Abstract
Bone morphogenetic protein 6 (BMP6) is a transforming growth factor beta superfamily member produced by mammalian oocytes as well as other cell types. Despite well-characterized effects of recombinant BMP6 on granulosa cells in vitro, the function of BMP6 in vivo has been ill-defined. Therefore, the effects of genetic deletion of the Bmp6 gene on female mouse fertility were assessed. The mean litter size of Bmp6−/− females was reduced by 22% (P < 0.05) compared to Bmp6+/+ controls. Not only did Bmp6−/− females naturally ovulate 24% fewer eggs, but competence of in vitro-matured oocytes to complete preimplantation development after fertilization in vitro was decreased by 50%. No apparent effect of Bmp6 deletion on either the morphology or the dynamics of follicular development was apparent. Nevertheless, levels of luteinizing hormone (LH)/human chorionic gonadotropin (hCG)-induced transcripts, which encode proteins required for cumulus expansion (HAS2, PTGS2, PTX3, and TNFAIP6), and of epidermal growth factor-like peptides (AREG, BTC, and EREG) were lower in Bmp6−/− mice than in controls after administration of a reduced dose of hCG (1 IU) in vivo. LH receptor (Lhcgr) transcript levels were not significantly lower in Bmp6−/− granulosa cells, suggesting that BMP6 is required for processes downstream of LH receptors. To assess whether another oocyte-derived BMP, BMP15, could have BMP6-redundant functions in vivo, the fertility of Bmp15/Bmp6 double mutants was assessed. Fertility was not significantly reduced in double-homozygous mutants compared with that in double-heterozygous controls. Therefore, BMP6 promotes normal fertility in female mice, at least in part, by enabling appropriate responses to LH and normal oocyte quality. Thus, Bmp6 probably is part of the complex genetic network that determines female fertility.
Keywords: BMP6, Bmp6, early development, fertility, follicles, follicular development, oocyte quality, oocytes, ovary, ovulation rate
BMP6 promotes normal fertility in female mice by enabling appropriate responses to LH and normal oocyte quality.
INTRODUCTION
Development of mammalian ovarian follicles is coordinated by a complex interaction of multiple signals, including gonadotropins and intrafollicular paracrine/autocrine and juxtacrine signals [1]. Bone morphogenetic proteins (BMPs), members of the transforming growth factor beta superfamily, are produced in ovarian follicles. Attenuating BMP signals in mouse follicular granulosa cells by genetic deletion of its receptors or signal mediators results in impaired female fertility [2–5]. Therefore, BMP signals play a critical role in coordinating mammalian follicular development [6–8].
The BMP ligand BMP6 is produced by mammalian oocytes as well as by other cell types [9–13]. The effect of recombinant BMP6 on granulosa cell functions has been studied extensively in vitro. For example, recombinant BMP6 suppresses follicle-stimulating hormone (FSH)- and/or insulin-like growth factor-induced progesterone production by rat, bovine, and ovine granulosa cells [14–17]. BMP6 decreases FSH receptor (Fshr) mRNA levels in rat granulosa cells [14] but enhances FSHR mRNA levels in human granulosa cells [12]. Furthermore, BMP6 prevents apoptosis of bovine cumulus cells, which are the granulosa cells associated with oocytes [18]. In addition to these studies in vitro, direct ovarian infusion of BMP6 in ewes advances the time of the luteinizing hormone (LH) surge, probably because of a transient increase in serum estradiol (E2) levels in the BMP6-infused animals [19]. This finding was consistent with a previous report showing that recombinant BMP6 promotes FSH-induced E2 production by ovine granulosa cells [20]. However, BMP6 has no effect on FSH-induced E2 production by cultured rat granulosa cells [14]. Therefore, although not conclusive, it appears that BMP6 affects gonadotropin signals in granulosa cells of several mammalian species. Previous studies using Bmp6-null (Bmp6−/−) mice found a consistent delay in ossification of the developing sternum and did not detect a significant reduction in female fertility [9]; however, it is important to note that the reproductive phenotype of Bmp6−/− mice, such as ovarian morphology, granulosa cell development, or sensitivity to exogenous gonadotropin treatments, was not assessed.
To define the reproductive function of BMP6 in vivo, the present study attempted to characterize the effects of deletion of the Bmp6 gene in vivo on female mouse fertility and granulosa cell function. Moreover, possible BMP6-redundant functions of the other oocyte-derived BMP, BMP15 [21, 22], on fertility of female mice was assessed using double-mutant (Bmp6−/−/Bmp15−/−) mice.
MATERIALS AND METHODS
Mice
Mice carrying a Bmp6-null (Bmp6tm1Lex; hereafter designated as Bmp6−) allele, in which coding exons 5–7 of Bmp6 were targeted by homologous recombination, were obtained from Lexicon Genetics, Inc., and were maintained on a C57BL/6J;129S5/SvEvBrd hybrid background in the research colony of the investigators at The Jackson Laboratory. The detailed values for physiological parameters are available online (http://www.informatics.jax.org/external/ko/lexicon/2523.html). The Bmp6-null allele (Bmp6tm1Lex) used in the present study was different from that (Bmp6tm1Rob) used in a previous study, in which a part of the Bmp6 second coding exon, encoding a portion of the pro region, as well as some downstream sequence was targeted [9]. Bmp6 mutant mice were identified by real-time PCR assay for the exons 6 and 7 and sequences of Bmp6 gene over its exon 2 sequence (GenBank accession no. NM_007556). The PCR primers used were 5′-GCCAACTACTGTGATGGAGAGTGTT-3′ and 5′-GTACTCGGGATTCATAAGGTGGA-3′ for exons 6 and 7 and 5′-ACCTATCCCAGATTCCTGAGGG-3′ and 5′-GCTGATAAGAAAGGTTTGGTTTTTAAAA-3′ for exon 2. Mice carrying both Bmp6− and Bmp15-null (Bmp15tm1Zuk; hereafter designated as Bmp15−) [23] alleles were produced by mating double-heterozygous (Bmp6+/−/Bmp15+/−) females with double-mutant (Bmp6−/−/Bmp15Y/−) males and were maintained on a C57BL/6J;129S5/S hybrid background. All experiments were conducted according to the Guide for the Care and Use of Laboratory Animals (Institute for Learning and Animal Research).
