Abstract
In situations of stress the heart beats faster and stronger. According to Marks and colleagues, this response is, to a large extent, the consequence of facilitated Ca2+ release from intracellular Ca2+ stores via ryanodine receptor 2 (RyR2), thought to be due to catecholamine-induced increases in RyR2 phosphorylation at serine 2808 (S2808). If catecholamine stimulation is sustained (for example, as occurs in heart failure), RyR2 becomes hyperphosphorylated and “leaky,” leading to arrhythmias and other pathology. This “leaky RyR2 hypothesis” is highly controversial. In this issue of the JCI, Marks and colleagues report on two new mouse lines with mutations in S2808 that provide strong evidence supporting their theory. Moreover, the experiments revealed an influence of redox modifications of RyR2 that may account for some discrepancies in the field.
The heart has a remarkable capacity to react to altered demand by changing the rate at which it beats and the force with which it contracts, thereby changing its output. Both the reduction of cardiac output in phases of rest and its increase in physical and emotional exercise (the fight or flight response) are essential for normal body homeostasis and long-term survival. It is not surprising therefore that cardiac rate and force are regulated at multiple levels, extrinsic and intrinsic to the heart, and in a highly complex and secured fashion. Stimulation of β1-adrenergic receptors by the sympathetic neurotransmitter norepinephrine induces increased production of the second messenger cAMP. cAMP directly and indirectly (via activation of PKA) induces faster depolarization in sinoatrial node cells (the cells that generate the action potentials that trigger cardiac contraction) and thus acceleration of heart rate (i.e., it has a “positive chronotropic effect”) and stronger contraction (i.e., it has a “positive inotropic effect”) and faster relaxation (i.e., it has a “positive lusitropic effect”) in working myocytes. In chronic heart failure, one of the most frequent life-threatening diseases in Western societies, norepinephrine levels are chronically elevated, which leads to desensitization of the β-adrenergic signalling cascade and blunted responses. β-Blockers, introduced by Waagstein and colleagues in the mid 1970s, protect the heart from chronic sympathetic stimulation and provide the largest prognostic benefit for patients with chronic heart failure.
Cardiac excitation-contraction coupling
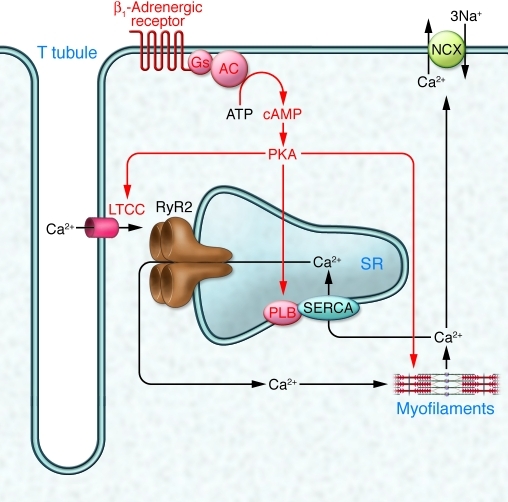
The positive inotropic and lusitropic consequences of β1-adrenergic receptor stimulation in cardiomyocytes are explained by changes in excitation-contraction coupling, i.e., the relationship between the cardiac action potential and myofilament activation (Figure 1 of this commentary). When the cell depolarizes during a cardiac action potential, L-type Ca2+ channels (LTCCs) open, allowing Ca2+ to enter the cell. This so-called trigger Ca2+ induces a much larger Ca2+ release from intracellular Ca2+ stores, known as the sarcoplasmic reticulum (SR), via large tetrameric ryanodine-sensitive channels, referred to as ryanodine receptor 2 (RyR2). The increase in Ca2+ concentration initiates a conformational change in the myofilaments and thereby contraction. Removal of Ca2+ from the cytosol via the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) and the Na+/Ca2+ exchanger (NCX) in the plasmalemma reverses the process. Importantly, the amount of Ca2+ entering the cell is, under normal conditions, exactly matched by the amount of Ca2+ leaving it via the NCX. β1-Adrenergic receptors stimulate the system at numerous levels via PKA (Figure 1 of this commentary). Phosphorylation of LTCCs increases their open probability, allowing more Ca2+ to enter the cell. Phosphorylation of phospholamban (PLB), a small protein that when unphosphorylated inhibits SERCA, leads to disinhibition, i.e., increased reuptake of Ca2+ into the SR. This has at least two consequences: first, more Ca2+ in the SR and therefore more Ca2+ release during systole, which has a positive inotropic effect; and second, faster Ca2+ removal from the cytoplasm and thus faster relaxation (i.e., a positive lusitropic effect). PKA also phosphorylates the myofilament proteins troponin inhibitor and myosin-binding protein C, which desensitizes the myofilaments to Ca2+ and facilitates relaxation (i.e., positive lusitropic effects).
