, PO females mated to BW males (female PO × male BW) produce overgrown but dysmorphic conceptuses. Most female PO × male BW offspring are dead by midgestation; those surviving to later time points display numerous defects reminiscent of several diseases. The hybrid effects are particularly pronounced in the placenta. Here we examine placental morphological defects via histology and in situ hybridization as well as the relationship between growth and mortality in the female PO × male BW cross. These assays indicate altered hybrid fetal:placental ratios by the equivalent of mouse (Mus) Embryonic Day (E) 13 and disorganization and labyrinth defects in female PO × male BW placentas and confirm earlier suggestions of a severely reduced junctional zone in the female bw × male po hybrids. Further, we show that both cellular proliferation and death are abnormal in the hybrids through BrdU incorporation and TUNEL assays, respectively. Together the data indicate that the origin of the effects is prior to the equivalent of Mus E10. Finally, as the majority of these assays had not previously been performed on Peromyscus, these studies provide comparative data on wild-type placentation.
Keywords: apoptosis, developmental biology, placenta, trophoblast
Pattern formation, cellular proliferation, and cell death are aberrant in Peromyscus hybrid placentas; these defects begin in the first half of gestation.
INTRODUCTION
The placenta and associated extraembryonic tissues perform multiple functions during mammalian prenatal development [1, 2]. In addition to mediating maternal-fetal nutrient/waste transfer and immune relationships, these tissues are major endocrine organs and contribute to early pattern formation and hematopoiesis [3]. Thus, perturbations of placental function may result in fetal and/or maternal health consequences [4–6]. However, there are few mammalian models for studying the potential interactions of natural genetic variants to produce developmental dysplasias. For example, the genomes of commonly used rodent strains do not represent naturally occurring allelic combinations and have been affected by inbreeding [7, 8]. Similarly, other domesticated mammals are known to have been altered by human selection (e.g., dogs, cows) [9].
Deer mice (Peromyscus) offer such an opportunity. These native North American cricetid rodents are >25 million years diverged from both laboratory mice (Mus) and Rats (Rattus) and not capable of interbreeding with them. Crosses between two species, P. maniculatus (stock = BW) and P. polionotus (stock = PO), produce asymmetric effects on growth and development. Both parental stock strains were derived from single wild populations [10].
While PO and BW animals have identical term fetal and placental weights, BW females crossed with PO males produce growth-retarded offspring [11, 12]. These animals remain smaller than either parental species throughout life (denoted as female bw × male po to indicate the effect). Litter sizes of the female bw × male po hybrid cross are slightly smaller than those of either parental strain, indicating some prenatal lethality [13]. However, the growth-retarded survivors do not exhibit a noticeably shortened life span (PO, BW live to 4+ yr), are fertile, and the sexes are equally represented.
In contrast, PO females mated to BW males (female PO × male BW) produce overgrown and severely dysmorphic conceptuses. Roughly half of all female PO × male BW breedings end in complete death of the litter by the time gestation is two-thirds complete [14, 15]. Female PO × male BW hybrids that survive to late gestation display numerous developmental defects, many reminiscent of human syndromes [14]. Rare female PO × male BW litters that reach parturition typically result in maternal death because of an inability to pass the hybrid offspring through the birth canal [13].
The placenta is particularly affected in both crosses [13, 16, 17]. The female bw × male po placentas average half the weight of those from the parental strains, while those of the female PO × male BW cross weigh about three times that of the parental strains. An additional portion (∼10%) of female PO × male BW conceptuses lack any obvious embryonic structures [14]. These conceptuses typically resemble placental tissue and thus may be analogous to hydatidiform moles.
The hybrid placentas (particularly female PO × male BW) have been shown to misexpress extracellular matrix (ECM)-related loci, loci-encoding cell-cycle regulators, and loci subject to genomic imprinting [18–20]. Numerous imprinted gene products have been documented as playing significant roles in mammalian placental growth and development [21]. Similar misexpression or loss of imprinting (LOI) of imprinted genes has been linked to the molar phenotype [22, 23]. However, neither the relationship of the altered gene expression to the hybrid phenotypes nor the nature of the placental defects have yet been elucidated.
To understand the basis of these defects, we therefore assessed hybrid placental patterning, growth dynamics, and their potential association with female PO × male BW hybrid mortality. We also assessed parental strain and hybrid placental growth, morphology, and gene expression over the time corresponding to Mus Embryonic Day (E) 10–16. Peromyscus development differs from that of Mus in that it is approximately 4 days longer prior to implantation. That is, a Peromyscus embryo E14 is the equivalent of a Mus E10 embryo. However, we refer to Mus equivalent developmental time points to avoid confusion.
Peromyscus placentas are discoidal and similar in morphology to those of other muroid rodents [24]. As in other muroids, Peromyscus placentation results in three distinct layers: 1) the labyrinth, where maternal and fetal blood supplies meet; 2) the junctional zone (or spongiotrophoblast), which gives rise to invasive and endocrine cells; and 3) the maternal decidua—that part of the endometrium that has been remodeled by invasive zygotic cells and serves as the attachment point. The morphology of the junctional zone/labyrinth boundary and maternal interface exhibit some differences from those of house mice (Mus) and other genera. Studies to date indicate that the female bw × male po placental phenotype is due to a reduction of the junctional zone [16, 17]. The structural changes occurring in female PO × male BW placentation are less clear, however, as are the dynamics of the abnormal proliferation. Here we investigate these changes through histology and gene expression of loci known to exhibit hybrid misexpression and/or trophoblast lineage markers. In addition, we assess BrdU incorporation and TUNEL assays to pursue these questions.