Fertility Assessment
To assess reproductive performance, 6-wk-old mutant or control female mice were mated with age-matched, wild-type (Bmp6+/+) B6SJLF1 male mice. The number of pups per litter was recorded for a 6-mo period.
Histological Assessment of Ovaries
Ovaries of 18-day-old or 6-mo-old mutant and littermate control female mice were fixed in Bouin solution, embedded in paraffin, serially sectioned (thickness, 5 μm), and stained with hematoxylin-eosin. The numbers of follicles at each developmental stage in ovaries of 18-day-old mice were estimated by counting follicles in every third section where the germinal vesicle of the oocyte was observed [24]. The criteria used for classifying developmental stages of follicles were as follows: primordial follicle, oocyte surrounded by a single squamous layer of follicular somatic cells; primary follicle, single layer of cuboidal granulosa cells; early or late secondary follicles, more than one or two layers, respectively, of granulosa cells and no antrum; early antral follicle, small antrum is in the process of forming; midantral follicle, a single antral space separating the mural granulosa cells from the cumulus cells surrounding the oocyte; and atretic follicle, contains a degenerated oocyte, an oocyte that has resumed meiosis, or cumulus cells dissociated from oocyte, and pyknotic granulosa cells are common.
Assessment of Natural and Gonadotropin-Induced Ovulation Rate
To assess the number of oocytes ovulated naturally, 2-mo-old Bmp6+/+ or Bmp6−/− female mice were mated to vasectomized males. The female mice were assessed for a vaginal plug every morning. Oviductal oocytes were counted when a plug was found. To assess the ovulatory response to exogenous gonadotropin, 20-day-old female mice were injected with 5 IU of equine chorionic gonadotropin (eCG) and, 48 h later, were given 1 or 5 IU of human chorionic gonadotropin (hCG). Oviductal oocytes were counted 14 to 16 h after hCG injection.
Hormone Analyses
Levels of FSH, LH, E2, and testosterone in serum of 20-day-old female mice were measured by the Ligand Assay and Analysis Core Laboratory (University of Virginia, Charlottesville, VA). The serum was prepared by centrifugation and stored at −80°C until assayed for hormonal levels. The limits of detection for each assay were 1.3 ng/ml, 0.04 ng/ml, 0.081 ng/ml, and 23 pg/ml for the FSH, LH, testosterone, and E2 assay, respectively. Samples that exhibited values below the limits of detection were assigned the threshold value.
Reverse-Transcription Real-Time PCR
To assess steady-state levels of transcripts, reverse-transcription real-time PCR was conducted as reported previously [25]. The PCR primers used were 5′-CTCTCAGAATGATGTCTTGGAGGTAAT-3′ and 5′-GATGTACAGCAGATTGTTAGCCTTTTC-3′ for Fshr (GenBank accession no. NM_013523), 5′-GGAGAACAATTCGCCCTTTCTT-3′ and 5′-GATGGACTCCACACAAACTTCCA-3′ for Cyp19a1 (NM_007810), 5′-CAAGAACATCCAGGCCAACATT-3′ and 5′-ACCTTCAAGTTGTGTGCCATCTC-3′ for Cyp11a1 (NM_019779), and 5′-AAGGCCTTGGGCATACTCAAC-3′ and 5′-CCCACATCTGGCACCATCTT-3′ for Star (NM_011485). The other PCR primers used were reported previously [25–28]. The results were normalized to a housekeeping gene, ribosomal protein L19 (Rpl19), by the 2−ΔΔCt method [29] and then presented as the levels relative to a control group.
Induction and Evaluation of Cumulus Expansion In Vitro
Bicarbonate-buffered Minimum Essential Medium α (Life Technologies, Inc.) with Earle salts, supplemented with 75 μg/ml of penicillin G, 50 μg/ml of streptomycin sulfate, 0.23 mM pyruvate, and 3 mg/ml of bovine serum albumin (Sigma-Aldrich Co.), was used as a basic culture medium. Cumulus cell-oocyte complexes (COCs) were isolated from 22- to 24-day-old Bmp6+/+ or Bmp6−/− female mice that had been primed with 5 IU of eCG 44 to 48 h earlier [30]. To induce cumulus expansion, the COCs were cultured in the basic culture medium supplemented with 5% serum and 10 ng/ml of epidermal growth factor (EGF; BD Biosciences). Some of the COCs were taken for analysis of transcript levels after 3 h of EGF treatment. Cumulus expansion index [31] was assessed after 14 h of culture.
Assessment of Developmental Competence of the Oocytes
To assess the preimplantation developmental competence of oocytes, Bmp6+/+ and Bmp6−/− oocytes were matured and fertilized with Bmp6+/+ sperm in vitro. Subsequent preimplantation development up to the blastocyst stage was assessed as reported previously [32].
Statistical Analysis
All experiments were repeated at least three times, and results are presented as the mean ± SEM. The Tukey-Kramer honestly significant differences test was used to compare multiple groups, and a t-test was used for paired comparison using the computer software JMP (SAS Institute, Inc.). A P value of less than 0.05 was considered to be statistically significant.
RESULTS
Fertility of Bmp6−/− Mutant Female Mice
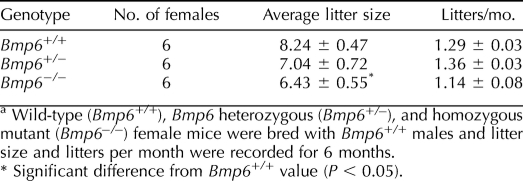
Wild-type (Bmp6+/+), heterozygous (Bmp6+/−), or Bmp6−/− female mice were mated with Bmp6+/+ males, and the numbers of pups and litters per month were recorded. Although no significant difference was found in frequency of litters, the mean litter size of Bmp6−/− female mice (6.43 ± 0.55 pups/litter) was reduced significantly (P < 0.05), by approximately 22%, compared to that of Bmp6+/+ mice (8.24 ± 0.47 pups/litter) (Table 1). Therefore, Bmp6−/− female mice are subfertile.