Figure 1. The classical view of cardiomyocyte excitation-contraction coupling and its regulation by β-adrenergic receptors.
Under unstimulated conditions (black arrows), depolarization during an action potential opens LTCCs in T tubules, allowing Ca2+ to enter the cell. This trigger Ca2+ induces a larger Ca2+ release from the SR via RyR2. The increase in Ca2+ concentration initiates conformational changes of the myofilaments and thereby contraction. Removal of Ca2+ via SERCA and NCX reverses the process. Catecholamines stimulate excitation-contraction coupling (red symbols and lettering) by phosphorylating LTCCs (increased Ca2+ influx), PLB (increased Ca2+ reuptake into the SR), and myofilament-based troponin I and myosin-binding protein C (increased relaxation). AC, adenylyl cyclise; Gs, stimulatory G protein. Adapted with permission from Nature (35).
RyR2 phosphorylation determines excitation-contraction coupling gain
In the signalling scheme outlined in Figure 1 of this commentary, which prevailed until the end of the last century, the two major determinants of intracellular Ca2+ transients and thereby the contractile force of the heart were (a) the size of the Ca2+ current entering via the LTCC (well exemplified by the negative inotropic effects of LTCC blockers) and (b) the activity of SERCA and thus the Ca2+ load of the SR. The critical role of the latter was convincingly demonstrated by the fact that Plb–/– mice, which have maximal SERCA activity, exhibit higher basal force and reduced inotropic response to isoprenaline (1). In this scheme, RyR2 was just a passive gatekeeper, opening in response to the trigger Ca2+ and releasing Ca2+ at a quantity proportional to SR Ca2+ concentrations. It is the merit of Marks and colleagues to have challenged that view with a whole series of papers (starting with their seminal paper in 2000; ref. 2), suggesting that PKA also increases the open probability of RyR2 by phosphorylation of serine 2808 (S2808) and the subsequent dissociation of FK506-binding protein 12.6 (FKBP12.6; also known as calstabin), a small regulatory protein that had been known since the early 1990s as a target of two immunosuppressant drugs, tacrolimus (also known as FK506) and rapamycin. Moreover, Marks and colleagues provided evidence that RyR2 is chronically hyperphosphorylated in human heart failure and therefore “leaky” (2). Leaky RyR2 channels in turn could explain both the reduced Ca2+ load of the SR (which leads to reduced contractile force of the failing heart) and the increased risk of spontaneous diastolic Ca2+ release, which, secondary to Ca2+ extrusion via the electrogenic NCX, could explain arrhythmias. In this theory, PKA phosphorylation of RyR2 and the subsequent dissociation of FKBP12.6 is the unifying mechanism underlying both the normal fight or flight response and heart failure.