MATERIALS AND METHODS
Animals
We purchased PO and BW animals from the Peromyscus Genetic Stock Center (http://stkctr.biol.sc.edu). Animals were housed and bred under approved institutional animal care protocols of the University of California, Irvine, or the University of South Carolina and in accordance with the Guide for the Care and Use of Laboratory Animals. Animals were housed with food and water ad libitum on a 16:8-h light:dark cycle. We bred PO females with BW males and BW females with PO males to obtain reciprocal hybrids. Males and females were placed in the same cage with a separation cage top to allow the estrous cycle of the female to be induced by the presence of a male without contact. After 3 days, a normal cage top was used so that females and males could mate at will. Vaginal smears were performed two times a day (morning and evening) and visually assessed for sperm. We designated Day 0 of development when sperm was found in vagina of cycling females. Placentas (and associated fetuses) were dissected at each time point listed and for each grouping (PO and BW, female bw × male po, and female PO × male BW). Placentas were then weighed (wet), and the average for each group and time point was determined.
Histological Analysis
Placentas were fixed in 4% paraformaldehyde. Fixed samples were dehydrated through ascending concentrations of ethanol and then sent to Histoserv Inc. (www.histoservinc.com) for sectioning and hematoxylin and eosin staining. The laminin (catalog #ab7463) antibody was purchased from Abcam (www.abcam.com) and used according to the manufacturers' protocol.
In Situ Hybridization
Detection of messenger RNA by in situ hybridization in tissue sections was performed by standard methods [25]. Peromyscus sequences (∼500 base-pair [bp] cDNA fragment from exons 1–2 of the H19 gene, ∼400-bp fragment spanning exons 2–3 of Cdkn1c, and a full-length Esx1 cDNA) were cloned into the Topo TA dual promoter cloning vector (Invitrogen). Sp6- and T7-derived RNA probes were generated from linearized plasmids in the presence of digoxygenin to generate both sense and anti-sense hybridization probes. Tpbpa in situ hybridization was performed with a full-length Mus cDNA probe. The Tpbpa and Esx1 procedures were performed by Histoserv, Inc. For both histology and in situ hybridizations, at least three sections from three individuals per time point and subgrouping were examined. Sense-strand probes for each gene were also hybridized; none exhibited staining.
Gene Expression
Quantitative real-time PCR for Esx1 was performed as previously described. The Peromyscus Rpl32 gene was used as a control. Reactions were done in triplicate in 96-well plates and run on an ABI 7900 real-time PCR instrument. Quantitative analysis was conducted according to Critical Factors for Successful Real-Time PCR from Qiagen located at www.Qiagen.com. A minimum of three samples were used for each subgrouping (PO, BW, female PO × male BW and female bw × male po) in the analysis.
Proliferation and Apoptosis Assays
Females with litters at the equivalent of Mus E10, E13, and E16 were injected with BrdU at 1.5 ml/100 g and euthanized 1 h later. Sections were stained for apoptosis and amount of proliferation with the In situ Cell Death Detection Kit, AP (catalog #11684809910) and 5-bromo-2′-deoxy-uridine Labeling Detection Kit II (catalog #1299964) assay kits from Roche Applied Science. The number of BrdU-incorporated and TUNEL-positive cells were counted under 30× magnification. The numbers presented were derived by averaging at least three sections from four individuals per time group from each subgrouping (the two parental strains—denoted as PO/BW, female bw × male po, and female PO × male BW).
RESULTS
Placental Growth Versus Hybrid Survival
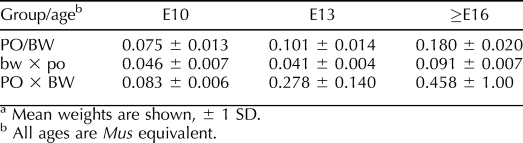
The placental weights of the parental strains and reciprocal hybrids at three time points (equivalent to Mus ages E10, 13, and 16) are summarized in Table 1. The reduction in female bw × male po placental weight is apparent at the earliest stage examined and throughout the remainder of gestation (i.e., including times later than E16).
TABLE 1.
Peromyscus placental weights (g) at selected ages.a
Surprisingly, placentas of the female PO × male BW conceptuses we examined at E10 do not vary significantly in weight from those of the parental stock animals. However, we did observe developmental defects in the E10 female PO × male BW conceptuses. By E13, however, the average female PO × male BW placental weight is greater than that of mature PO/BW embryos (∼E15 or later).
Our studies indicate that the majority of female PO × male BW conceptuses are dead by E16. While many of these deaths occur prior to E10, overall female PO × male BW mortality continues to increase from E10 to E16. We had previously observed that greater placental weight was correlated with the degree of LOI in backcross (PO females × F1 males) that mimics the female PO × male BW cross [26]. Similarly, the degree of LOI in mixed-background animals is associated with prenatal mortality. We therefore considered whether increased placental growth might be generally associated with the female PO × male BW mortality.