TABLE 1.
Fertility analysis of Bmp6 mutant female mice.a
Morphology of the Bmp6−/− Ovary
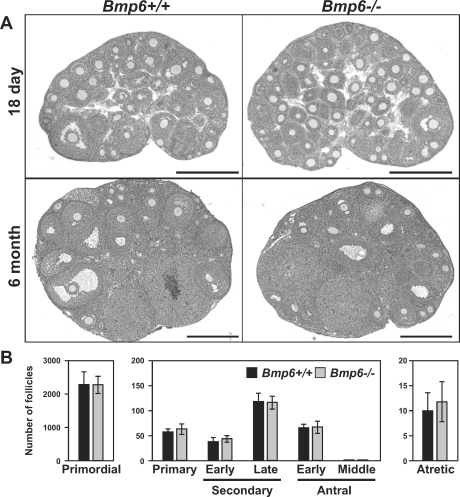
The ovaries of 18-day-old and 6-month-old Bmp6−/− mice displayed normal histology when compared with those of Bmp6+/+ mice (Fig. 1A). No significant differences were found in numbers of follicles at each developmental stage between 18-day-old Bmp6+/+ and Bmp6−/− ovaries (Fig. 1B). Therefore, development of ovarian follicles in Bmp6 mutant mice appears to be morphologically normal.
FIG. 1.
Morphology of Bmp6 mutant ovaries. A) Representative photographs of ovaries of 18-day-old (top) and 6-mo-old (bottom) Bmp6+/+ and Bmp6−/− mice are shown. Bar = 500 μm. B) Follicular count of unstimulated 18-day-old Bmp6+/+ (black bars) and Bmp6−/− (gray bars) mice. Follicle counts were conducted for primordial follicles and the other developmental stages of follicles using three or nine ovaries of each genotype, respectively.
Reduced Response of the Bmp6−/− Ovary to LH/hCG
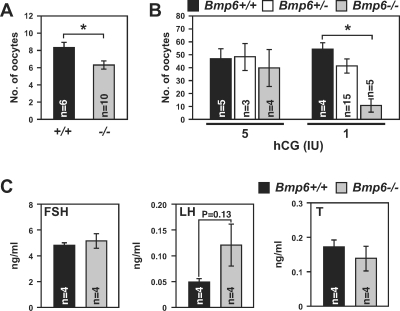
To resolve the etiology of reduced fertility in Bmp6−/− female mice, the number of naturally ovulated oocytes in vivo was compared between adult Bmp6+/+ and Bmp6−/− mice without exogenous gonadotropin treatment. The number of oocytes ovulated by Bmp6−/− mice (6.30 ± 0.47 oocytes) was significantly lower than that ovulated by wild-type mice (8.33 ± 0.61 oocytes) (P < 0.05) (Fig. 2A).
FIG. 2.
Ovulation rates and serum hormone levels in Bmp6 mutant mice. A and B) Numbers of oocytes ovulated in vivo in adult Bmp6+/+ (black bars) and Bmp6−/− (gray bars) mice (A) and gonadotropin-induced ovulation in immature Bmp6+/+ (black bars), Bmp6+/− (open bars), and Bmp6−/− (gray bars) mice (B) were examined. C) Serum FSH, LH, and testosterone (T) levels in immature Bmp6+/+ (black bars) and Bmp6−/− (gray bars) mice without exogenous gonadotropin treatment were examined. Serum levels of E2 were below the limit of detection in both Bmp6+/+ and Bmp6−/− mice (not shown). *P < 0.05.
The effect of exogenous gonadotropin treatment on ovulation rate was examined using immature mice. The eCG-primed Bmp6+/+, Bmp6+/−, or Bmp6−/− mice were injected with hCG, which mimics the LH surge, and the number of ovulated oocytes was determined after 14 h (Fig. 2B). Bmp6−/− mice treated with 5 IU of hCG ovulated oocytes at numbers comparable to those in Bmp6+/+ and Bmp6+/− mice. However, when Bmp6−/− mice were treated with a lower dose of hCG (1 IU), Bmp6−/− mice ovulated significantly fewer oocytes, whereas littermate Bmp6+/+ and Bmp6+/− mutant mice ovulated the same numbers of oocytes as at the 5 IU dose (54.2 ± 5.1, 41.3 ± 5.4, and 10.8 ± 5.0 oocytes for Bmp6+/+, Bmp6+/−, and Bmp6−/−, respectively; P < 0.05) (Fig. 2B). These results suggest that Bmp6−/− female mice are subfertile, at least in part because of reduced ovulatory response to LH/hCG.
Serum levels of FSH, LH, and testosterone in 20-day-old female mice without exogenous gonadotropin treatment were examined (Fig. 2C). Serum E2 levels were below the limit of detection in both Bmp6+/+ and Bmp6−/− mice (not shown). Whereas the serum FSH and testosterone levels were comparable between Bmp6+/+ and Bmp6−/− mice, the LH level trended to be higher in Bmp6−/− mice compared to Bmp6+/+ mice, but this difference was not statistically different (P = 0.13).
Cumulus Expansion
The LH signal to the COC is indirect and mediated by EGF-like growth factors encoded by Areg, Btc, and Ereg transcripts that are expressed by mural granulosa cells [33]. The EGF-like growth factor signal in cumulus cells is amplified by a positive-feedback mechanism, which involves expression of the EGF-like growth factors by the cumulus cells [34]. The EGF-like growth factor signal induces cumulus expansion, which requires expression of proteins encoded by Has2, Ptgs2, Ptx3, and Tnfaip6 transcripts by cumulus cells [27, 35–40].