The controversy about the “leaky RyR2” hypothesis
Appealing as Marks’ theory is, the concept has been challenged and remains controversial (Tables 1 and 2). On the one hand, some theoretical considerations argue against it. For example, it seems counterintuitive that phosphorylation at a single residue in a protein of more than 5,000 amino acids could profoundly affect channel open probability. Second, S2808, the proposed site of phosphorylation by PKA, is located in an area distant from the FKBP12.6/RyR2 interaction site (3), making it somewhat unlikely that phosphorylation affects FKPB12.6 binding. Third, it seems unlikely and to contradict experimental results (4) that an isolated increase in RyR2 open probability has more than a transient consequence on Ca2+ handling, because an isolated increase in Ca2+ release from the RyR2 will automatically lead to reduced Ca2+ load in the SR and therefore fast normalization of Ca2+ transients (autoregulation). Thus, leaky RyR2 channels alone, in contrast to super active SERCA (as in the case of Plb knockout; ref. 1), should not affect the basal force of contraction. Of course, theoretical arguments can be disputed and require experimental validation. It is now well established that point mutations in RyR2 can cause catecholaminergic polymorphic ventricular tachycardia (CPVT) — an electrophysiological disorder of the heart that can cause sudden death in young people as a result of arrhythmia — and hence, single-site phosphorylation could do so as well. In addition, the autoregulation argument does not necessarily hold if one accepts the idea that PKA phosphorylation of RyR2 is always accompanied by phosphorylation of LTCC and PLB and that RyR2 is just one out of (at least) three players in the concert.
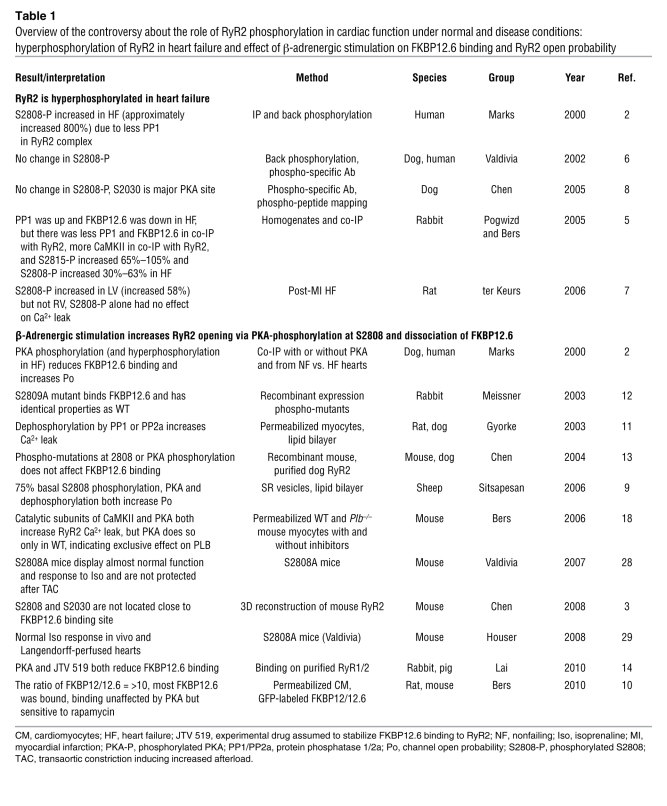
Table 1 .
Overview of the controversy about the role of RyR2 phosphorylation in cardiac function under normal and disease conditions: hyperphosphorylation of RyR2 in heart failure and effect of β-adrenergic stimulation on FKBP12.6 binding and RyR2 open probability
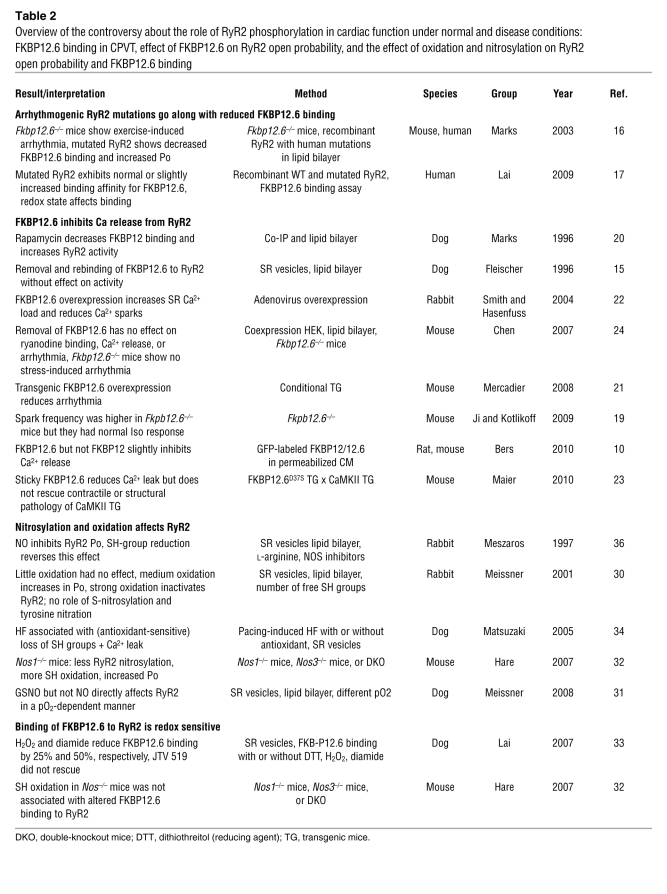
Table 2 .