To test this hypothesis, we plotted placental weights of timed pregnancies vs. the percentage of female PO × male BW conceptuses alive in litters at that age (Supplemental Fig. S1 available at www.biolreprod.org). These data provided little evidence of a specific association at any particular age. A possible exception is seen from E12 to E13; the percentage of live conceptuses observed at E12 was only ∼50%, while only ∼30% of those observed at E13 were alive. However, examination of timed pregnancies indicates that the cumulative total of live conceptuses by this point is well below 50% (meaning that there may be a selection bias such that conceptuses that survive to E13 are atypical). This observation, combined with the phenotypic heterogeneity of the female PO × male BW conceptuses, makes determining such associations difficult.
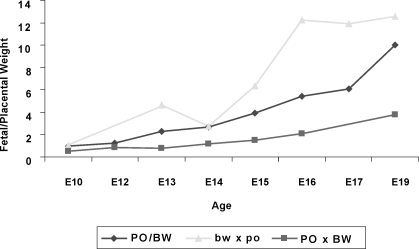
We also assessed fetal:placental weight ratios as a measure of placental efficiency [27]. As expected, the average value of this ratio increases steadily over the second half of gestation in parental strain (PO or BW) animals (Fig. 1); it reaches 10 the day prior to parturition. While the trajectory of the female bw × male po ratio is more erratic, it reaches slightly greater values than that of the parental strains. This increased ratio reflects the observation that female bw × male po placental weight is more affected than fetal weight. In contrast, the maximum average female PO × male BW ratio is approximately six. The lowered ratio in female PO × male BW conceptuses may reflect both placental defects and cell lineage shifts toward extraembryonic fates (as suggested by the molar conceptuses).
FIG. 1.
Average placental:fetal weight ratios from E10 to E16. Genotype is indicated in key. With the exception of the parental strains (PO/BW) and female bw × male po at E14, all three classes differed significantly from each other after E12 as judged by the Student t-test; (P < 0.001) as implemented in Microsoft Excel. All ages are Mus equivalent.
Female PO × Male BW Hybrid Placental Hemorrhaging
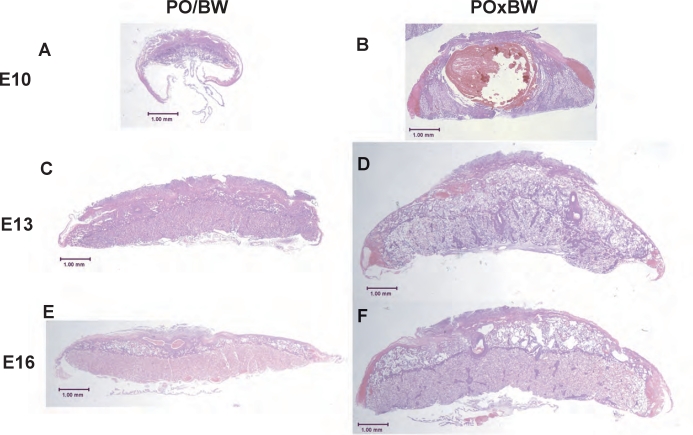
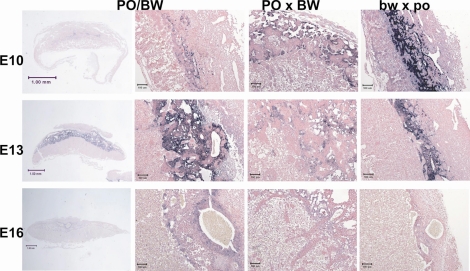
One cause of the female PO × male BW placental inefficiency is suggested by the frequent occurrence of macroscopic blood pools (i.e., indicating intraplacental hemorrhaging). We used hematoxylin and eosin staining to assess the nature and ontogeny of this major defect in placental pattern formation (Fig. 2). The female PO × male BW blood pools we have observed are more often located at the placental edges. However, as shown in Figure 2, large pools occupying a central sinus occur at least as early as E10 (note that the degree of hemorrhaging is heterogeneous). This suggests that a possible underlying cause of embryonic death may be failure of maternal-fetal nutrient transfer due to blood vessel instability in the labyrinth. Moreover, our previous research suggested a deficiency of some ECM-related proteins in female PO × male BW placentas [20].
FIG. 2.
Hematoxylin and eosin-stained histological sections illustrating the hemorrhaging and disorganization observed in the female PO × male BW placentas. Genotype is indicated at top and age (Mus equivalent) at left. Original magnification ×1; bars = 1 mm.
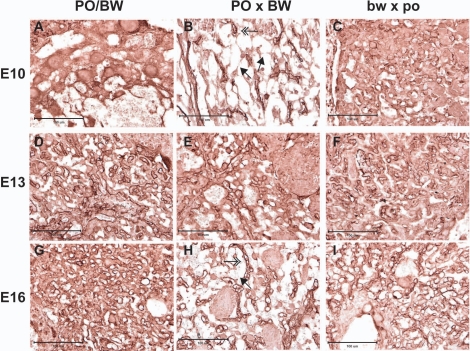
To further investigate this possibility, we stained placental sections with a laminin antibody. These proteins are key components of the basal lamina and therefore useful for enhancing the clarity of placental blood vessels [28]. Examination of the stained sections suggests a reduction in female PO × male BW embryonic blood vessels (i.e., relative to PO/BW; Fig. 3). Moreover, the walls of the maternal blood sinuses within the female PO × male BW labyrinth typically appear thinner and often compromised. These defects are consistent with the previously documented reduction in female PO × male BW placental collagen expression as well as similar hemorrhaging in the associated embryos [11, 20].
