Liposarcoma of the cord is very rare and needs to be differentiated from other para-testicular sarcomas and inguinal hernias.
Introduction
Malignant lesions of the spermatic cord are rare with only around 100 cases reported in the world literature.1–5 Most of these cancers are sarcomas that present as slow-growing inguinal or scrotal masses. There have been occasions when an effort had to be made to differentiate them from inguinal hernias, funicular cysts, lipomas of the cord, et cetera.3–6 Preoperative diagnosis is not common and usually they present as operative or histological surprises. The recommended treatment is surgery in the form of wide local excision which in most cases is in the form of radical high orchiectomy from as close to the deep ring as possible. There is no indication for routine lymph node dissections as the loco-regional lymph nodes are rarely involved. The outcome is fairly good in most cases if the resection is R0 (microscopic freedom from cancer). Adjuvant radiotherapy is usually not required except in cases with positive margins or local recurrence and poor prognostic factors. There is no definite role of chemotherapy and most of the recommendations are based on cases with recurrences. These cancers are, however, known for local recurrences and long-term follow-up of up to 10 years is mandatory; even recurrences after 20 years have been reported.5–7
Case report
A 60-year-old man from the hills presented with a right inguinal swelling of six years duration that had started growing rapidly in the last six months and had reached the size of 5 cm × 4 cm. There was no pain in the swelling at the onset and during this period of rapid progression. The initial diagnosis made by his general practitioner was that of right-sided inguinal hernia and the patient was referred for surgery. There was, however, no cough impulse and it was firm to hard in consistency. Ultrasound examination revealed a heterogenous mass with mixed echogenicity involving the right spermatic cord (highly suspicious of malignancy); the testes was, however, normal. The patient was taken up for surgery through the inguinal approach. The spermatic cord was dissected and delivered out and it showed a hard lipomatous mass (5 cm × 4 cm) involving the entire circumference that was inseparable from the cord (Figures 1, 2 and 3).The testes was also delivered in to the wound and was found to be normal on gross examination. The rest of the cord was normal. This mass had a ‘bunch of grapes’ appearance and consisted of several masses of various sizes surrounding the spermatic cord. An occlusion clamp was applied on the spermatic cord close to the deep ring and the swelling was cut open to take the imprint smears. The cut surface showed a variegated appearance (Figure 3). In view of the high suspicion of malignancy, right radical high orchiectomy was done. The histopathological examination revealed a well-differentiated liposarcoma of the cord with clear margins of resection. The patient was not given any adjuvant treatment and a follow-up of three years revealed no loco-regional recurrence or progression of tumour.
Figure 1.
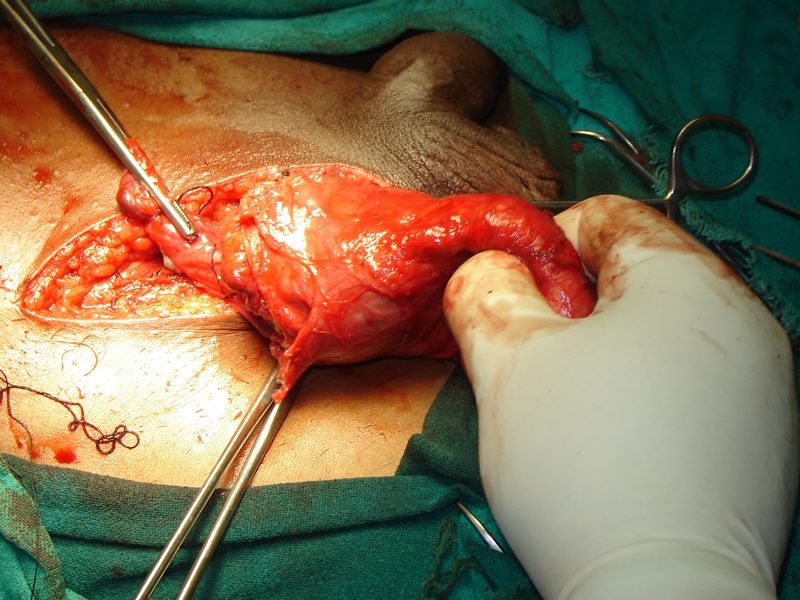
The tumour (5 cm × 4 cm) can be seen. The testes and the rest of the spermatic cord are normal
Figure 2.