Overview of the controversy about the role of RyR2 phosphorylation in cardiac function under normal and disease conditions: FKBP12.6 binding in CPVT, effect of FKBP12.6 on RyR2 open probability, and the effect of oxidation and nitrosylation on RyR2 open probability and FKBP12.6 binding
More concerning than theoretical considerations are numerous reports that failed to reproduce important aspects of the data that support the leaky RyR2 hypothesis and the critical importance of S2808 (Tables 1 and 2). (a) Phosphorylation of RyR2 at S2808 has been found by others to be either not altered in heart failure at all or to be only moderately increased (5–8). Others have reported that 75% of the available RyR2 S2808 sites are phosphorylated under normal conditions, making a 9-fold change in chronic heart failure somewhat unlikely (9). (b) Whereas general consensus exists that β-adrenergic stimulation increases spontaneous Ca2+ release (the “Ca2+ leak”) from the SR, the role of RyR2 phosphorylation and FKBP12.6 dissociation remains controversial. Importantly, PKA had no effect on Ca2+ release in permeabilized Plb–/– mouse myocytes, i.e., cells in which the SR is maximally loaded with Ca2+ and one would have expected a particularly strong effect of increasing RyR2 open probability (10). These data suggest that the effect in WT myocytes is exclusively due to increased SR Ca2+ loading (via PLB phosphorylation). This experiment also shows that the contribution of RyR cannot be judged without controlling for SR Ca2+ load. Others have studied isolated RyR2 preparations and observed that not only phosphorylation at S2808, but also dephosphorylation, increased its open probability (9, 11). Several groups have failed to reproduce the effect observed by Marks and colleagues (2) of PKA phosphorylation at S2808 or mutations of this site on FKBP12.6 binding (2, 10, 12, 13), but others have (14). Moreover, a recent study by the Bers group (10) reported very low FKBP12.6 concentrations in rodent cardiomyocytes, resulting in only 10%–20% of RyR2 being occupied by FKBP12.6. These data, although in contrast to earlier data on isolated dog SR that reported more than 80% occupancy (15), suggest that a change of RyR2 affinity for FKBP12.6 would not affect more than a minority of RyR2. (c) Another exciting finding by the Marks group (16), namely that CPVT-associated mutations in RyR2 are associated with decreased FKBP12.6 binding, has also been challenged by reports of unaltered or even increased binding affinity of mutant RyR2 channels for FKBP12.6 (17). (d) Several reports support the idea that removal of FKBP12.6 from the RyR2 complex results in increased open probability, spark frequency, and arrhythmias (18, 19) and thus support this aspect of the Marks theory (2, 16, 20). Conversely, overexpression of FKBP12.6 (or sticky mutants) was accompanied by a reduced propensity for cardiac arrhythmia (21–23). However, this did not improve cardiac pathology in a transgenic mouse model of heart failure (with increased Ca2+ leak), arguing against the universal role of leaky RyR2 in the pathophysiology of heart failure. Finally, others (24) could neither reproduce the effect of FKBP12.6 dissociation on RyR2 open probability nor the proarrhythmic phenotype of Fkbp12.6-knockout mice observed by the Marks group (16).