FIG. 3.
Representative examples of laminin immunohistochemistry to assess labyrinth vasculature. Genotype is indicated at top and age at left. Columns represent groups of placentas by genotype: PO/BW, female PO × male BW and female bw × male po, respectively. A–C = E10 placentas, D–F = E13 placentas and G–I = E16 placentas (all ages are Mus equivalent). Double arrowheads represent embryonic blood vessels; single arrowheads indicate compromised maternal blood sinuses. Such tears were never observed in PO/BW or female bw × male po sections. All pictures were taken of the labyrinth at ×40 magnification.
Labyrinth and Junctional Zone Markers
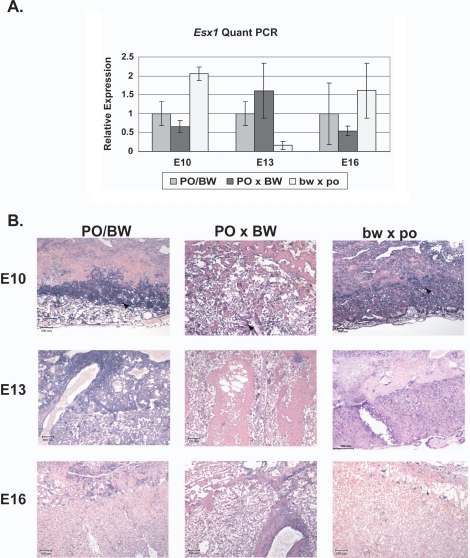
The product of the X-chromosome-linked Esx1 gene is a homeodomain transcription factor critical for proper morphogenesis of vascularization within the labyrinth layer [29, 30]. Ablation of mouse Esx1 expression results in enlarged placentas with little demarcation between the labyrinth and junctional zone. The Esx1 mutant placentas also exhibit abnormal capillary dilation and branching. In addition to these similarities between the knockout and female PO × male BW placentas, Esx1 is tightly linked to a Peromyscus locus mapped on the basis of association with the placental hypertrophy [26, 31]. Thus, variation in this gene may contribute to aspects of the female PO × male BW dysgenesis.
Our previous study indicated Esx1 overexpression in female bw × male po placentas—possibly reflecting the relatively high proportion of labyrinthine cells in this hybrid cross. We reassessed Esx1 expression using the same quantitative PCR assay at E10, E13, and E16 (Fig. 4A). The temporally expanded data reveals that the female bw × male po Esx1 overexpression is transitory; at E13, female bw × male po expression drops significantly below that of PO/BW. While the average female PO × male BW expression is lower than that of the parental strains at E10 and E16, the values substantially overlap. Therefore, while it is unlikely that reduced Esx1 expression levels contribute to the female PO × male BW defects, it is possible that spatial misexpression plays a role.
FIG. 4.
Quantitative PCR (A) and in situ hybridization assays (B) of placental Esx1 expression. In A, genotype is indicated at bottom. Bars indicate standard error. Female bw × male po expression was significantly different (P < 0.001) than the other two classes at both E10 and E13 (Mus equivalent). In B, genotype is indicated at top and age at left. Note the expanded area of Esx1 expression at E10 in both hybrid types relative to the parental strains (PO/BW). However, the expressing cells are more dispersed in the female PO × male BW hybrids. Arrows indicate areas of relatively intense staining. Original magnification ×10; bars = 100 μm.
To investigate this possibility, we performed in situ hybridization with a Peromyscus Esx1 probe on placental sections. As expected, the highest proportion of expressing cells is seen at E10, with few positive cells at E16 in the parental strains or either hybrid type. As in Mus, E10 expression appeared highest in the developing labyrinth. At E13 and E16, however, expressing cells are more dispersed throughout the placenta (Fig. 4B). Esx1 staining in the E10 female bw × male po hybrid placentas reflects the apparent reduction of junctional zone trophoblast. Similarly, female PO × male BW E10 Esx1 expression is more dispersed, presumably reflecting that spatial disorganization has already occurred.
