Abstract
In both male and female germ cells of mice, retinoic acid (RA) is a meiosis-inducing factor. In the present study, we used a germ cell culture system to examine the direct effects of RA on meiotic initiation in male germ cells at the stage when they normally enter mitotic arrest to determine the extent to which fetal male germ cells can respond to exogenous RA to alter their sex-specific pathway. Male germ cells between 13.5 and 15.5 days postcoitum (dpc) were isolated from Pou5fl-green fluorescent protein transgenic fetuses and cultured with or without RA for up to 6 days. In the absence of RA, male germ cells did not undergo DNA replication and did not enter meiosis in culture. However, in the presence of RA, male germ cells isolated at 13.5 dpc expressed Stra8 and initiated the meiotic process. The ratio of cells entering meiosis gradually decreased as cells were isolated progressively at later stages. By 15.5 dpc, isolated male germ cells lost their ability to respond to RA signaling. These cells remained dispersed as single cells and progressed along the male differentiation pathway, as evidenced by the establishment of male-specific methylation imprints regardless of the presence or absence of RA. We conclude that male germ cells maintain sexual bipotency until 14.5 dpc that can be reversed by the addition of RA. Once male germ cells enter mitotic arrest, however, they appear to be committed irreversibly to the male-specific differentiation pathway even in the presence of exogenously added RA.
Keywords: fetal male germ cells, meiosis, mitotic arrest, retinoic acid, sex differentiation
Fetal male germ cells maintain a sexual bipotency until 14.5 dpc that can be reversed by the addition of retinoic acid (RA), but once mitotic arrest occurs, they irreversibly commit to the male-specific pathway, even in the presence of RA.
INTRODUCTION
Germ cells are unique, because they undergo meiosis to generate haploid cells. In mammals, both male and female germ cells perform meiosis, but the timing of entry into meiosis differs significantly between the sexes. Mouse primordial germ cells (PGCs) are sexually bipotent before gonadal sex differentiation [1, 2]. Once sex differentiation occurs, the PGCs enter oogenesis in females or spermatogenesis in males to develop as oocytes or as prospermatogonia, respectively [1, 3]. This developmental switch appears to occur at approximately 13.5 days postcoitum (dpc). At this stage, female PGCs immediately initiate meiosis, progress into the first meiotic prophase, and then arrest at the dictyate stage. At this same stage, male PGCs begin to enter mitotic arrest and remain suspended in the G0/G1 stage of the cell cycle until a few days after birth. Following birth, male germ cells resume mitotic proliferation and subsequently enter meiosis during puberty, whereas female germ cells remain arrested in the first meiotic prophase until ovulated [4]. This initial sexual differentiation of fetal germ cells appears to be dependent on the sexual phenotype of the gonadal somatic cells rather than on the chromosomal sex of the germ cells [5, 6].
In the male gonads, expression of Sry, the testis-determining gene, triggers testis cord formation at approximately 12.5 dpc, and the male germ cells become enclosed by pre-Sertoli cells [1, 7–10]. At this time, the male germ cells also begin to enter mitotic arrest [11]. During mitotic arrest, the male germ cells initiate de novo methylation of genomic imprints at 15.5 dpc and establish biallelic, male-specific imprints by birth or shortly thereafter [12–14]. It has been reported that male PGCs at 12.5 dpc are already committed to the male differentiation pathway and that they will become “prespermatogonia” even without the male gonadal environment [1, 9]. Our own previous study showed that the male-specific pathway is autonomously regulated in male germ cells that have been exposed to the male gonad before 13.5 dpc [15]. Thus, these results suggest that male germ cells might be committed to the male differentiation pathway before they enter the G0/G1 phase of the cell cycle.
Recently, it has been shown that retinoic acid (RA) can act as a meiosis-inducing factor in the mouse gonad [16, 17]. RA induces Stra8 (stimulated by retinoic acid 8), an RA-responsive gene, in female PGCs at 12.5 dpc, which then leads to meiotic initiation in fetal female germ cells [16, 17]. In the male gonad, pre-Sertoli cells produce CYP26B1, an RA-metabolizing enzyme, to prevent the male PGCs from entering meiosis during the fetal period [16, 17]. In postnatal testes, however, RA stimulates Stra8 expression in spermatogenic cells [18, 19]. In addition, it has been reported that RA treatment induces Stra8 expression in both fetal and neonatal male germ cells when they are removed from the counteracting influence of pre-Sertoli cells [18, 20]. Furthermore, Stra8-deficient mice have been used to demonstrate that Stra8 is required for meiotic initiation and/or meiotic processes in both males and females [21–23]. These findings strongly suggest that despite the different timing of meiotic initiation in each sex, male and female germ cells appear to share a similar meiotic initiation pathway based on induction of Stra8 expression by RA in premeiotic germ cells.
To further define the molecular mechanisms responsible for the sexual differentiation of germ cells, we have employed an organ culture system that affords us maximum opportunity to manipulate specific molecular constituents [10, 16–18, 20], an approach that was critical to the initial discovery of the significant role played by RA in regulating meiotic initiation in germ cells [16, 17]. Recently, some reports have suggested that RA treatment cannot induce meiosis in male germ cells following the initiation of testis differentiation but can induce meiosis in sexually bipotent PGCs before gonadal sex differentiation [1, 10, 20, 24]. It is not clear, however, if this effect is regulated by the various types of somatic cells that surround the germ cells in the developing testis or if this is an intrinsic feature of developing male germ cells. We previously established a germ cell culture system to directly study intrinsic features of germ cells while excluding any effects from gonadal somatic cells [15]. In the present study, we utilized this germ cell culture system to investigate the direct effects of RA on entry into meiosis of male germ cells between 13.5 and 15.5 dpc—the stage at which these cells normally enter mitotic arrest. We used this approach to determine the extent to which male germ cells maintain sexual bipotency following the initiation of sexual differentiation of the testis.