Evidence from gene-targeted, phospho-mutant RyR2 mouse models
In this confusing state of the RyR2 literature, one would expect “clean” knockin mouse models to finally provide a definite answer. Yet, this is only partially the case, as shown by two studies from the Marks laboratory published in this issue of the JCI (25, 26). The two papers report on two new mouse models: one in which the S2808 site was mutated to a non-phosphorylatable alanine (S2808A; ref. 25), and another in which S2808 was replaced by aspartic acid (S2808D), a “phosphomimetic” amino acid (26). The expectation was that S2808A mice would be less sensitive to the force-increasing acute effects of isoprenaline (blunted fight or flight response) but also protected from adverse long-term consequences of β-adrenergic stimulation, such as cardiac dilatation and dysfunction (as seen in mice lacking phosphatase inhibitor-1; ref. 27). Conversely, S2808D mice would be expected to exhibit spontaneous FKBP12.6 depletion from the RyR2 complex, initial hypercontractility (because of facilitated Ca2+ release from the SR), spontaneous development of a cardiomyopathic phenotype, and exaggerated pathology under chronic stress. The results obtained by Marks and colleagues almost exactly matched these expectations and go beyond (25, 26). S2808A mice also exhibited blunted chronotropic responses (i.e., blunted acceleration of the heart rate) to isoprenaline and reduced exercise capacity, suggesting that RyR2 phosphorylation at S2808 regulates not only contractile force but heart rate too and that it is a critical determinant of physical fitness. The S2808D experiments also enlarge the picture by showing that the β-blockers metoprolol and carvedilol, while effective in infarcted WT mice, had no effect in S2808D mice. In contrast, the Ca2+ release channel–stabilizing drug S107 stabilized FKBP12.6 binding to RyR2 and ameliorated cardiac dysfunction in S2808D mice. Taken together, the new results from the Marks group strongly support a key role for phosphorylation of RyR2 S2808 in β-adrenergic regulation of cardiac force and frequency, in setting maximal exercise capacity, in determining cardiac responses to chronic stress, and in the protective effect of β-blockers.
The new findings in the context of the published state of the art?
Unfortunately, these new results from the Marks laboratory (25, 26) are in striking contrast to studies from the groups of Valdivia and Houser in an independently generated S2808A knockin mouse line (28, 29). These mice not only displayed normal heart dimensions before and after aortic banding but also normal myocyte shortening and Ca2+ transients, normal responses to isoprenaline, and, interestingly, normal PKA-catalyzed incorporation of radioactive phosphate into RyR2, despite effective elimination of S2808 phosphorylation. The latter result supports data suggesting that S2030 is the dominant PKA phosphorylation site on RyR2 and that S2808 is of minor quantitative relevance (8).
How can one reconcile these different results with apparently identical mutant mouse lines? Marks and colleagues present two major lines of reasoning (25, 26). The first one is that the other studies used doses/concentrations of isoprenaline and experimental conditions (pacing) that obscured differences in sensitivity. This explanation is based on a key experiment performed by the Marks group (25), showing that low doses of isoprenaline (2 μg/kg) had markedly smaller chronotropic and inotropic effects in S2808A than in WT mice but a 1,000-fold higher dose exerted similar maximal effects (Figure 1 of ref. 25). Indeed, the Valdivia and the Houser studies used saturating concentrations/doses of isoprenaline in vitro (1 μmol/l) and in vivo (2 mg/kg), respectively (28, 29). However, dosing alone cannot account for the strikingly different observations for two reasons. First, the Houser group also tested the effects of isoprenaline on Langendorff-perfused mouse hearts at 10 nmol/l, a concentration that is clearly not saturating, and observed essentially identical effects in S2808A and WT hearts (29). Second, Figure 3 of ref. 25 shows, somewhat in contrast to their own findings presented in Figure 1 in the same paper , that the contractile force (dP/dt) response was reduced in S2808A mouse hearts over the entire dose range (exhibiting a clear plateau at approximately 0.3 μg/kg/min in both WT and S2808A mice). Moreover, a saturating concentration of isoprenaline (100 nmol/l) exerted less than a maximal force response in free-running Langendorff-perfused S2808A mice. Marks and colleagues therefore propose (25) that the differences between their results and those of the Houser group (at 10-fold lower concentrations) were due to the fact that Houser’s group used paced Langendorff-hearts (480 beats/min). However, as demonstrated in Figure 3 of ref. 25, pacing (at 600 beats/min) reduced but did not abolish the differences between WT and S2808A mice. The Marks group also argues that in the Valdivia study (28) S2808A mouse myocytes did not react exactly as WT myocytes in response to isoprenaline (Figure 5C in ref. 28). Whereas this is formally true, the slightly (but statistically significantly) higher Ca2+ transient in WT myocytes at 3 Hz does not strike as a relevant difference to the author of this commentary. Finally, the Marks group shows in the second paper (26) that Ca2+ spark frequency in 6- to 8-month-old S2808D mouse myocytes was larger than in WT, despite lower SR Ca2+ load. It is difficult to explain these data by an isolated change in RyR2 open probability, because such situation automatically leads to a new equilibrium at a lower SR load (4).