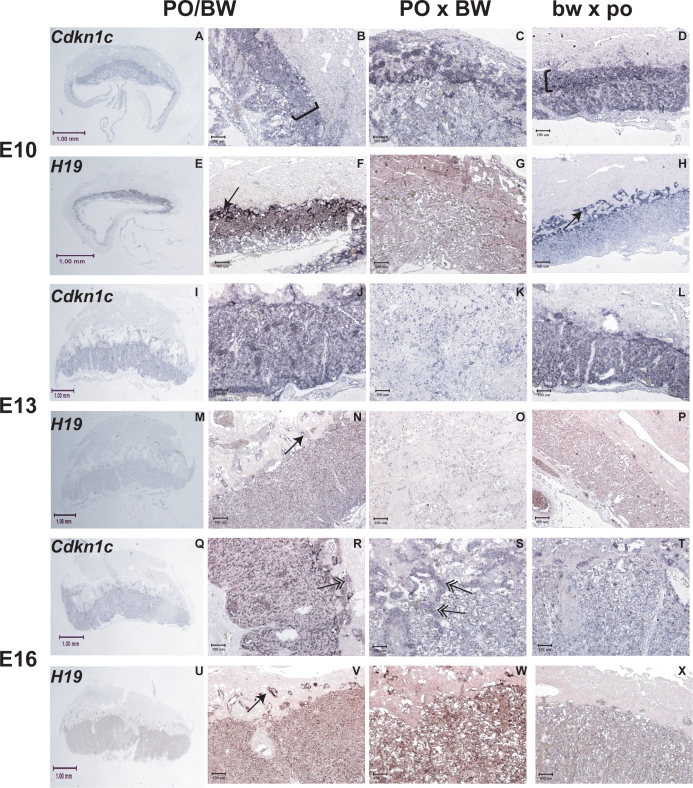
We also performed in situ hybridization experiments with probes from two additional loci whose primary placental expression is within the labyrinthine layer. Cdkn1c and H19 are linked imprinted loci that we have previously shown to exhibit female PO × male BW hybrid misexpression [18–20]. While both are normally expressed from the maternal allele, H19 exhibits increased (biallelic) female PO × male BW expression, while placental Cdkn1c expression is reduced. The potential effects of increased H19 expression are unclear [32]. However, the Cdkn1c product is a negative regulator of growth, and its downregulation is associated with hyperproliferation in normal development and diseases, including hydatidiform moles [33].
Figure 5 illustrates that Cdkn1c stains both the Peromyscus junctional zone and the labyrinth at E10. Thereafter, expression is confined largely to the labyrinth, trophoblast giant cells (TGCs), and probable immature TGCs. In contrast, H19 is found in the labyrinth and mature TGCs at all ages. The H19 TGC expression illustrates a similar migration of these cells in Peromyscus as in other rodent species: at E10, the majority of TGCs are seen between the labyrinth and spongiotrophoblast layer but by E16 are within the decidua and junctional zone. However, the staining patterns of these two genes suggest a lack of demarcation between the two zones in female PO × male BW and are consistent with an early reduction in the female bw × male po junctional zone/spongiotrophoblast.
FIG. 5.
Representative results of placental in situ hybridization experiments with Cdkn1c and H19 probes. Columns represent groups of placentas (PO/BW, female PO × male BW and female bw × male po, respectively). A–H = E10 placentas, I–P = E13 placentas, Q–X = E16 placentas (all ages are Mus equivalent). Rows alternate between the two genes as indicated in the leftmost panels. Single arrow = mature TGCs, double arrow = probable immature TGCs. Brackets in B and D outline spongiotrophoblast. Column 1 pictures were taken at ×1 magnification; column 2–4 pictures were taken at ×15 magnification. Note that the PO/BW ×10 magnification picture at E10 (B) appears rotated, as it is a close-up of the rightmost section of A.
We used the well-established marker Tpbpa (i.e., 4311) to further assess possible aberrant junctional zone patterning in the hybrids (Fig. 6). In Mus, this gene is expressed in all junctional zone cell types except TGCs [34]. Surprisingly, E10 Tpbpa staining was most intense in female bw × male po placental sections. However, by E13 the female bw × male po staining region was considerably narrower than that of the parental strains and nearly absent at E16. This result suggests an initial surfeit of female bw × male po spongiotrophoblast progenitors that fail to multiply and/or survive at a normal rate.
FIG. 6.
Representative results of Tpbpa in situ placental hybridization. Genotypes and ages indicated at the top and left of figure, respectively (all ages are Mus equivalent). The first column pictures were taken at ×1 magnification, while all others were taken at ×10 magnification. Note that the ×10 pictures are rotated 90 degrees clockwise relative to the ×1 pictures.
In contrast, female PO × male BW Tpbpa staining was less cohesive than that of the parental strains at all three ages: E10 expression is scattered and found on the edges of the areas stained by H19 and Cdkn1c. By E13, parental strain Tpbpa staining has expanded considerably, while female PO × male BW is even more scattered. This suggests that very early female PO × male BW placental patterning is at least slightly perturbed, and the disorganization is magnified as development proceeds.
Proliferation and Cell Death
The observed aberrant growth and pattern formation in the hybrid placentas suggests possible imbalances between proliferation and apoptosis. As we previously found genes encoding cell-cycle regulators to be affected in the hybrid placentas [20], we investigated whether gross differences in cellular proliferation and death were apparent in the hybrid placentas. We utilized BrdU incorporation to measure proliferation and TUNEL staining, which identifies cells with DNA fragmentation typically resulting from apoptosis.