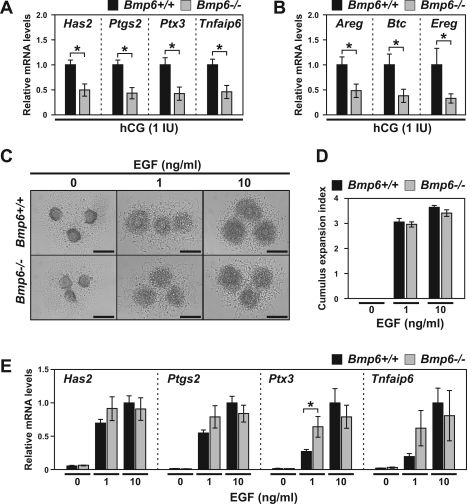
To test whether the response to the LH signal is lower in cumulus cells of Bmp6 mutant mice, eCG-primed Bmp6+/+ or Bmp6−/− mice were injected with 1 IU of hCG, and after 3 h, the steady-state levels of these transcripts were determined in COCs in vivo. The levels of these LH-induced transcripts were all significantly down-regulated in Bmp6−/− COCs compared with those in Bmp6+/+ COCs (P < 0.05) (Fig. 3, A and B). The levels of Btc and Ereg transcripts in Bmp6−/− mural granulosa cells 3 h after treatment with hCG in vivo tended to be lower than those in Bmp6+/+ mural granulosa cells; however, the differences were not statistically significant because of a high variation among the experiments (data not shown).
FIG. 3.
Steady-state levels of LH-induced transcripts in hCG-treated Bmp6 mutant mice. A and B) The eCG-primed immature Bmp6+/+ (black bars) or Bmp6−/− (gray bars) mice were injected with 1 IU of hCG, and levels of transcripts encoding cumulus expansion-related proteins (A) and EGF-like growth factors (B) in COCs at 3 h post-hCG injection were examined. C–E) COCs isolated from eCG-primed immature Bmp6+/+ (black bars) or Bmp6−/− (gray bars) mice were treated with 1 or 10 ng/ml of EGF in vitro. Cumulus expansion index (D) and levels of transcripts (E) were assessed after 14 and 3 h of EGF treatment, respectively. Bar = 200 μm. *P < 0.05.
Although the above results suggest that Bmp6−/− follicles are less responsive to LH/hCG, it was possible that Bmp6−/− COCs express lower levels of these transcripts because of an impaired development of cumulus cells or oocytes. To address this, COCs of eCG-primed Bmp6+/+ or Bmp6−/− mice without hCG treatment were treated with EGF to induce cumulus expansion in vitro. The degree of EGF-induced cumulus expansion by Bmp6−/− COCs in vitro was comparable to that of Bmp6+/+ COCs (Fig. 3, C and D). In addition, the expression levels of the cumulus expansion-related transcripts by Bmp6−/− COCs were comparable with, or even greater than, those detected in Bmp6+/+ COCs when they were treated with EGF in vitro (Fig. 3D). Therefore, Bmp6 mutant COCs can express comparable levels of transcripts required for cumulus expansion in vitro; however, they do not express full levels of these transcripts in vivo in response to the lower dose of hCG (1 IU). These results strongly support the idea that follicular cells of Bmp6 mutant mice are less responsive to LH/hCG and that reduced numbers of ovulated eggs in the mutant oocytes may be caused, at least in part, by defects in cumulus expansion.
Quality of Bmp6−/− Oocytes
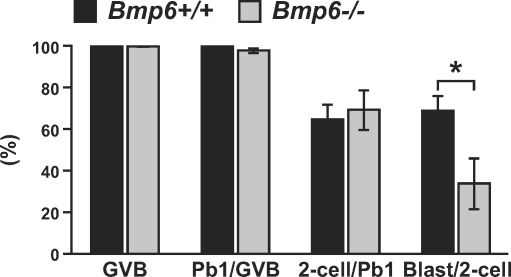
Developmental competence of Bmp6−/− oocytes to complete preimplantation development was assessed in vitro (Fig. 4). The percentages of oocytes that resumed meiosis (germinal vesicle breakdown), extruded the first polar body, and developed to the 2-cell stage were comparable for Bmp6+/+ and Bmp6−/− oocytes fertilized with Bmp6+/+ sperm in vitro. However, the ratio of 2-cell embryos that developed to the blastocyst stage was significantly lower in Bmp6−/− oocytes than in Bmp6+/+ oocytes. Therefore, in addition to the reduced LH response in Bmp6−/− mice, lower oocyte quality may contribute to the subfertility of Bmp6−/− female mice.
FIG. 4.
Developmental competence of Bmp6 mutant oocytes. Oocytes of Bmp6+/+ (black bars) and Bmp6−/− (gray bars) mice were matured and fertilized in vitro, and the preimplantation embryonic development was assessed (n = 6). Blast, blastocyst-stage embryo; GVB, oocytes that underwent germinal vesicle breakdown; Pb1, oocytes extruded the first polar body; 2-cell, 2-cell embryo. *P < 0.05.
Transcript Levels in Bmp6 Mutant Follicles
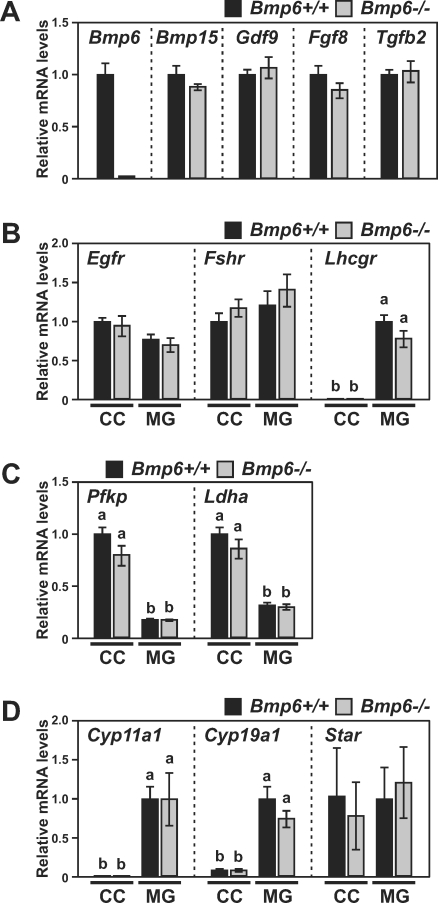
Levels of transcripts encoding receptors for EGF (Egfr), FSH (Fshr), or LH (Lhcgr), as well as those of glycolytic enzymes (Pfkp and Ldha) [30] and steroidogenic proteins (Cyp11a1, Cyp19a1, and Star), were compared in cumulus and mural granulosa cells of eCG-primed Bmp6+/+ and Bmp6−/− mice. Levels of transcripts encoding oocyte-produced growth factors (Bmp15, Gdf9, Fgf8, and Tgfb2) were examined as well.