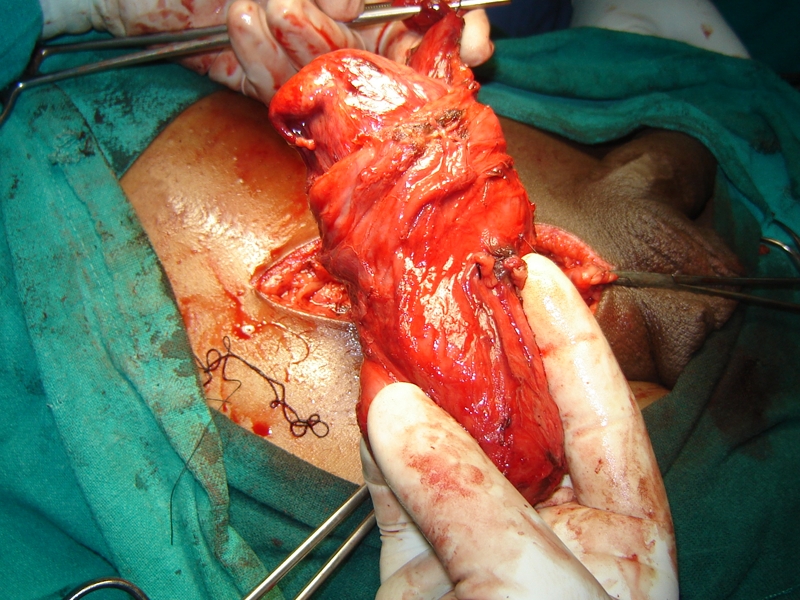
The lipomatous hard tumour dissected off the inguinal canal
Figure 3.
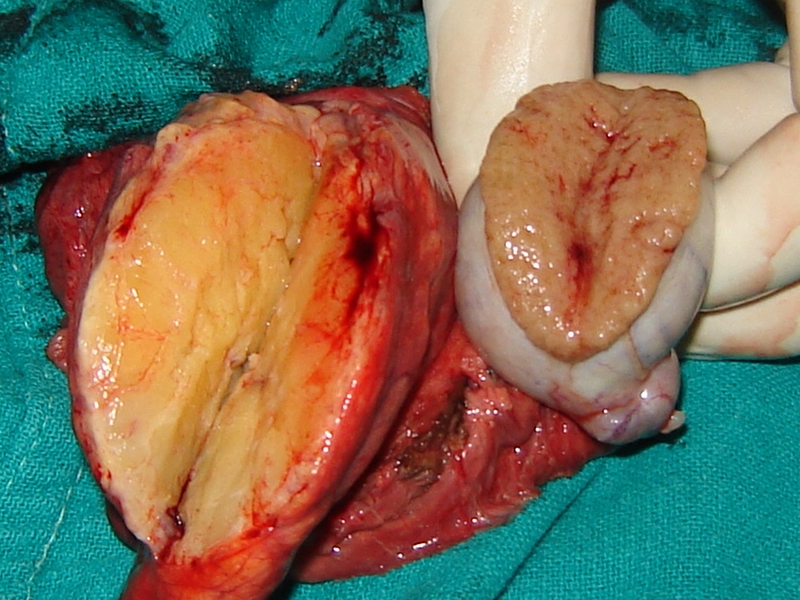
Cut section showing the variegated appearance of the tumour and the grossly normal-looking testes
Discussion
The first case of sarcoma of the cord was reported by Lesauvage in 1845.1–5 Less than 100 cases of spermatic cord liposarcoma have been reported in the world literature so far. Most patients present in their 50s or 60s in the form of a slow-growing inguinal or inguino-scrotal mass. A relatively higher incidence of this cancer has been reported in Japanese men (nearly one-fourth of the reported cases) but the cancer is known to occur all over the world.7 No reason for this is known. Regarding the origin of this cancer there are various theories, but most arise de novo. Simple lipomas that constitute nearly 80% of all neoplasms almost never transform into sarcomas.5–9 Various authors have hypothesized that a mesenchymal origin rather than malignant transformation of lipomatous cells leads to liposarcomas.7–9
Staging of these sarcomas is based on histological grade and presence of metastasis. WHO classification of soft tissue tumour recognizes five categories of liposarcomas in order of increasing malignancy: (a) well differentiated adipocytic, inflammatory, sclerosing subtypes; (b) de-differentiated; (c) myxoid; (d) round cell; and (e) pleomorphic.4–6
The liposarcomas of the cord need to be differentiated from the paratesticular tumours. Paratesticular tumours can be benign (lipoma, leiomyoma, haemangioma) or malignant (rhabdomyosarcoma, melanotic neuroectodermal tumour of infancy). Lipoma is the most common primary benign paratesticular neoplasm and the most common tumor of the spermatic cord. The optimum management of these tumours continues to be unclear because of their rarity. Although rare, these are clinically significant lesions that affect patients of all ages. They remain generally asymptomatic but may have potentially life-threatening sequelae.7–13