MATERIALS AND METHODS
Mice
Pou5fl-green fluorescent protein (GFP) transgenic mice (Tg OG2), generated by microinjecting CBA/CaJ × C57BL/6J F2 zygotes, express germ cell-specific GFP driven by the Pou5fl gene promoter/enhancer (a generous gift from Dr. Jeff R. Mann, University of Melbourne, Victoria, Australia) [25]. CD1 females were mated with male Tg OG2 mice to produce CD-1 × OG2 F1 hybrid fetuses. GFP-positive germ cells were collected from these F1 fetuses as described below to perform all experiments. All relevant experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Hawaii.
Media
Gonads and germ cells were collected in Hepes-Dulbecco modified Eagle medium (DMEM; Invitrogen) with 20% fetal bovine serum (FBS; HyClone Laboratories). Germ cells were cultured in high-glucose DMEM supplemented with 0.1 mM nonessential amino acid, 0.1 mM 2-mercaptoethanol, 2 mM glutamine, 1 mM sodium pyruvate, 100 IU/ml of penicillin, 100 μg/ml of streptomycin, and 20% FBS with or without 1 μM RA (Sigma-Aldrich).
Germ Cell Culture
Male gonads were obtained from F1 hybrid fetuses at 13.5, 14.5, and 15.5 dpc. Gonads were dissected in Hepes-DMEM with 10% FBS and incubated in 0.2% collagenase (Sigma-Aldrich) and Accumax (Innovative Cell Technologies) for 10 min at 37°C. After incubation, gonads were gently pipetted to prepare a single-cell suspension. GFP-positive cells were sorted using a FACSAria cell sorter (BD Bioscience). After sorting, the purity of GFP-positive cells was greater than 98%. Approximately 8000 germ cells were cultured on collagen-coated mesh inserts (Corning Life Science) with or without RA. The germ cells were cultured at 37°C with 5% CO2 in air for 1–6 days.
DNA Replication Assay
A DNA replication assay was performed using a Click-iT 5-ethynyl-2′-deoxyuridine (EdU) Alexa Fluor HCS assay kit (Invitrogen). On each of the culture days (1–6 days), male germ cells were incubated with EdU for 5 h. After incubation, the germ cells were placed on glass slides and treated according to the manufacturer's instructions. EdU-positive cells were counted using an Axio Skop 2 Plus microscope (Carl Zeiss Microimaging).
Quantitative Gene Expression Analysis
After culturing for 1–6 days, single or clumped germ cells were collected one by one with glass pipettes to make 200–400 cells as one set, and cDNA was synthesized using the Superscript III CellsDirect cDNA synthesis system (Invitrogen). We used three to five sets of germ cells from each stage for the gene expression assay as described previously [15]. We examined the quantitative expression of the following genes: DNA methyltransferase 3-like (Dnmt3l), stimulated by retinoic acid 8 (Stra8), and synaptonemal complex protein 3 (Sycp3). The sequences of the primers used were as follows (forward and reverse, respectively): for Dnmt3l, 5′-GTGCGGGTACTGAGCCTTTTTAGA-3′ and 5′-CGACATTTGTGACATCTTCCACGTA-3′; for Stra8, 5′- GTTTCCTGCGTGTTCCACAAG-3′ and 5′-CACCCGAGGCTCAAGCTTC-3′; and for Sycp3, 5′-GTGCCTGGTGGAAGAAAGCA-3′ and 5′-GGAGCCTTTTCATCAGCAACAT-3′.
Quantitative real-time PCR was performed using the iCycler system (Bio-Rad Laboratories). Results were normalized to β-actin gene expression and calibrated according to the result from germ cells cultured without RA (in vitro control). Data are presented as the means of three to five replicate analyses of each cell type.
Immunocytochemical Staining
After 1–4 days of culture, the germ cells were placed on glass slides and fixed with 4% paraformaldehyde for 15 min. After washing, they were permeabilized with 0.2% Triton X-100 and 3% BSA in PBS (−) for 15 min. They were then incubated with anti-STRA8 (1:500; Abcam) and anti-SYCP3 (1:500; Abcam) overnight at 4°C. After washing, the germ cells were incubated with a 1:500 dilution of goat anti-rabbit Alexa 568 or goat anti-mouse Alexa 568 (Invitrogen), respectively, as a secondary antibody. Stra8- and Sycp3-positive cells were detected using an Axio microscope (Carl Zeiss).
Bisulfite Genomic Sequencing
The DNA methylation patterns at imprinted loci were examined using bisulfite genomic sequencing as previously described [15, 26]. Briefly, one sample of genomic DNA prepared from 500-1000 GFP-positive germ cells was used for each PCR amplification. Bisulfite modification of DNA was performed using a methylation assay kit (Zymo Research). Primer pairs specific for the differentially methylated domains (DMDs) of the paternally imprinted H19 gene were used for nested amplification as previously described [15, 26]. Three sets of experiments were performed for each stage.
Statistical Analysis
All values are expressed as the mean ± SEM. For comparisons of DNA replication and quantitative gene expression levels, data were analyzed by ANOVA followed by least-significant-difference comparison test. Significance was considered as a P-value of less than 0.05.