As expected, Bmp6 transcripts were not detected in Bmp6−/− oocytes (Fig. 5A). Transcript levels of the oocyte-produced growth factors other than Bmp6 mRNA were not significantly different in Bmp6+/+ and Bmp6−/− oocytes, suggesting that deficiency in Bmp6 did not affect production of these other oocyte factors (Fig. 5A). Although the levels of Lhcgr and Cyp19a1 transcripts in mural granulosa cells and of Pfkp and Ldha in cumulus cells of Bmp6−/− mice tended to be lower than those in Bmp6+/+ mice, the differences were not statistically significant (Fig. 5, B and C). Overall, levels of transcripts examined were not significantly different between Bmp6+/+ and Bmp6−/− granulosa cells.
FIG. 5.
Transcript levels in oocytes (A) or granulosa cells (B–D) of eCG-primed Bmp6 mutant mice. Levels of transcripts encoding growth factors in oocytes (n = 5; A) as well as receptors (n = 6 for Fshr and Egfr and 11 for Lhcgr; B), glycolytic enzymes (n = 6; C), and steroidogenic proteins (n = 8; D) in granulosa cells of Bmp6+/+ (black bars) and Bmp6−/− (gray bars) mice were assessed. Values indicated by different letters (a and b) are significantly different (P < 0.05). CC, cumulus cells; MG, mural granulosa cells.
Bmp6/Bmp15 Double-Mutant Mice
Although significant species differences exist in functions of, and response to, BMP ligands [16, 41, 42], BMP6 and the other oocyte-derived BMP, BMP15 [21, 22], appear to have several redundant roles in regulating granulosa cell functions. They can act through the same BMP receptor [6], and their intercellular signals are mediated by the MAD homolog 1/5/9 (SMAD1/5/9; formerly known as SMAD1/5/8) pathway in granulosa cells [15, 17, 43, 44]. Both recombinant BMP6 and BMP15 suppress apoptosis in bovine granulosa cells [18] and increase mRNA levels of inhibitor of DNA binding 1 (ID1) in ovine granulosa cells [45]. Therefore, a possible redundant function of BMP6 and BMP15 was assessed using mutant mice homozygous for both Bmp6 and Bmp15 (Bmp6−/−/Bmp15−/−).
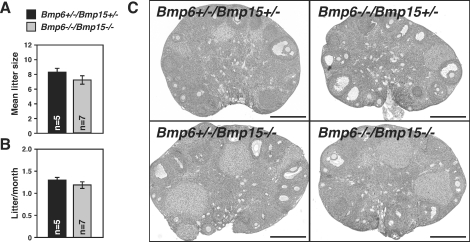
The mean litter size and number of litters per month of Bmp6−/−/Bmp15−/− female mice tended to be lower than those of double heterozygous (Bmp6+/−/Bmp15+/−) littermates; however, these differences were not statistically significant (Fig. 6, A and B). The histology of 6-mo-old Bmp6−/−/Bmp15−/− ovaries was grossly normal when compared with Bmp6+/−/Bmp15+/− littermates (n = 3) (Fig. 6C). Formation of ovarian granulosa cell tumors was reported in several mutant-mouse models in which follicular BMP signal had been attenuated [3–5]. For example, all of Smad1/Smad5 double-homozygous mutant female mice exhibit evidence of tumors after 3 mo of age [4]. We examined more than 10 female Bmp6−/−/Bmp15−/− mice older than 6 mo but did not observe granulosa cell tumors.
FIG. 6.
Ovarian morphology and fertility analysis of female mice deficient in both Bmp6 and Bmp15. A and B) Double-heterozygous (Bmp6+/−/Bmp15+/−, black bars) and double-homozygous (Bmp6−/−/Bmp15−/−, gray bars) mutant female mice were bred with Bmp6+/+/Bmp15+/+ male, and litter size (A) and number of litters per month (B) were recorded for 6 mo. C) Representative photographs of ovaries of 6-mo-old mutant mice are shown. Bar = 500 μm.
DISCUSSION
Despite extensive studies conducted using isolated granulosa cells in vitro, evidence for a role of BMP6 in female fertility in vivo has been lacking. The present study shows that female mice deficient in BMP6, at least in the strain of mice used for the present study, exhibit a 22% reduction in litter size. Bmp6−/− ovaries are less responsive to LH/hCG, and cumulus cells of Bmp6−/− mice express decreased levels of the LH-induced transcripts after treatment with lower dose of hCG in vivo. However, transcript levels for LH receptor are not significantly lower in Bmp6−/− ovaries, suggesting that BMP6 is required for processes downstream of LH receptor expression. In addition, Bmp6−/− oocytes are less competent to complete preimplantation development in vitro when fertilized with wild-type sperm. Therefore, BMP6 is required for normal fertility of female mice by enabling appropriate responses to LH and promoting normal oocyte quality. The underlying mechanisms by which BMP6 affects the actions of the ovulatory surge of LH remain to be investigated. Mouse oocytes express Bmp6 [9], but other cell types also express this ligand [10–13]. Therefore, effects of Bmp6 deletion on fertility, responses to LH/hCG, or oocyte quality could result from systemic effects rather than from the absence of oocyte-derived BMP6.