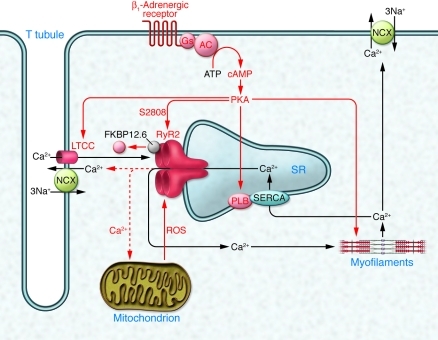
A second hypothesis to explain the controversy relates to redox modifications of RyR2. It is well known and undisputed that RyR1 (the skeletal isoform) and RyR2 are redox responsive (Tables 1 and 2). RyR1 contains 101 thiols/subunit, and their oxidation (e.g., by ROS) increases RyR1 open probability at low/moderate levels of oxidation (and irreversibly inactivates it at high levels; ref. 30). RyR2 is also subject to oxidation and S-nitrosylation (likely via S-nitrosoglutathione) in a pO2-dependent manner (31). Recent work suggests competition between NOS1/NO-dependent S-nitrosylation and ROS-dependent SH oxidation, with the latter leading to increased leakiness of the channel (32). One report indicates that SH oxidation reduces the affinity of RyR2 for FKBP12.6 (33), but another shows that the level of FKBP12.6 associated with RyR2 is normal despite its increased oxidation in Nos–/– mice (32). In any case, there is little doubt that redox modifications can profoundly affect RyR2 function. Moreover, redox conditions can easily differ between different protocols and laboratories and are rarely explicitly reported (e.g., some investigators use ascorbic acid in their Tyrode’s solution, others do not). Thus, the idea that different redox states of RyR2 account for at least some of the published discrepancies is appealing. Based on experiments presented in Figure 1D of ref. 25, Marks and colleagues propose that oxidation of RyR2 sensitizes the channel for PKA phosphorylation at S2808 and destabilizes FKBP12.6 binding. Conversely, under non-oxidizing conditions, PKA may not induce FKBP12.6 dissociation, and this might then explain why others did not find it. The spontaneous cardiomyopathic phenotype of S2808D mice observed by Marks and colleagues was accompanied by increased S-nitrosylation and more pronounced SH oxidation (Figure 2B of ref. 26). This heart failure–associated increase in oxidation could then explain why it took 6 months for S2808D mice to exhibit significant loss of FKBP12.6 from the RyR2 complex (Figure 2, A and B, of ref. 26), despite the proposed “hyperphosphorylation state” of RyR2 being present throughout life. The hypothesis derived by Marks and colleagues from these results is that both oxidative stress (as commonly seen in heart failure; ref. 34) and PKA phosphorylation cooperate to destabilize the RyR2-FKBP12.6 complex (Figure 2 of this commentary). The increased Ca2+ leak then promotes ROS generation (e.g., from mitochondria) and thus accelerates the vicious circle (Figure 2 of this commentary). This elegant hypothesis could indeed explain some of the experimental discrepancies, particularly those in vitro. It is more difficult to assume that it can explain discrepancies between experiments in whole heart homogenates, intact cells/hearts, and entire animals.