RESULTS
Cell Proliferation and DNA Replication
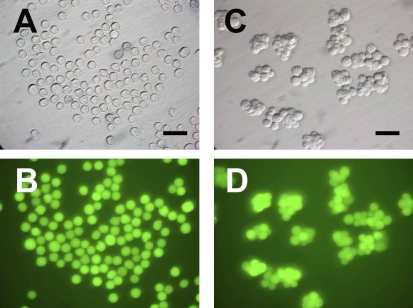
When male germ cells recovered at 13.5, 14.5, and 15.5 dpc were cultured without RA, most (97%) remained as single cells after 4 days of culture (Fig. 1, A and B). However, in the presence of RA, the live cell population became a mixture of single cells and cell clumps. Approximately 65% of the male germ cells recovered at 13.5 dpc formed cell clumps consisting of 2–8 cells after 4 days of culture (Fig. 1, C and D). In contrast, only 7% of male germ cells recovered at 14.5 dpc formed such clumps when cultured with RA, and no cell clumps formed when male germ cells recovered at 15.5 dpc were cultured in the presence of RA (data not shown). These results suggest that the tendency of fetal male germ cells to form clumps when cultured in the presence of RA declines as a function of the developmental stage at which the germ cells are recovered.
FIG. 1.
Morphology of the male germ cells cultured with RA. Male germ cells isolated from Pou5fl-GFP transgenic fetuses at 13.5 dpc were cultured with or without RA for 4 days. A and B) In the absence of RA, most of the germ cells maintained a single cell condition. C and D) In the presence of RA, some of the cells formed clumps. Bright-field micrographs are shown in A and C; fluorescence micrographs of the same fields are shown in B and D. Bar = 50 μm.
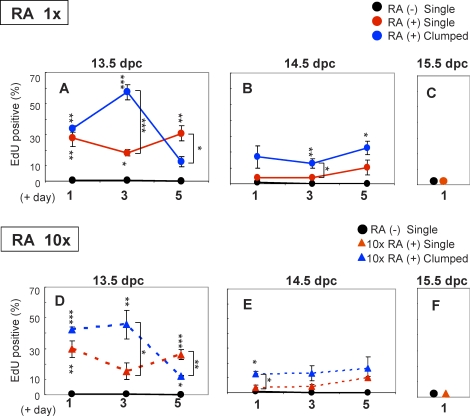
To assess the effect of RA on DNA replication, we used the EdU assay system (Invitrogen). The EdU assay does not require DNA denaturation and antibody treatment, which are required for the 5-bromodeoxyuridine assay. Male germ cells recovered at 13.5, 14.5, and 15.5 dpc were incubated with EdU for 5 h after 1, 3, or 5 days of culture, and the ratio of EdU-positive cells was then determined (Fig. 2). In the controls cultured without RA, EdU-positive cells were very rare, and most of the male germ cells did not undergo DNA replication regardless of the stage at which they were isolated (Fig. 2, A–C). In the presence of RA, on the other hand, approximately 30% of the male germ cells recovered at 13.5 dpc became EdU-positive after 1 day of culture (Fig. 2A). Significantly, up to 57% of germ cells in the clumps became EdU-positive after 3 days of culture. Approximately 20% of germ cells recovered at 14.5 dpc became EdU-positive after 1 day of culture in the presence of RA, but a greater incidence of EdU-positive cells was still found among clumped cells, similar to that seen for germ cells recovered at 13.5 dpc (Fig. 2B). In contrast, male germ cells recovered at 15.5 dpc did not undergo DNA replication even in the presence of RA (Fig. 2C). To determine if the rate of DNA replication is directly proportional to the RA concentration, male germ cells were next cultured with 10 μM RA (10-fold higher than the concentration used for the previous experiments). However, this significantly higher RA concentration did not increase the DNA replication rate in male germ cells isolated at any stage (Fig. 2, D–F).
FIG. 2.
RA stimulates DNA replication in the male germ cells. Male germ cells isolated at 13.5 dpc (A), 14.5 dpc (B), and 15.5 dpc (C) were cultured with or without 1 μM RA for 1, 3, and 5 days. On each of these days, the germ cells were incubated with EdU for 5 h. After incubation, the germ cells were categorized into three groups—single cells without RA (RA [−] Single), single cells with RA (RA [+] Single), and clumped cells with RA (RA [+] Clumped)—and examined for EdU-positive cell numbers. Without RA, the cells did not perform DNA replication in all stages. In the presence of RA, the germ cells isolated at 13.5 dpc increased DNA replication. Especially in the clumped cells, more than 50% of them were EdU positive after 3 days of culture. In the germ cells isolated at 14.5 dpc, the EdU-positive cell number decreased. The male germ cells isolated at 15.5 dpc did not perform DNA replication even in the presence of RA. Male germ cells isolated at 13.5 dpc (D), 14.5 dpc (E), and 15.5 dpc (F) were also cultured with 10 μM RA (RA 10×) for 1, 3, and 5 days. The ratios of DNA replication were the same as the original concentration of RA in all stages. Each column represents the mean of three replicate experiments. Error bars show the SEM. Asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between the RA negative control (RA [−] Single) and RA-treated samples or between RA-treated single cells and clumped cells in each culture day.
Quantitative Gene Expression
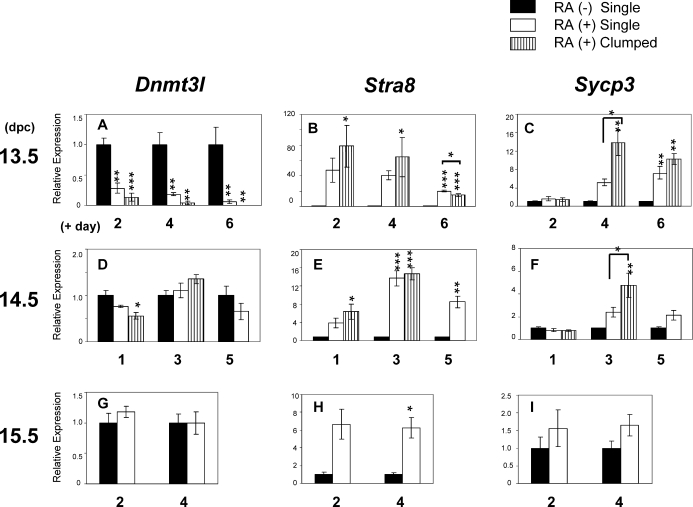
We next examined the effects of ectopic RA administration on gene expression patterns in fetal male germ cells maintained in culture. Dnmt3l is required to direct proper de novo DNA methylation in fetal male germ cells [27–29]. Stra8 is essential for the onset of meiosis [21, 22]. Sycp3 is a marker for meiosis. The expression profiles of these genes in endogenous male and female germ cells between 13.5 and 19.5 dpc are shown in Supplemental Figure S1 (available at www.biolreprod.org). In male germ cells recovered at 13.5 dpc, Dnmt3l expression was significantly diminished both among dispersed single cells and in cell clumps in the presence of RA (Fig. 3A). In the cell clumps, Dnmt3l expression levels were decreased to approximately 10% of those in control cultures (not treated with RA) after 2–4 days of culture. However, in male germ cells recovered at 14.5 and 15.5 dpc, Dnmt3l expression levels were very similar regardless of the presence or absence of RA in the medium (Fig. 3, D and G).