In vitro studies using recombinant ligands showed that BMP6 can affect FSH signaling in granulosa cells either positively or negatively, depending on mammalian species. BMP6 suppresses Fshr mRNA expression and FSH-induced progesterone production in diethylstilbestrol-primed rat granulosa cells without affecting E2 production or Cyp19a1 transcript levels [14, 17]. In contrast, BMP6 promotes FSH-induced E2 production by ovine granulosa cells [20]. Furthermore, BMP6 enhances FSHR mRNA levels in human granulosa cells [12]. In the present study, expression levels of well-known FSH-regulated transcripts in granulosa cells, such as Lhcgr, Cyp19a1, and Fshr transcripts, were not significantly changed in Bmp6−/− granulosa cells. BMP6 belongs to the 60A subfamily of the BMP ligands, which also includes BMP5, BMP7, BMP8A, and BMP8B [46, 47]. In rodent ovaries, BMP5 and BMP7 are produced by granulosa cells and theca cells, respectively [6, 48], and regulate FSH signaling in granulosa cells in vitro [48, 49]. Therefore, these BMPs may compensate for the absence of BMP6 with regard to regulating FSH signaling in vivo.
In the present study, we observed a tendency for increased basal LH level in the serum of Bmp6−/− female mice. The E2 levels in the serum of both wild-type and Bmp6−/− mice were below the limit of detection and, therefore, could not be compared. The apparently higher basal LH level in Bmp6−/− mice probably results from the relatively poor response of granulosa cells to LH, because mice with reduced LH sensitivity, such as Lhcgr knockout female mice, also exhibit increased serum LH levels [50, 51]. Alternatively, it is possible that although differences in the kinetics of follicular development were not detected between 18-day-old wild-type and Bmp6 mutant mice, the progression of follicular development in 20-day-old mice may vary between these genotypes, and it may result in different feedback effects on the pituitary secretion of LH. These possibilities remain to be elucidated. It is unlikely that these levels can be attributed to the direct effect of loss of BMP6 in the pituitary, because BMP6 has no effects on secretion of LH by rat and ovine pituitary cells [52, 53].
In addition to BMP6, BMP15 is produced by mammalian oocytes [21, 22]. Bmp15-null (Bmp15−/−) female mice are subfertile because of defective granulosa cell functions and reduced developmental competence of oocytes [23, 54]. Cumulus cells of Bmp15−/− mice exhibit reduced levels of transcripts encoding enzymes for glycolysis and cholesterol biosynthesis before the LH surge [26, 55, 56]. Furthermore, recombinant human BMP15 promotes cumulus expansion in vitro [57]. Therefore, BMP15 plays a critical role in regulating granulosa cell functions. Surprisingly, in the present study, we did not observe significant reduction in fertility of double-mutant (Bmp6−/−/Bmp15−/−) female mice compared with that of double-heterozygous (Bmp6+/−/Bmp15+/−) mice, despite the fact that the double-mutant mice are deficient in BMP15. In addition to the redundant functions of BMP15 and BMP6, some nonredundant functions of these BMPs are apparent [14, 58, 59]. BMP15 promotes follicular development in mice [60], but BMP6 appears to suppress the FSH signaling in vitro, which would be required for normal follicular development in vivo. Nevertheless, we did not observe any morphological differences in follicular development and FSH signaling in immature Bmp6−/− mice. One possible explanation for the absence of significant effects of Bmp15 deletion on fertility of the double-mutant mice is that deletion of the Bmp6 gene may rescue some of the defects caused by BMP15 deficiency in the follicles. This possibility remains to be tested.
Female fertility in various species is affected by BMP signaling in different ways. Ewes carrying homozygous mutation in BMP15 are infertile because of a block in folliculogenesis at the primary stage [61, 62]. Likewise, immunization against BMP15 in ewe and cattle results in impaired follicular development and ovulation rate [63, 64]. Ewes carrying a mutation in a gene encoding one of the BMP receptors, BMPR1B (also known as ALK6), exhibit increased fertility [65–68]. On the other hand, mice deficient in Bmp15 [23, 54], Bmp6 ([9] and present study), and both Bmp6 and Bmp15 (present study) exhibit relatively modest defects in female fertility compared with those in other mammalian species. However, prevention of BMP signaling in granulosa cells by conditional deletion of their downstream signaling molecules, SMAD1/5/9, results in development of metastatic granulosa cell tumors, and the female mutant mice become infertile [4, 5]. Likewise, female mice deficient in Bmpr1b exhibit defective cumulus expansion and are infertile [2]. In addition, conditional deletion of the other BMP receptor, BMPR1A, in granulosa cells produces a severe reduction in female fertility, and female mice deficient in both Bmpr1a and Bmpr1b suffer from ovarian granulosa cell tumors [3]. Therefore, BMP signaling is, indeed, critical for ovarian follicular development and female fertility in mice. Oocyte-derived BMP ligands and the other BMP ligands produced by follicular somatic cells may constitute a total and complementary BMP signaling system in follicles, and this entire BMP signaling system is probably essential for normal fertility in female mice.
Multiple processes, including follicular development, ovulation, fertilization, and subsequent preimplantation development, determine female fertility. Each process is affected by multiple factors, including genetic variability as well as environmental influences. For example, previous studies have identified several quantitative trait loci (QTL) that determine hormone-induced ovulation rate or litter size in mice [69–73]. Therefore, ovulation and litter size are quantitative processes controlled by a complex interaction among multiple genes. The Bmp6 locus was not identified by the previous QTL linkage analyses as a candidate locus that determines ovulation rate or litter size [69–73]; however, the present study has clearly shown that a deletion in the Bmp6 gene does affect ovulation rate and litter size in mice. Therefore, Bmp6 is probably part of the complex genetic network that determines female fertility. Although a 22% decrease in fertility, whether caused by Bmp6 deletion or any other genetic or environmental factor, may seem mild in the context of a laboratory model, this difference could determine the success or failure of a species or population in the wild and have significant agricultural economic impact.
In summary, the present study has shown that BMP6 is required for normal fertility in female mice, at least in part, by enabling appropriate responses to LH and promoting normal oocyte quality in vivo. However, in addition to BMP15, other BMP ligands produced in ovarian follicles, such as BMP5 and BMP7, may compensate for the absence of BMP6 and prevent more drastic consequences to ovarian function and fertility in female mice.