The tumour may assume large proportions and up to 13.5 kg liposarcomas of the cord have been reported. Clinical assessment may not be accurate and inguinal hernia, hydrocoele and chronic epididymitis are the most common differential diagnoses.9–16 An ultrasound examination may help in confirming the consistency of the mass and the status of testes and the cord. The use of CT scans is not widely reported, but has been found to be useful, as liposarcomas are of low density and can be well-demarcated. There are no pathognomonic features for the differentiation of benign versus malignant masses defined in the literature.7,9,16 Use of MRI provides good information on the local situation, but an exact evaluation of any masses again cannot be obtained. FDG–PET scan may be of use in recurrent cases but their routine use is not indicated.17,18
Histologically, almost all of these cancers are well-differentiated, low-grade malignancies with no or minimal tendency to metastasize but they may be locally invasive.1–7 High-grade round cell and pleomorphic variety of liposarcomas are rare (16 patients reported so far of which four died) but may metastasize to the regional lymph nodes.
The recommended treatment of choice is surgery in the form of radical high orchiectomy. Retroperitoneal lymph node dissection is not indicated unless there is evidence of tumour. Recurrence is usually on account of the pseudocapsule (through which tumour cells infiltrate in to adjoining structures) such as in other soft tissue sarcomas. These are managed by surgery or radiotherapy, or rarely chemotherapy. Long-term follow-up is mandatory as recurrences even after 18 years have been reported.5,11,19,20
In regions like the inguinal canal, achieving an R0 resection may not always be feasible and some authors recommend adjuvant radiotherapy for these liposarcomas. The recommended dosage is 60 Gy over 6 weeks and the radiation field should cover the internal inguinal ring. Due to their relative resistance against chemotherapy, a routine adjuvant systemic therapy is not justified in lipo or any other seminal cord sarcoma. In view of the limited number of published cases, no specific outcome data are available but late recurrences have been reported and a long-term follow-up (of at least 10 years) is recommended.10
Conclusions
Liposarcomas of the seminal cord are rare. Therapy should include a radical surgical excision usually administered by radical inguinal orchiectomy. If the margin status is in doubt, adjuvant radiation is indicated. Distant disease has not been reported, but local relapse is common and may occur several years after primary therapy. Thus, follow-up periods have to be of sufficient duration.