FIG. 3.
Quantitative expression of Dnmt3l, Stra8, and Sycp3 genes in the germ cells cultured with RA. A–C) Gene expression in male germ cells isolated at 13.5 dpc and cultured with or without RA for 2, 4, and 6 days. Dnmt3l expression was drastically decreased in the cells cultured with RA. In contrast, Stra8 and Sycp3 expressions were increased in the presence of RA, peaking at 2 and 4 days of culture, respectively. D–F) Gene expression in male germ cells isolated at 14.5 dpc and cultured with or without RA for 1, 3, and 5 days. G–I) Gene expression in male germ cells isolated at 15.5 dpc and cultured with or without RA for 2 and 4 days. Dnmt3l (A, D, and G), Stra8 (B, E, and H), Sycp3 (C, F, and I) gene expression patterns at different stages are shown. Each column represents the mean of three to five replicate experiments. Error bars show the SEM. Asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between the RA-negative control (RA [−] Single) and RA-treated samples or between RA-treated single cells (RA [+] Single) and RA-treated clumped cells (RA [+] Clumped) in each culture group.
When male germ cells recovered at 13.5 dpc were cultured with RA, Stra8 expression in the clumps after 2 days of culture was sharply increased—up to 80-fold higher than control levels (Fig. 3B). Subsequently, Stra8 mRNA levels gradually decreased when the culture period was extended. Expression of Sycp3 was also increased in the clumped cells cultured in the presence of RA, and these levels peaked at a 14-fold increase after 4 days of culture (Fig. 3C). Although the single cells cultured with RA also showed increased Stra8 and Sycp3 expression levels, these levels were consistently lower than those observed in the clumped cells.
When male germ cells recovered at later stages were cultured with RA, Stra8 expression increased to 15- and 7-fold higher than control levels in germ cells recovered at 14.5 and 15.5 dpc, respectively (Fig. 3, E and H). However, in spite of this increased expression of the Stra8 gene in these cells, little or no elevation of Sycp3 gene expression was detected (Fig. 3, F and I).
Localization of STRA8 Protein
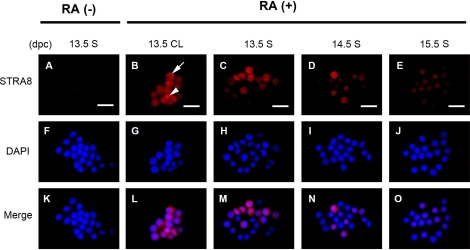
To assess the extent of RA stimulation of gene expression in the cultured germ cells, we examined expression patterns of a known RA-responsive gene using immunostaining for STRA8 protein in male germ cells recovered at 13.5, 14.5, and 15.5 dpc and cultured with RA. In the controls cultured without RA, none of the male germ cells expressed STRA8 regardless of the stage at which they were isolated (Fig. 4A). In the presence of RA, however, germ cells recovered at 13.5 dpc showed strong expression of STRA8 protein in both the clumped cells and the single cells after 2 days of culture (Fig. 4, B and C). The localization of STRA8 was divided into two types: only in the nucleus or in both the nucleus and the cytoplasm (Fig. 4B). Some of the cells expressed STRA8 predominantly in the cytoplasm compared to the nucleus. In the clumped cells after 3 days of culture, STRA8 was strongly expressed only in the nucleus or in both the nucleus and the cytoplasm. Most of the single cells also showed STRA8 expression after 3 days of culture in the presence of RA, and its localization was very similar to that in the cells in the clumps (data not shown). In germ cells recovered at 14.5 dpc and cultured in the presence of RA, the proportion of STRA8-positive cells was decreased relative to that observed in germ cells recovered at 13.5 dpc (Fig. 4D), and in germ cells recovered at 15.5 dpc and cultured with RA, STRA8 localization was mainly detected in the nucleus after 2 days of culture (Fig. 4E). For the latter cells, the localization of STRA8 was predominantly in the nucleus, and the expression levels were very low.
FIG. 4.
STRA8 immunolocalization in the male germ cells cultured with RA. Male germ cells at 13.5 dpc (A–C), 14.5 dpc (D), and 15.5 dpc (E) were cultured for 2 days with RA (RA [+]) or without RA (RA [−]) and immunostained with an anti-STRA8 antibody. STRA8 protein was localized in the nucleus (arrowhead) or in both the nucleus and the cytoplasm (arrow). Nuclei were visualized with 4′,6′-diamidino-2-phenylindole (Tris-like) (F–J). Merge images (K–O) are also shown. CL, clumped cells; S, single cells. Bar = 30 μm.