Acknowledgments
We thank Dr. Martin M. Matzuk for providing Bmp15-mutant mice and Drs. Mary Ann Handel and Sophie La Salle for their helpful suggestions during preparation of the present paper.
Footnotes
Supported by grants HD23839 from the Eunice Kennedy Shriver NICHD and CA34196 from the NIH.
REFERENCES
- Edson MA, Nagaraja AK, Matzuk MM.The mammalian ovary from genesis to revelation. Endocr Rev 2009; 30: 624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, Lyons KM.The type I BMP receptor BmprIB is essential for female reproductive function. Proc Natl Acad Sci U S A 2001; 98: 7994–7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson MA, Nalam RL, Clementi C, Franco HL, Demayo FJ, Lyons KM, Pangas SA, Matzuk MM.Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol 2010; 24: 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM.Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 2008; 28: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook BS, Eldin K, Li X, Shivasankaran S, Pangas SA.Smad1-Smad5 ovarian conditional knockout mice develop a disease profile similar to the juvenile form of human granulosa cell tumors. Endocrinology 2009; 150: 5208–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF.The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 2004; 25: 72–101. [DOI] [PubMed] [Google Scholar]
- Otsuka F.Multiple endocrine regulation by bone morphogenetic protein system. Endocr J 2009; 57: 3–14. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C.TGF-beta superfamily members and ovarian follicle development. Reproduction 2006; 132: 191–206. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ.Mice lacking Bmp6 function. Dev Genet 1998; 22: 321–339. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Matzuk MM.Oocyte-expressed TGF-beta superfamily members in female fertility. Mol Cell Endocrinol 2000; 159: 1–5. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL.Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev 1989; 3: 1657–1668. [DOI] [PubMed] [Google Scholar]
- Shi J, Yoshino O, Osuga Y, Koga K, Hirota Y, Hirata T, Yano T, Nishii O, Taketani Y.Bone morphogenetic protein-6 stimulates gene expression of follicle-stimulating hormone receptor, inhibin/activin beta subunits, and anti-Mullerian hormone in human granulosa cells. Fertil Steril 2009; 92: 1794–1798. [DOI] [PubMed] [Google Scholar]
- Paradis F, Novak S, Murdoch GK, Dyck MK, Dixon WT, Foxcroft GR.Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction 2009; 138: 115–129. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Moore RK, Shimasaki S.Biological function and cellular mechanism of bone morphogenetic protein-6 in the ovary. J Biol Chem 2001; 276: 32889–32895. [DOI] [PubMed] [Google Scholar]
- Glister C, Kemp CF, Knight PG.Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 2004; 127: 239–254. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Reader KL, Bibby AH, Lun S, Ross I, Haydon LJ, McNatty KP.The role of bone morphogenetic proteins 2, 4, 6 and 7 during ovarian follicular development in sheep: contrast to rat. Reproduction 2006; 131: 501–513. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Otsuka F, Inagaki K, Otani H, Takeda M, Suzuki J, Goto J, Ogura T, Makino H.Differential regulation of steroidogenesis by bone morphogenetic proteins in granulosa cells: involvement of extracellularly regulated kinase signaling and oocyte actions in follicle-stimulating hormone-induced estrogen production. Endocrinology 2007; 148: 337–345. [DOI] [PubMed] [Google Scholar]
- Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB.Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci 2005; 118: 5257–5268. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Kendall NR, Baird DT.Effect of direct ovarian infusion of bone morphogenetic protein 6 (BMP6) on ovarian function in sheep. Biol Reprod 2009; 81: 1016–1023. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Souza CJ, Skinner AJ, Webb R, Baird DT.Enhanced response of granulosa and theca cells from sheep carriers of the FecB mutation in vitro to gonadotropins and bone morphogenic protein-2, -4, and -6. Endocrinology 2006; 147: 1608–1620. [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM.The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol 1998; 12: 1809–1817. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Vuojolainen K, Jaatinen R, Ketola I, Aaltonen J, Lehtonen E, Heikinheimo M, Ritvos O.A novel growth differentiation factor-9 (GDF-9) related factor is coexpressed with GDF-9 in mouse oocytes during folliculogenesis. Mech Dev 1998; 78: 135–140. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM.Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 2001; 15: 854–866. [DOI] [PubMed] [Google Scholar]
- Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J.Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development 2001; 128: 1119–1126. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ.Fibroblast growth factors and epidermal growth factor cooperate with oocyte-derived members of the TGFbeta superfamily to regulate Spry2 mRNA levels in mouse cumulus cells. Biol Reprod 2009; 81: 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O'Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ.Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 2007; 134: 2593–2603. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Eppig JJ.Targeted suppression of Has2 mRNA in mouse cumulus cell-oocyte complexes by adenovirus-mediated short-hairpin RNA expression. Mol Reprod Dev 2009; 76: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ.Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol 2010; 24: 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD.Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Pendola FL, Eppig JJ.Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 2005; 279: 20–30. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Chesnel F.Secretion of cumulus expansion enabling factor by mouse oocytes: relationship to oocyte growth and competence to resume meiosis. Dev Biol 1993; 158: 400–409. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ.Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 1996; 54: 197–207. [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M.EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004; 303: 682–684. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS.Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 2006; 20: 1352–1365. [DOI] [PubMed] [Google Scholar]
- Fulop C, Salustri A, Hascall VC.Coding sequence of a hyaluronan synthase homologue expressed during expansion of the mouse cumulus-oocyte complex. Arch Biochem Biophys 1997; 337: 261–266. [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM.Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol 2002; 16: 1154–1167. [DOI] [PubMed] [Google Scholar]
- Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K.Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 2003; 130: 2253–2261. [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS.Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology 2003; 144: 4376–4384. [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Russell DL, Day AJ, Breyer RM, Richards JS.Decreased expression of tumor necrosis factor-alpha-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology 2003; 144: 1008–1019. [DOI] [PubMed] [Google Scholar]
- Richards JS.Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 2005; 234: 75–79. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, Western A, Meerasahib MF, Mottershead DG, Groome NP, Ritvos O, Laitinen MP.Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction 2005; 129: 481–487. [DOI] [PubMed] [Google Scholar]
- Juengel JL, McNatty KP.The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 2005; 11: 143–160. [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S.Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem 2003; 278: 304–310. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo-Oja N, Dragovic RA, Hickey TE, Ritvos O, Mottershead DG.Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J Cell Sci 2006; 119: 3811–3821. [DOI] [PubMed] [Google Scholar]
- Hogg K, Etherington SL, Young JM, McNeilly AS, Duncan WC.Inhibitor of differentiation (Id) genes are expressed in the steroidogenic cells of the ovine ovary and are differentially regulated by members of the transforming growth factor-beta family. Endocrinology 2010; 151: 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley DM.The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 1994; 8: 133–146. [DOI] [PubMed] [Google Scholar]
- Hogan BL.Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 1996; 10: 1580–1594. [DOI] [PubMed] [Google Scholar]
- Pierre A, Pisselet C, Dupont J, Bontoux M, Monget P.Bone morphogenetic protein 5 expression in the rat ovary: biological effects on granulosa cell proliferation and steroidogenesis. Biol Reprod 2005; 73: 1102–1108. [DOI] [PubMed] [Google Scholar]
- Lee WS, Otsuka F, Moore RK, Shimasaki S.Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod 2001; 65: 994–999. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I.Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol 2001; 15: 172–183. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV.Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol 2001; 15: 184–200. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S.A novel function of bone morphogenetic protein-15 in the pituitary: selective synthesis and secretion of FSH by gonadotropes. Endocrinology 2002; 143: 4938–4941. [DOI] [PubMed] [Google Scholar]
- Faure MO, Nicol L, Fabre S, Fontaine J, Mohoric N, McNeilly A, Taragnat C.BMP-4 inhibits follicle-stimulating hormone secretion in ewe pituitary. J Endocrinol 2005; 186: 109–121. [DOI] [PubMed] [Google Scholar]
- Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ.Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol 2004; 276: 64–73. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ.Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 2008; 135: 111–121. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Eppig JJ.Society for Reproductive Biology Founders' Lecture 2005. Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fertil Dev 2005; 17: 667–674. [DOI] [PubMed] [Google Scholar]
- Yoshino O, McMahon HE, Sharma S, Shimasaki S.A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci U S A 2006; 103: 10678–10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F, Yamamoto S, Erickson GF, Shimasaki S.Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem 2001; 276: 11387–11392. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shimasaki S.Bone morphogenetic protein-15. Identification of target cells and biological functions. J Biol Chem 2000; 275: 39523–39528. [DOI] [PubMed] [Google Scholar]
- McMahon HE, Hashimoto O, Mellon PL, Shimasaki S.Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice. Endocrinology 2008; 149: 2807–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 2000; 25: 279–283. [DOI] [PubMed] [Google Scholar]
- Davis GH, McEwan JC, Fennessy PF, Dodds KG, Farquhar PA.Evidence for the presence of a major gene influencing ovulation rate on the X chromosome of sheep. Biol Reprod 1991; 44: 620–624. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Hudson NL, Heath DA, Smith P, Reader KL, Lawrence SB, O'Connell AR, Laitinen MP, Cranfield M, Groome NP, Ritvos O, McNatty KP.Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biol Reprod 2002; 67: 1777–1789. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Hudson NL, Berg M, Hamel K, Smith P, Lawrence SB, Whiting L, McNatty KP.Effects of active immunization against growth differentiation factor 9 and/or bone morphogenetic protein 15 on ovarian function in cattle. Reproduction 2009; 138: 107–114. [DOI] [PubMed] [Google Scholar]
- Wilson T, Wu XY, Juengel JL, Ross IK, Lumsden JM, Lord EA, Dodds KG, Walling GA, McEwan JC, O'Connell AR, McNatty KP, Montgomery GW.Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol Reprod 2001; 64: 1225–1235. [DOI] [PubMed] [Google Scholar]
- Mulsant P, Lecerf F, Fabre S, Schibler L, Monget P, Lanneluc I, Pisselet C, Riquet J, Monniaux D, Callebaut I, Cribiu E, Thimonier J, et al. Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Merino ewes. Proc Natl Acad Sci U S A 2001; 98: 5104–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza CJ, MacDougall C, Campbell BK, McNeilly AS, Baird DT.The Booroola (FecB) phenotype is associated with a mutation in the bone morphogenetic receptor type 1 B (BMPR1B) gene. J Endocrinol 2001; 169: R1–R6. [DOI] [PubMed] [Google Scholar]
- Bindon BM.Reproductive biology of the Booroola Merino sheep. Aust J Biol Sci 1984; 37: 163–189. [DOI] [PubMed] [Google Scholar]
- Spearow JL, Bradford GE.Genetic variation in spontaneous ovulation rate and LH receptor induction in mice. J Reprod Fertil 1983; 69: 529–537. [DOI] [PubMed] [Google Scholar]
- Spearow JL.Major genes control hormone-induced ovulation rate in mice. J Reprod Fertil 1988; 82: 787–797. [DOI] [PubMed] [Google Scholar]
- Spearow JL, Nutson PA, Mailliard WS, Porter M, Barkley M.Mapping genes that control hormone-induced ovulation rate in mice. Biol Reprod 1999; 61: 857–872. [DOI] [PubMed] [Google Scholar]
- Liljander M, Sallstrom MA, Andersson S, Wernhoff P, Andersson A, Holmdahl R, Mattsson R.Identification of genetic regions of importance for reproductive performance in female mice. Genetics 2006; 173: 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljander M, Andersson A, Holmdahl R, Mattsson R.Increased litter size and superovulation rate in congenic C57BL mice carrying a polymorphic fragment of NFR/N origin at the Fecq4 locus of chromosome 9. Genet Res 2009; 91: 259–265. [DOI] [PubMed] [Google Scholar]