Figure 2. The leaky RyR2 hypothesis: phosphorylation and oxidation of RyR2 in the center of a vicious circle in heart failure.
According to Marks and colleagues, catecholamines control excitation-contraction coupling gain not only at the level of LTCCs and PLB, but also at the level of RyR2 by phosphorylating it at S2808. The latter reduces RyR2 affinity for the stabilizing accessory protein, FKBP12.6, and increases its open probability. In heart failure, sustained catecholamine stimulation leads to hyperphosphorylation, leaky RyR2, spontaneous Ca2+ release and, via NCX, spontaneous depolarizations. The new data presented in this issue of the JCI (25, 26) now suggest that phosphorylation at S2808 alone does not suffice to dissociate FKBP12.6 but that it needs oxidation of the channel plus phosphorylation. The level of oxidizing ROS is commonly increased in heart failure and, importantly, the increased Ca2+ leak from RyR2 (in consequence of oxidation and phosphorylation) further increases ROS production, e.g., from mitochondria. This constitutes a classical vicious circle. Black arrows and lettering indicate basic excitation-contraction coupling; red arrows indicate changes under chronic catecholamine stimulation and heart failure. The red dotted line indicates Ca2+ leak. Adapted with permission from Nature (35).
Conclusion
Although the controversies surrounding RyR2 phosphorylation are sure to continue, the means to directly test evolving hypotheses are at hand. Direct head-to-head comparisons of available animal models and other reagents in multiple laboratories and the further development of ever-more refined reagents will help to clarify this fundamental aspect of cardiovascular physiology.
Acknowledgments
The work of the author is supported by grants from the German Research Foundation (DFG FOR 604, Es 88/10-1), the European Union (EUGeneHeart, Angioscaff), and the German Heart Foundation.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(12):4197–4203. doi:10.1172/JCI45251
See the related article beginning on page 4388.
References
- 1.Luo W, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75(3):401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 2.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101(4):365–376. doi: 10.1016/S0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 3.Jones PP, et al. Localization of PKA phosphorylation site, Ser(2030), in the three-dimensional structure of cardiac ryanodine receptor. Biochem J. 2008;410(2):261–270. doi: 10.1042/BJ20071257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res. 2007;100(1):105–111. doi: 10.1161/01.RES.0000252828.17939.00. [DOI] [PubMed] [Google Scholar]
- 5.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97(12):1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 6.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002;91(11):1015–1022. doi: 10.1161/01.RES.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 7.Obayashi M, et al. Spontaneous diastolic contractions and phosphorylation of the cardiac ryanodine receptor at serine-2808 in congestive heart failure in rat. Cardiovasc Res. 2006;69(1):140–151. doi: 10.1016/j.cardiores.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Xiao B, et al. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96(8):847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 9.Carter S, Colyer J, Sitsapesan R. Maximum phosphorylation of the cardiac ryanodine receptor at serine-2809 by protein kinase a produces unique modifications to channel gating and conductance not observed at lower levels of phosphorylation. Circ Res. 2006;98(12):1506–1513. doi: 10.1161/01.RES.0000227506.43292.df. [DOI] [PubMed] [Google Scholar]
- 10.Guo T, et al. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res. 2010;106(11):1743–1752. doi: 10.1161/CIRCRESAHA.110.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terentyev D, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Gyorke S. Protein phosphatases decrease sarcoplasmic reticulum calcium content by stimulating calcium release in cardiac myocytes. J Physiol. 2003;552(pt 1):109–118. doi: 10.1113/jphysiol.2003.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. . J Biol Chem. 2003;278(51):51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 13.Xiao B, Sutherland C, Walsh MP, Chen SR. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6). Circ Res. 2004;94(4):487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- 14.Blayney LM, Jones JL, Griffiths J, Lai FA. A mechanism of ryanodine receptor modulation by FKBP12/12.6, protein kinase A, and K201. Cardiovasc Res. 2010;85(1):68–78. doi: 10.1093/cvr/cvp273. [DOI] [PubMed] [Google Scholar]