Entry into the Meiotic Process
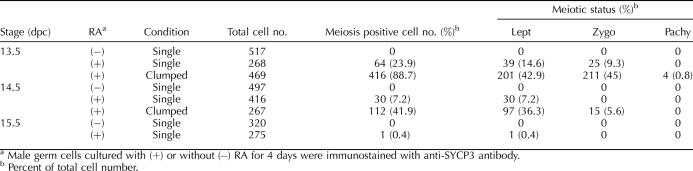
We next used immunocytochemical staining for SYCP3 to assess the direct effect of RA on induction of entry into meiosis in male germ cells recovered at 13.5, 14.5, and 15.5 dpc. After 4 days of culture with or without RA, germ cells were immunostained for SYCP3, and their status was then listed in one of four different stages—1) interphase (no entry into meiosis), 2) leptotene, 3) zygotene, or 4) pachytene—as previously described [15]. When male germ cells recovered at 13.5, 14.5, and 15.5 dpc were cultured without RA, none proceeded into meiosis (Table 1). In the presence of RA, however, 89% of the clumped germ cells recovered from male fetuses at 13.5 dpc had entered meiosis to some extent, about half of the meiosis-positive cells (45% of the total germ cells) had reached the zygotene stage, and a few had progressed to the pachytene stage (Fig. 5 and Table 1). Interestingly, only 24% of the single cells recovered from male fetuses at 13.5 dpc entered meiosis in the presence of RA, and approximately 40% of those meiosis-positive cells (9% of the total germ cells) showed progression to the zygotene stage (Table 1).
TABLE 1.
Retinoic acid induces fetal male germ cells to enter meiosis.
FIG. 5.
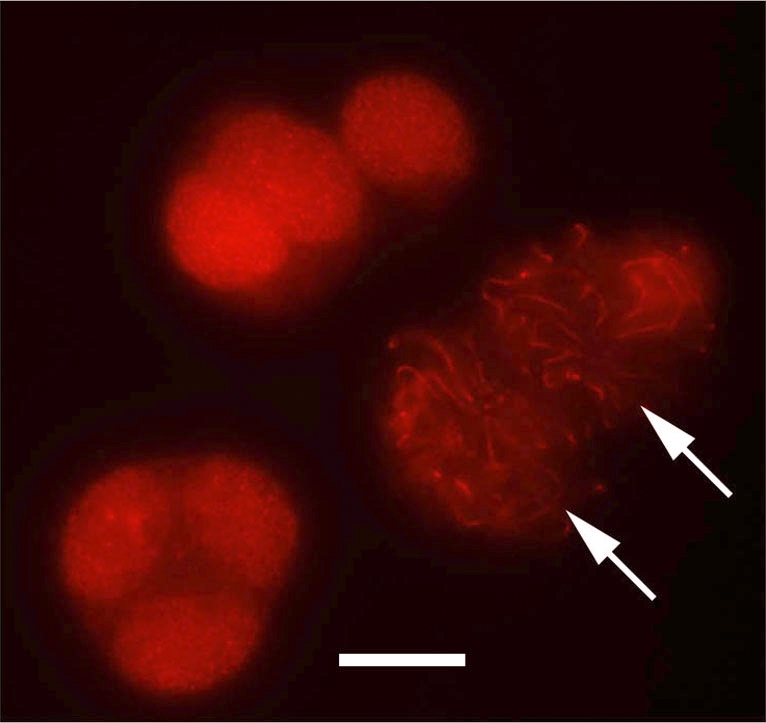
SYCP3 immunolocalization in the male germ cells cultured with RA. Clumped cells obtained after 4 days of culture of the male germ cells isolated at 13.5 dpc in the presence of RA were immunostained to detect SYCP3. Arrows show the germ cells that reached the pachytene stage. All other cells were in the zygotene stage. Bar = 10 μm.
After culturing the male germ cells recovered at 14.5 dpc, most of the single cells (93%) did not enter meiosis even in the presence of RA. Among the clumped cell population, 42% entered meiosis, but only a small fraction of these cells (5% of the total germ cells) reached the zygotene stage. Finally, none of the male germ cells recovered at 15.5 dpc progressed into meiosis even in the presence of RA (Table 1).
Establishment of Genomic Imprints of H19
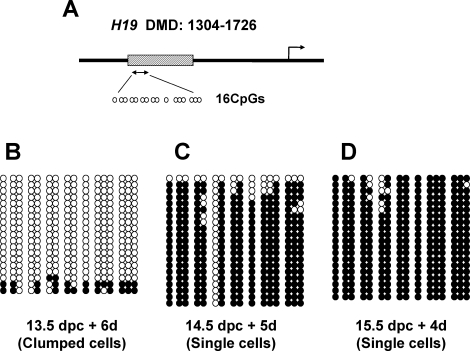
In mouse germ cells, the inherited, allele-specific methylation imprints are completely erased by 13.5 dpc, and sex-specific imprints are subsequently reestablished on both alleles according to the sex of the individuals. In males, reestablishment of biallelic paternal imprints is initiated by 15.5 dpc and is completed shortly after birth. Our previous study showed that male germ cells recovered at 13.5 dpc and cultured without RA for 6 days establish paternal methylation imprints in the H19 DMDs similar to those found in endogenous male germ cells at 19.5 dpc [15]. To determine if ectopic RA affects this process, we used bisulfite genomic sequencing to examine the methylation status of the H19 DMDs in male germ cells after 4–6 days of cell culture in the presence of RA. We first cultured male germ cells recovered at 13.5 dpc for 6 days without RA as a control. Approximately 70% of total CpG sites in the H19 DMDs became methylated in these cells (data not shown), similar to results reported from our previous study [15]. This confirms that male germ cells isolated at 13.5 dpc and maintained in the absence of any gonadal somatic cells are able to perform de novo methylation in culture. Next, we examined the methylation status in the clumped germ cells derived from male fetuses at 13.5 dpc and maintained in culture with added RA. Only 10% of H19 CpG sites were methylated, and the H19 DMDs remained entirely hypomethylated in these cells (Fig. 6B). This result suggests that de novo methylation of the H19 gene in the clumped cells was significantly suppressed by the presence of RA. When germ cells recovered at 14.5 dpc were cultured with RA for 5 days, most of the H19 CpG sites (83%) in the single-cell population became methylated (Fig. 6C), suggesting that most of these cells have the ability to establish male-specific imprints regardless of the presence or absence of ectopic RA. Finally, almost all CpG sites (97%) in the H19 DMDs in germ cells isolated at 15.5 dpc were completely methylated after 4 days of culture in the presence of RA (Fig. 6D), indicating that these cells do not respond to the presence of ectopic RA.