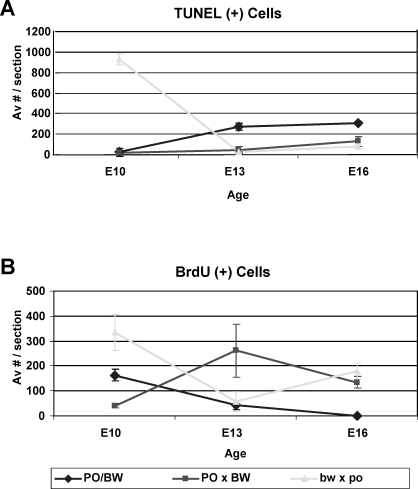
In Mus, placental growth shows increasing proliferation and organization of the placenta until approximately E14.5, when it is considered mature [35]. Reciprocally, regionalized apoptosis in the maternal decidua and fetal glycogen cells start at mid- to late gestation. Figure 7 indicates that these patterns are very similar in wild-type Peromyscus: the number of BrdU-positive cells declines past E10, becoming virtually absent by E16. Correspondingly, the number of PO/BW TUNEL-positive placental cells increased over this period.
FIG. 7.
Quantitation of BrdU (A) and TUNEL (B) assays. Average number of positive cells per section is shown for each genotype (indicated in key); bars indicate standard error. Significant differences (P < 0.001) in A are female bw × male po (from the other two classes) at E10 and both hybrid types (from PO/BW) at E13 and E16. All classes significantly differ from each other in B except female bw × male po from PO/BW at E13 and the two hybrid types from each other at E16 (all ages are Mus equivalent).
Both the female PO × male BW and the female bw × male po placentas exhibited shifted proliferation/apoptosis curves. Surprisingly, both hybrid types had higher numbers of BrdU-positive cells, albeit at different points in development: at E10, female bw × male po placentas displayed both greatly increased numbers of proliferating and dying cells relative to the parental strains. By E13, however, proliferation had been reduced to wild-type levels, and cell death was virtually absent. TUNEL-positive cells increased at E16 but remained below wild-type levels.
In contrast, female PO × male BW cell death remained well below that of the parental strains at all three time points examined. While BrdU incorporation did rise to levels well in excess of those exhibited by the parental strains, this increase was not apparent until E13. The number of female PO × male BW proliferative cells decreased somewhat from E13 to E16, reaching roughly equivalent numbers as that of apoptotic cells at the latter time point.
The localization of cell death and proliferation in PO/BW and female PO × male BW placentas were similar to those observed in Mus (e.g., late gestation apoptosis in the decidua and glycogen cells) [36, 37]. The abnormally high levels of proliferation in the female bw × male po placentation were centered within the spongiotrophoblast layer and maternal decidua, with apoptotic cell located throughout the placenta (data not shown).
DISCUSSION
The placenta is a transient organ whose proper function is nevertheless critical for both maternal and fetal health [6]. Placental hypoplasia is associated with intrauterine and other growth retardation [2, 4] syndromes, while hyperplasia is associated with fetal overgrowth syndromes such as Beckwith-Wiedemann [38] as well as gestational diabetes [39] and various forms of gestational trophoblast disease [40]. Peromyscus offer the opportunity for assessing the effects of natural genetic variation on placental growth, development, and physiology as well as an additional comparative model for these processes as well as disease. We have previously shown altered expression and epigenetic state of a number of imprinted loci implicated in human diseases that correlate with the Peromyscus hybrid phenotypes (e.g., reductions in Cdkn1c and Phlda2 expression) [41].
We have focused primarily on the dramatic placental defects in female PO × male BW dysgenesis and how they may underlie fetal mortality. Female PO × male BW placental efficiency (as defined by fetal:placental weight ratio) is reduced after E12. This period of placental weight increase is immediately preceded by a rise in fetal mortality. Among the potential causes of mortality in this time frame are the observed labyrinth defects; these include unstable maternal blood spaces (i.e., presumably due to the thinner walls) and a decrease in the number of fetal capillaries. These defects would be expected to inhibit maternal-fetal nutrient and waste exchange.
The in situ hybridization experiments suggest that both labyrinth and junctional zone patterning are affected. Because of our previous demonstration of a reduction in Phlda2 expression [14], we predicted an increase in the number of female PO × male BW Tpbpa staining cells by comparison with the Mus knockout of this gene [42]. However, this was not the case; we hypothesize that female PO × male BW overproliferation (and simultaneous reduction in apoptosis) results in an elevated proportion of improperly differentiated cells. For example, we frequently observed cells that may be immature/improperly differentiated TGCs (Fig. 5). Concurrent experiments assessing TGC and stem-cell markers support this hypothesis. We suggest that the maternal effect that results in female PO × male BW genomic imprinting perturbations [43] also results in epigenetic misregulation of lineage allocation and/or differentiation.
While the maternal effect locus appears to be the major determinant of the female PO × male BW phenotypes, Esx1 is tightly linked to a locus influencing placental overgrowth in that cross. Similarities to the Mus targeted mutation strengthen the case of Esx1 as a candidate for this locus. While there is no apparent reduction in female PO × male BW Esx1 expression, its spatial patterning is clearly perturbed. As the analyses clearly indicate, the female PO × male BW defects genesis occurs prior to E10, necessitating earlier analyses.
Preliminary analyses also suggest a role for the X chromosome in the genetics of the female bw × male po growth retardation. While it is not clear that this potential linkage will include Esx1, its transient overexpression and subsequent sharp decline are intriguing. While the female bw × male po E16 expression is wild type, the earlier misexpression and differences in spatial expression may be sufficient to contribute to the aberrant placental patterning.