DECLARATIONS
Competing interests
None declared
Funding
None
Ethical approval
Written informed consent to publication has been obtained from the patient or next of kin
Guarantor
CC
Contributorship
C was the chief operating surgeon; MT, RK, SJ, NN, YK were the assisting surgeons and helped with the preparation of the manuscript; SS was the pathologist in charge of the case
Acknowledgements
None
Reviewer
Sachin Malde
References
- 1.Schwartz SL, Swierzewski SJ 3rd, Sondak VK, Grossmann HB Liposarcoma of the spermatic cord: report of 6 cases and review of the literature. J Urol 1995;153:154–7 [DOI] [PubMed] [Google Scholar]
- 2.Dündar M, Erol H, Koçak I, Kaçar F Liposarcoma of the spermatic cord. Urol Int 2001;67:102–3 [DOI] [PubMed] [Google Scholar]
- 3.Ushida H, Johnin K, Koizumi S, Okada Y [Liposarcoma of the spermatic cord in the left scrotum and inguinal region: a case report.] Hinyokika Kiyo 2000;46:349–51 [PubMed] [Google Scholar]
- 4.Logan JE, Williams MB, Shaves ME, McCammon KA Liposarcoma of the spermatic cord: a case report and review of management. UroToday Int J 2010; doi:10.3834/uij.1944-5784.2010.08.08 [Google Scholar]
- 5.Hinman F, Gibson TE Tumors of the epididymis, spermatic cord and testicular tunics: a review of literature and report of three new cases. Arch Surg 1924;8:100 [Google Scholar]
- 6.Demirci U, Buyukberber S, Cakir A, et al. Synchronous testicular liposarcoma and prostate adenocarcinoma: a case report. Cases J 2010;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman J, Brennan MF, Alektiar K, Russo P Adult spermatic cord sarcomas: management and results. Ann Surg Oncol 2003;10:669–75 [DOI] [PubMed] [Google Scholar]
- 8.Sogani PC, Grabstald H, Withmore WJ Spermatic cord sarcoma in adults. J Urol 1978;120:301. [DOI] [PubMed] [Google Scholar]
- 9.Cardenosa G, Papinicolaou W, Fung CY, et al. Spermatic cord sarcomas: sonographic and CT features. Urol Radiol 1990;12:136–7 [DOI] [PubMed] [Google Scholar]
- 10.Ballo MT, Zagars GK, Pisters PW, Feig BW, Patel SR, von Eschenbach AC Spermatic cord sarcoma: outcome, patterns of failure and management. J Urol 2001;166:1306–10 [DOI] [PubMed] [Google Scholar]
- 11.Blitzer PH, Dosoretz DE, Proppe KH Treatment of malignant tumors of the spermatic cord: a study of 10 cases and a review of the literature. J Urol 1981;126:611–14 [DOI] [PubMed] [Google Scholar]
- 12.Fagundes MA, Zietman AL, Althausen AF The management of spermatic cord sarcoma. Cancer 1996;77:1873–6 [DOI] [PubMed] [Google Scholar]
- 13.Catton C, Cummings BJ, Fornasier V Adult paratesticular sarcoma: a review of 21 cases. J Urol 1991;146:342–5 [DOI] [PubMed] [Google Scholar]
- 14.Ishida A, Takeuchi H, Tomoyoshi T Giant liposarcoma of the spermatic cord: report of a case. Acta Urol Japan 1985;31:1059–64 [PubMed] [Google Scholar]
- 15.Vorstman B, Block NL, Politano VA The management of spermatic cord liposarcomas. J Urol 1984;131:66–9 [DOI] [PubMed] [Google Scholar]
- 16.Hoshino T, Yajima M, Iwasaki A, Hirokawa M, Matsushita K Liposarcoma of the spermatic cord: a case report. Acta Urol Japan 1987;33:1296–9 [PubMed] [Google Scholar]
- 17.Goodman FR, Staunton MD, Rees HC Liposarcoma of the spermatic cord. J R Soc Med 1991;84:499–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipset RE, Kirpekar M, Cooke KS, Abiri MM US case of the day. Myxoid liposarcoma of the spermatic cord. Radiographics 1997;17:1316–18 [DOI] [PubMed] [Google Scholar]
- 19.Anjum MI, Eccersley J, Bhat A, Azzopard A, Khan O, Palmer JH Liposarcoma of the spermatic cord: a report of two cases. Int Urol Nephrol 1997;29:227–32 [DOI] [PubMed] [Google Scholar]
- 20.Kosuda S, Wahl RL, Grossman HB Demonstration of recurrent dedifferentiated liposarcoma of the spermatic cord by FDG-PET. Ann Nucl Med 1997;11:263–6 [DOI] [PubMed] [Google Scholar]