FIG. 6.
De novo methylation status of the H19 DMD in the male germ cells cultured with RA. A) Map indicates the location of the H19 upstream DMD as a box with 16 CpG dinucleotides represented as open circles within the analyzed region in the DMD. Sequence is based on GenBank database information (GenBank accession no. U19619). B–D) DNA methylation status of the H19 DMD in the germ cells cultured with RA. The male germ cells isolated at 13.5 dpc (B), 14.5 dpc (C), and 15.5 dpc (D) were cultured with RA for 6, 5, and 4 days, respectively. DNA methylation was analyzed on three sets of germ cells at each stage. Closed circles represent methylated CpGs, and open circles represent unmethylated CpGs. Each line of circles corresponds to an individually analyzed strand of DNA.
DISCUSSION
Retinoic acid has been reported to be a meiosis-inducing substance [16, 17]. However, past studies have not clearly elucidated the extent to which this effect of RA acts directly on germ cells or, perhaps, is mediated by gonadal somatic cells. We used a defined cell culture system in which purified germ cells can be maintained in the absence of gonadal somatic cells to examine the ability of RA to induce entry of male germ cells into meiosis at a stage when they would normally enter mitotic arrest. We found that the addition of exogenous RA caused a portion of male germ cells recovered at 13.5 and 14.5 dpc to initiate meiosis in culture, whereas the addition of RA to cultures of male germ cells isolated at 15.5 dpc failed to induce any entry into meiosis. These results support the previously reported contention that RA is, indeed, a meiosis-inducing factor, and they indicate this is a direct effect on the germ cells that does not require the presence of surrounding gonadal somatic cells. In addition, these results suggest that at the beginning of the period of sexual differentiation of germ cells (13.5–14.5 dpc), RA can induce a reversal of the sexual phenotype by causing male germ cells that would normally enter mitotic arrest, characteristic of the male differentiation pathway, to instead enter the meiotic prophase characteristic of the female differentiation pathway. However, once male germ cells have progressed further into the male differentiation pathway (i.e., by 15.5 dpc), they become insensitive to this effect and appear to be committed irreversibly to the male pathway even in the presence of exogenously added RA.
Some previous reports claimed that exogenous RA could not induce meiosis in fetal germ cells and/or that it could only induce entry into meiosis in PGCs before gonadal sex differentiation [1, 10, 20, 24]. Most of these studies were based on the use of a gonadal organ culture system. Best et al. [10] concluded that exogenous RA does not induce meiosis or change the sex fate of XY germ cells. Trautmann et al. [20] suggested that RA treatment can induce meiosis in male PGCs at 11.5–12.5 dpc but not in male germ cells present in the gonads at 13.5 dpc. This suggests the possibility that when exogenous RA is added to fetal gonadal organ cultures, it may be metabolized normally or its action may be somehow masked by the gonadal somatic cells present in the organ culture system. The purified germ cell culture system used in the present study allowed us to eliminate this effect so that we could assess the direct effects of RA on developing germ cells. Although germ cells were treated with collagenase/Accumax and cultured without supporting somatic cells, our previous study [15] and the present study showed that these treatments do not negatively affect the response of the cells to RA.
Our study showed that exogenous RA stimulates male germ cells recovered at 13.5 dpc to form clumps of 2–12 cells in culture (Fig. 1). We wondered if these clumps were formed by aggregation of cells originally placed in culture or by proliferation of the original cells to form new cells. A previous report demonstrated that RA stimulates cell proliferation in cultured germ cells [30]. We observed meiotic cells in both the dispersed (single) and clumped cell populations derived from male germ cells isolated at 13.5 dpc, and the meiotic cells in both populations showed similar meiotic progress (to the leptotene/pachytene stages) after 4 days of culture with RA (Table 1). Our EdU assay demonstrated that the germ cells in the clumps performed DNA replication very frequently (Fig. 2), but this assay did not distinguish DNA replication in cells entering meiosis from that in cells maintaining mitotic proliferation [22, 31]. RA is known to stimulate Stra8 expression, which is required both for the premeiotic S phase and for subsequent events during meiotic prophase [21–23]. In our clumped cells in culture, Stra8 expression at both the mRNA and protein levels was dramatically elevated soon after RA treatment (Figs. 3 and 4). Therefore, we conclude that the EdU-positive cell population in the clumps represents primarily cells that have entered meiosis rather than mitotically active cells. Because these cells were all found to be at some stage of the first meiotic prophase, they had not yet undergone cytokinesis. Taken together, these results suggest that the cell clumps must be formed by aggregation of preexisting, individual dispersed cells rather than by proliferation of a few cells to form additional cells. Interestingly, it has been reported that fetal germ cells express adhesion molecules during their development [32–34]. Di Carlo and De Felici [33] demonstrated that E-cadherin mediates germ cell adhesion, especially in male germ cells dispersed from gonads in culture. Cadherins are the best-characterized family of adhesion molecules mediating homophilic, calcium-dependent cell adhesion. Di Carlo and De Felici also demonstrated that β-catenin plays an important role in regulating E-cadherin-mediated adhesion among germ cells. Although we have yet to investigate this possibility, it may be that RA signaling stimulates germ cells to produce adhesion molecules that favor the formation of cellular clumps when meiosis is initiated in male germ cells.