The junctional zone marker Tpbpa also appears overexpressed in E10 female bw × male po placentas as judged by in situ hybridization. However, this region is reduced relative to the parental strains by E13, corroborating our observation that the higher rate of cell death particularly targets this region. Concurrent analyses also indicate a premature reduction in TGC numbers.
It is as yet unclear whether the female bw × male po fetal and postnatal growth retardation is dependent on the placental defects. These and many other questions make this unique system ripe for additional study. More detailed analyses are now feasible because of recent increases in Peromyscus resources, including BW genome sequencing in progress (www.ncbi.nlm.nih.gov/Traces/home), more than 90 000 ESTs in public databases, a nascent genetic map [44], and improved reproductive technology (unpublished data).
Acknowledgments
The Tpbpa probe was courtesy of Dr. Steven Lipkin of Weill Cornell Medical College. We thank Dr. Grant MacGregor (University of California, Irvine) and Dr. Steven Kistler (University of South Carolina) for assistance in microscopy/digital photography.
Footnotes
Supported by NSF #MCB-0517754 and NIH grant #P40 RR014279.
REFERENCES
- Moffett A, Loke YW, McLaren A.Biology and Pathology of Trophoblast. Cambridge, UK:Cambridge University Press;2006. [Google Scholar]
- Fowden AL, Sferruzzi-Perri AN, Coan PM, Constancia M, Burton GJ.Placental efficiency and adaptation: endocrine regulation. J Physiol 2009; 587: 3459–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárcena A, Kapidzic M, Muench MO, Gormley M, Scott MA, Weier JF, Ferlatte C, Fisher SJ.The human placenta is a hematopoietic organ during the embryonic and fetal periods of development. Dev Biol 2009; 327: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cross JC.Placental function in development and disease. Reprod Fertil Dev 2006; 18: 71–76. [DOI] [PubMed] [Google Scholar]
- Myatt L.Placental adaptive responses and fetal programming. J Physiol 2006; 572: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SJ.The placenta dilemma. Semin Reprod Med 2000; 18: 1–6. [DOI] [PubMed] [Google Scholar]
- Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM.Genealogies of mouse inbred strains. Nat Genet 2000; 24: 23–25. [DOI] [PubMed] [Google Scholar]
- Smale L, Heideman PD, French JA.Behavioral neuroendocrinology in nontraditional species of mammals: things the “knockout” mouse can't tell us. Horm Behav 2005; 48: 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobney K, Larson G.Genetics and animal domestication: new windows on an elusive process. J Zool 2006; 269: 261–271. [Google Scholar]
- Dewey MJ, Dawson WD.Deer mice: “the Drosophila of North American mammalogy.” Genesis 2001; 29: 105–109. [DOI] [PubMed] [Google Scholar]
- Dawson WD.Fertility and size inheritance in a Peromyscus species cross. Evolution 1965; 19: 44–55. [Google Scholar]
- Dawson WD.Postnatal development in Peromyscus maniculatus-polionotus hybrids I, developmental landmarks and litter mortality. Ohio J Sci 1966; 66: 518–522. [Google Scholar]
- Maddock MB, Chang MC.Reproductive failure and maternal-fetal relationship in a Peromyscus species cross. J Exp Zool 1979; 209: 417–426. [DOI] [PubMed] [Google Scholar]
- Duselis AR, Vrana PB.Assessment and disease comparisons of hybrid developmental defects. Hum Mol Genet 2007; 16: 808–819. [DOI] [PubMed] [Google Scholar]
- Liu TT.Prenatal mortality in Peromyscus with special reference to its bearing on reduced fertility in some interspecific and intersubspecific crosses. Contrib Lab Vert Biol 1953; 60: 1–32. [Google Scholar]
- Rogers JF, Dawson WD.Foetal and placental size in a Peromyscus species cross. J Reprod Fertil 1970; 21: 255–262. [DOI] [PubMed] [Google Scholar]
- Vrana PB, Matteson PG, Schmidt JV, Ingram RS, Joyce A, Prince KL, Dewey MJ, Tilghman SM.Genomic imprinting of a placental lactogen in Peromyscus. Dev Genes Evol 2001; 211: 523–532. [DOI] [PubMed] [Google Scholar]
- Vrana PB, Guan XJ, Ingram RS, Tilghman SM.Genomic imprinting is disrupted in interspecific Peromyscus hybrids. Nat Genet 1998; 20: 362–365. [DOI] [PubMed] [Google Scholar]
- Wiley CD, Matundan HH, Duselis AR, Isaacs AT, Vrana PB.Patterns of hybrid loss of imprinting reveal tissue- and cluster-specific regulation. PLoS ONE 2008; 3: e3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duselis AR, Obergfell C, Mack JA, O'Neill MJ, Nguyen QK, O'Neill RJ, Vrana PB.Cell-cycle and extra-cellular matrix gene expression changes during deer mouse (Peromyscus) hybrid placental development. Reprod Fertil Dev 2007; 19: 695–708. [DOI] [PubMed] [Google Scholar]