- 15.Timerman AP, et al. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem. 1996;271(34):20385–20391. doi: 10.1074/jbc.271.34.20385. [DOI] [PubMed] [Google Scholar]
- 16.Wehrens XH, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113(7):829–840. doi: 10.1016/S0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 17.Zissimopoulos S, Thomas NL, Jamaluddin WW, Lai FA. FKBP12.6 binding of ryanodine receptors carrying mutations associated with arrhythmogenic cardiac disease. Biochem J. 2009;419(2):273–278. doi: 10.1042/BJ20082324. [DOI] [PubMed] [Google Scholar]
- 18.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99(4):398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, et al. Dissociation of FKBP12.6 from ryanodine receptor type 2 is regulated by cyclic ADP-ribose but not beta-adrenergic stimulation in mouse cardiomyocytes. Cardiovasc Res. 2009;84(2):253–262. doi: 10.1093/cvr/cvp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaftan E, Marks AR, Ehrlich BE. Effects of rapamycin on ryanodine receptor/Ca(2+)-release channels from cardiac muscle. Circ Res. 1996;78(6):990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- 21.Gellen B, et al. Conditional FKBP12.6 overexpression in mouse cardiac myocytes prevents triggered ventricular tachycardia through specific alterations in excitation-contraction coupling. Circulation. 2008;117(14):1778–1786. doi: 10.1161/CIRCULATIONAHA.107.731893. [DOI] [PubMed] [Google Scholar]
- 22.Loughrey CM, et al. Over-expression of FK506-binding protein FKBP12.6 alters excitation-contraction coupling in adult rabbit cardiomyocytes. J Physiol. 2004;556(pt 3):919–934. doi: 10.1113/jphysiol.2003.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seidler T, et al. Limitations of FKBP12.6-directed treatment strategies for maladaptive cardiac remodeling and heart failure [published online ahead of print August 24, 2010]. J Mol Cell Cardiol. doi: 10.1016/j.yjmcc.2010.08.016. doi: 10.1016/j.yjmcc.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Xiao B, et al. Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: sensitization of store overload-induced Ca2+ release. J Biol Chem. 2007;282(41):30256–30264. doi: 10.1074/jbc.M703510200. [DOI] [PubMed] [Google Scholar]
- 25.Shan J, et al. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120(12):4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan J, et al. Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice. J Clin Invest. 2010;120(12):4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittkopper K, et al. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest. 2010;120(2):617–626. doi: 10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benkusky NA, et al. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101(8):819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 29.MacDonnell SM, et al. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res. 2008;102(8):e65–e72. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun J, Xu L, Eu JP, Stamler JS, Meissner G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem. 2001;276(19):15625–15630. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Yamaguchi N, Xu L, Eu JP, Stamler JS, Meissner G. Regulation of the cardiac muscle ryanodine receptor by O(2) tension and S-nitrosoglutathione. Biochemistry. 2008;47(52):13985–13990. doi: 10.1021/bi8012627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104(51):20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zissimopoulos S, Docrat N, Lai FA. Redox sensitivity of the ryanodine receptor interaction with FK506-binding protein. J Biol Chem. 2007;282(10):6976–6983. doi: 10.1074/jbc.M607590200. [DOI] [PubMed] [Google Scholar]
- 34.Yano M, et al. Correction of defective interdomain interaction within ryanodine receptor by antioxidant is a new therapeutic strategy against heart failure. Circulation. 2005;112(23):3633–3643. doi: 10.1161/CIRCULATIONAHA.105.555623. [DOI] [PubMed] [Google Scholar]
- 35.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 36.Zahradnikova A, Minarovic I, Venema RC, Meszaros LG. Inactivation of the cardiac ryanodine receptor calcium release channel by nitric oxide. Cell Calcium. 1997;22(6):447–454. doi: 10.1016/S0143-4160(97)90072-5. [DOI] [PubMed] [Google Scholar]