Regardless of the mechanism that led to the clumping, the clumped germ cells entered meiosis more frequently than the dispersed germ cells in the presence of RA. Thus, among male germ cells originally recovered at 13.5 dpc, nearly 90% of the clumped cells entered meiosis, whereas only 24% of the dispersed cells became meiotic, after 4 days of culture in the presence of RA (Table 1). We also showed that RA treatment directly induces Stra8 expression in male germ cells at a stage when these cells would otherwise normally enter mitotic arrest. This effect was most prevalent in male germ cells isolated at 13.5 dpc, and the ability to respond to RA induction of Stra8 expression was then gradually lost in cells isolated at 14.5 and 15.5 dpc. Importantly, Stra8 expression colocalized with the male germ cell population that entered meiosis (Figs. 3 and 4 and Table 1). These results are consistent with the idea that the RA signaling pathway required to activate Stra8 expression, which appears to function in male germ cells isolated at 13.5 dpc, becomes progressively suppressed in male germ cells isolated at subsequent stages. We also found that RA receptor α was abundantly expressed in endogenous male germ cells at 13.5, 14.5, and 15.5 dpc (data not shown). Therefore, the decrease of the sensitivity to RA seems to be an intrinsic characteristic of germ cells that cannot be explained by the receptivity of the cells to RA. NANOS2, a male-specific RNA-binding protein, represents a candidate for one of the factors involved in this suppression [35, 36]. During normal development, CYP26B1 inhibits RA signaling in fetal male gonads at 12.5 dpc; however, its expression becomes reduced by 13.5 dpc [16]. Nanos2 begins to be expressed in male germ cells at the point when CYP26B1 is down-regulated [36]. Based on studies of knockout mice, Suzuki and Saga [36] proposed that Nanos2 inhibits meiotic initiation in male germ cells via suppression of Stra8 expression after 13.5 dpc.
We previously demonstrated that germ cells exposed to the male gonad before 13.5 dpc become autonomously committed to the male differentiation pathway and are subsequently able to progress through this pathway in the absence of a gonadal environment [15]. The present results show that at least some male germ cells recovered at 13.5 and 14.5 dpc can be induced by RA to escape the male-specific pathway and enter a female pathway characterized by entry into meiosis during the fetal period (Fig. 5 and Table 1). In addition to entering meiosis on a female schedule, these cells also did not establish male-specific imprinting at the H19 gene but, rather, maintained a hypomethylated status characteristic of the methylation pattern normally detected in female germ cells during the late fetal stages (Fig. 6B). However, germ cells recovered from testes at 14.5 and 15.5 dpc were less likely to enter meiosis and did establish male-specific methylation imprints in the presence of RA (Fig. 6, C and D), suggesting that the mechanisms regulating male-specific imprinting are closely related to those preventing entry of male germ cells into meiosis during the fetal period.
The loss of sexual bipotency in male germ cells seems to coincide with the progress of mitotic arrest in these cells, with most of them entering the G0/G1 stage by 14.5–15.5 dpc [11]. Previous reports indicated that XY PGCs commit to the male lineage by 12.5 dpc, because these PGCs autonomously differentiate into prespermatogonia even when aggregated with female gonadal cells or lung tissue [1, 9].
The present results suggest that commitment of male germ cells to the male differentiation pathway is reversible until these cells enter mitotic arrest. However, once they enter the G0/G1 phase of the cell cycle, the male pathway is then stably maintained regardless of their environment. Recently, molecular details of cell-cycle mechanisms in germ cells have been revealed [11, 37, 38]. A peptidyl isomerase, Pin1, is involved in the regulation of PGC proliferation [39]. Thus, Pin1−/− PGCs do not undergo cell-cycle arrest and display a prolonged cell cycle as a result of defective G1-S progression [39]. A G1-S checkpoint protein, retinoblastoma protein (pRB), is expressed in male germ cells at the stage of mitotic arrest [11, 38]. It has been proposed that signals from pre-Sertoli cells up-regulate CDK inhibitors to promote dephosphorylation of the pRB protein and that hypophosphorylated pRB then activates the G1-S phase checkpoint, allowing arrest to occur [11]. Retinoblastoma 1 (RB1) is a potent cell cycle regulator in germ cells. Actually, although Rb1−/− male germ cells fail to arrest in G0/G1 at 14.5 dpc, they still enter mitotic arrest by 16.5 dpc, and this is presumed to result from up-regulation of other cell-cycle suppressors [38]. It is not clear whether male-specific genes such as Nanos2 contribute to mitotic arrest of fetal male germ cells. Further studies of the mechanisms regulating cell-cycle function may reveal additional specific functions that contribute to the initial differentiation of male and female germ cells.
In summary, we have demonstrated that exposure of fetal male germ cells to RA at a stage when they would normally enter mitotic arrest can directly induce initiation of meiosis in these cells during the initial, but not the later, stages of germ cell sex differentiation. As a result, we conclude that male germ cells maintain sexual bipotency until they reach the stage of mitotic arrest at 14.5–15.5 dpc. Once the male germ cells enter mitotic arrest, however, they appear to be irreversibly committed to the male differentiation pathway leading to spermatogenesis.
Acknowledgments
We thank Dr. John R. McCarrey for critical reading of the manuscript. We also acknowledge Dr. Jeff R. Mann for generously providing OG2 transgenic mice. We are grateful to Ms. Alexandra Gurary for her technical support with cell sorting. The fluorescence microscope at the Kakaako Imaging Core was supported by National Institutes of Health grants G12RR003061 and P20RR016453.
Footnotes
Supported by National Institute of Health grant P20RR024206 to Y.Y., National Institutes of Health grant G12RR003061 to Y.Y., and the Japan Society for the Promotion of Science (JSPS) postdoctoral Fellowships for Research Abroad to K.O.