- Maccani MA, Marsit CJ.Epigenetics in the placenta. Am J Reprod Immunol 2009; 62: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson H, Hayward BE, Sheridan E, Bonthron DT.A global disorder of imprinting in the human female germ line. Nature 2002; 416: 539–542. [DOI] [PubMed] [Google Scholar]
- El-Maarri O, Seoud M, Coullin P, Herbiniaux U, Oldenburg J, Rouleau G, Slim R.Maternal alleles acquiring paternal methylation patterns in biparental complete hydatidiform moles. Hum Mol Genet 2003; 12: 1405–1413. [DOI] [PubMed] [Google Scholar]
- King BF, Hastings RA.The comparative fine structure of the interhemal membrane of chorioallantoic placentas from six genera of myomorph rodents. Am J Anat 1977; 149: 165–179. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Nieto MA.Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol 1993; 225: 361–373. [DOI] [PubMed] [Google Scholar]
- Vrana PB, Fossella JA, Matteson P, del Rio T, O'Neill MJ, Tilghman SM.Genetic and epigenetic incompatibilities underlie hybrid dysgenesis in Peromyscus. Nat Genet 2000; 25: 120–124. [DOI] [PubMed] [Google Scholar]
- van Rens BT, de Koning G, Bergsma R, van der Lende T.Preweaning piglet mortality in relation to placental efficiency. J Anim Sci 2005; 83: 144–151. [DOI] [PubMed] [Google Scholar]
- Natale D, Starovic M, Cross JC.Phenotypic analysis of the mouse placenta. Methods Mol Med 2006; 121: 275–293. [DOI] [PubMed] [Google Scholar]
- Li Y, Lemaire P, Behringer RR.Esx1, a novel X chromosome-linked homeobox gene expressed in mouse extraembryonic tissues and male germ cells. Dev Biol 1997; 188: 85–95. [DOI] [PubMed] [Google Scholar]
- Li Y, Behringer RR.Esx1 is an X-chromosome-imprinted regulator of placental development and fetal growth. Nat Genet 1998; 20: 309–311. [DOI] [PubMed] [Google Scholar]
- Loschiavo M, Nguyen QK, Duselis AR, Vrana PB.Mapping and identification of candidate loci responsible for peromyscus hybrid overgrowth. Mamm Genome 2007; 18: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsen JL, Duran KL, Bartolomei MS.Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev 1998; 12: 3693–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA, Hodges MD, Rees HC, Sebire NJ, Seckl MJ, Newlands ES, Genest DR, Castrillon DH.The maternally transcribed gene p57KIP2 (CDNK1C) is abnormally expressed in both androgenetic and biparental complete hydatidiform moles. Hum Mol Genet 2002; 11: 3267–3272. [DOI] [PubMed] [Google Scholar]
- Lescisin KR, Varmuza S, Rossant J.Isolation and characterization of a novel trophoblast-specific cDNA in the mouse. Genes Dev 1988; 2: 1639–1646. [DOI] [PubMed] [Google Scholar]
- Watson E, Cross JC.Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005; 20: 180–193. [DOI] [PubMed] [Google Scholar]
- Jurisicova A, Detmar J, Caniggia I.Molecular mechanisms of trophoblast survival: from implantation to birth. Birth Defects Res C Embryo Today 2005; 75: 262–280. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Herrler A.Regulation of proliferation and apoptosis during development of the preimplantation embryo and the placenta. Birth Defects Res C Embryo Today 2005; 75: 249–261. [DOI] [PubMed] [Google Scholar]
- McCowan LME, Becroft DMO.Beckwith-Wiedemann syndrome, placental abnormalities, and gestational proteinuric hypertension. Obstet Gynecol 1994; 83: 813–817. [PubMed] [Google Scholar]
- Yu Y, Singh U, Shi W, Konno T, Soares MJ, Geyer R, Fundele R.Influence of murine maternal diabetes on placental morphology, gene expression, and function. Arch Physiol Biochem 2008; 114: 99–110. [DOI] [PubMed] [Google Scholar]
- Cheung AN.Pathology of gestational trophoblastic diseases. Best Pract Res Clin Obstet Gynaecol 2003; 17: 849–868. [DOI] [PubMed] [Google Scholar]
- Apostolidou S, Abu-Amero S, O'Donoghue K, Frost J, Olafsdottir O, Chavele KM, Whittaker JC, Loughna P, Stanier P, Moore GE.Elevated placental expression of the imprinted PHLDA2 gene is associated with low birth weight. J Mol Med 2006; 85: 379–387. [DOI] [PubMed] [Google Scholar]
- Frank D, Fortino W, Clark L, Musalo R, Wang W, Saxena A, Li CM, Reik W, Ludwig T, Tycko B.Placental overgrowth in mice lacking the imprinted gene Ipl. Proc Natl Acad Sci U S A 2002; 99: 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duselis AR, Wiley CD, O'Neill MJ, Vrana PB.Genetic evidence for a maternal effect locus controlling genomic imprinting and growth. Genesis 2005; 43: 155–165. [DOI] [PubMed] [Google Scholar]
- Ramsdell CM, Lewandowski AA, Weston Glenn JL, Vrana PB, O'Neill RJ, Dewey MJ.Comparative genome mapping of the deer mouse (Peromyscus maniculatus) reveals greater similarity to rat (Rattus norvegicus) than to the lab mouse (Mus musculus). BMC Evol Biol 2008; 8: 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]