REFERENCES
- Adams IR, McLaren A.Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development 2002; 129: 1155–1164. [DOI] [PubMed] [Google Scholar]
- McLaren A.Germ cells and germ cell sex. Philos Trans R Soc Lond B Biol Sci 1995; 350: 229–233. [DOI] [PubMed] [Google Scholar]
- Speed RM.Meiosis in the fetal mouse ovary. I. An analysis at the light microscope level using surface-spreading. Chromosoma 1982; 85: 427–437. [DOI] [PubMed] [Google Scholar]
- McLaren A.Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol 1984; 38: 7–23. [PubMed] [Google Scholar]
- Ford CE, Evans EP, Burtenshaw MD, Clegg HM, Tuffrey M, Barnes RD.A functional ‘sex-reversed' oocyte in the mouse. Proc R Soc Lond B Biol Sci 1975; 190: 187–197. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS.In situ analysis of fetal, prepuberal and adult XX–XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development 1991; 112: 265–268. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E.XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 1990; 109: 635–646. [DOI] [PubMed] [Google Scholar]
- Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R.Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 1990; 348: 450–452. [DOI] [PubMed] [Google Scholar]
- McLaren A, Southee D.Entry of mouse embryonic germ cells into meiosis. Dev Biol 1997; 187: 107–113. [DOI] [PubMed] [Google Scholar]
- Best D, Sahlender DA, Walther N, Peden AA, Adams IR.Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development 2008; 135: 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH.Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells 2008; 26: 339–347. [DOI] [PubMed] [Google Scholar]
- Davis TL, Yang GJ, McCarrey JR, Bartolomei MS.The H19 methylation imprint is erased and re-established differentially on the parental alleles during male germ cell development. Hum Mol Genet 2000; 9: 2885–2894. [DOI] [PubMed] [Google Scholar]
- Lucifero D, Mertineit C, Clarke HJ, Bestor TH, Trasler JM.Methylation dynamics of imprinted genes in mouse germ cells. Genomics 2002; 79: 530–538. [DOI] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J.Epigenetic reprogramming in mammalian development. Science 2001; 293: 1089–1093. [DOI] [PubMed] [Google Scholar]
- Iwahashi K, Yoshioka H, Low EW, McCarrey JR, Yanagimachi R, Yamazaki Y.Autonomous regulation of sex-specific developmental programming in mouse fetal germ cells. Biol Reprod 2007; 77: 697–706. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P.Retinoid signaling determines germ cell fate in mice. Science 2006; 312: 596–600. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC.Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006; 103: 2474–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, Griswold MD.Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 2008; 78: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD.Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod 2008; 79: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann E, Guerquin MJ, Duquenne C, Lahaye JB, Habert R, Livera G.Retinoic acid prevents germ cell mitotic arrest in mouse fetal testes. Cell Cycle 2008; 7: 656–664. [DOI] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC.Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 2008; 105: 14976–14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC.In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006; 38: 1430–1434. [DOI] [PubMed] [Google Scholar]
- Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Feret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB.STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci 2008; 121: 3233–3242. [DOI] [PubMed] [Google Scholar]
- Li H, Kim KH.Retinoic acid inhibits rat XY gonad development by blocking mesonephric cell migration and decreasing the number of gonocytes. Biol Reprod 2004; 70: 687–693. [DOI] [PubMed] [Google Scholar]
- Szabo PE, Hubner K, Scholer H, Mann JR.Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev 2002; 115: 157–160. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Low EW, Marikawa Y, Iwahashi K, Bartolomei MS, McCarrey JR, Yanagimachi R.Adult mice cloned from migrating primordial germ cells. Proc Natl Acad Sci U S A 2005; 102: 11361–11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedin F, Lieber MR, Hsieh CL.The DNA methyltransferase-like protein DNMT3L stimulates de novo methylation by Dnmt3a. Proc Natl Acad Sci U S A 2002; 99: 16916–16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K, Okano M, Lei H, Li E.Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development 2002; 129: 1983–1993. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, Sasaki H.Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 2004; 429: 900–903. [DOI] [PubMed] [Google Scholar]
- Koshimizu U, Watanabe M, Nakatsuji N.Retinoic acid is a potent growth activator of mouse primordial germ cells in vitro. Dev Biol 1995; 168: 683–685. [DOI] [PubMed] [Google Scholar]
- Marston AL, Amon A.Meiosis: cell-cycle controls shuffle and deal. Nat Rev Mol Cell Biol 2004; 5: 983–997. [DOI] [PubMed] [Google Scholar]
- Anderson R, Schaible K, Heasman J, Wylie C.Expression of the homophilic adhesion molecule, Ep-CAM, in the mammalian germ line. J Reprod Fertil 1999; 116: 379–384. [DOI] [PubMed] [Google Scholar]
- Di Carlo A, De Felici M.A role for E-cadherin in mouse primordial germ cell development. Dev Biol 2000; 226: 209–219. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Hamada K, Yamamoto M, Suda T, Iseki S.The expression of platelet endothelial cell adhesion molecule-1 in mouse primordial germ cells during their migration and early gonadal formation. Histochem Cell Biol 2003; 119: 355–362. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y.Conserved role of nanos proteins in germ cell development. Science 2003; 301: 1239–1241. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Saga Y.Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev 2008; 22: 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller C, Wilhelm D, Koopman P.Cell cycle analysis of fetal germ cells during sex differentiation in mice. Biol Cell 2009; 101: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller CM, Wilhelm D, Koopman P.Retinoblastoma 1 protein modulates XY germ cell entry into G1/G0 arrest during fetal development in mice. Biol Reprod 2010; 82: 433–443. [DOI] [PubMed] [Google Scholar]
- Atchison FW, Capel B, Means AR.Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development 2003; 130: 3579–3586. [DOI] [PubMed] [Google Scholar]