Abstract
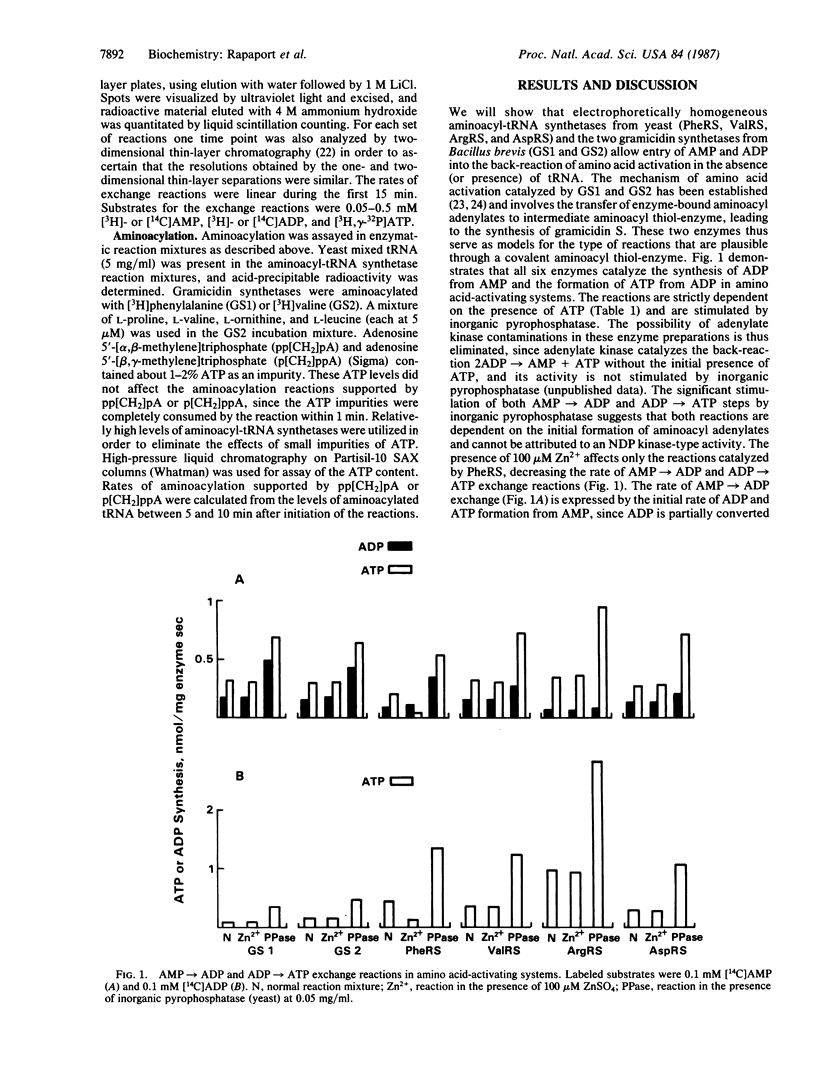
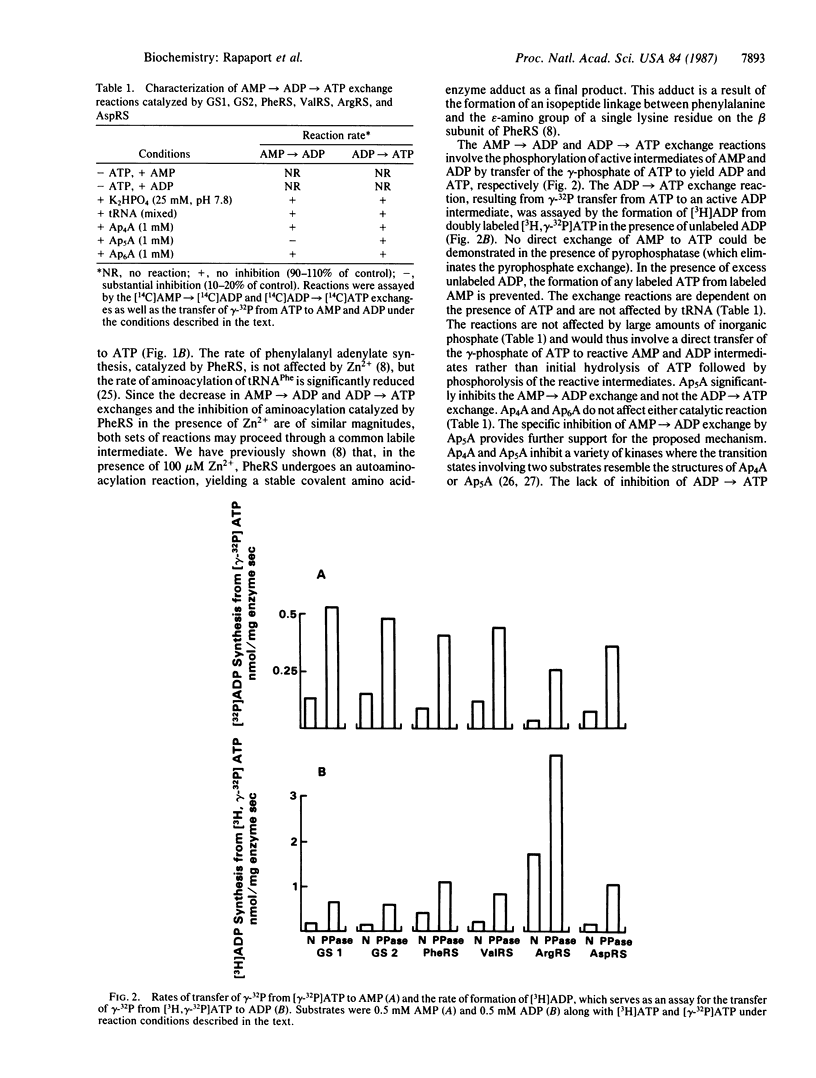
Aminoacyl-tRNA synthetases (amino acid-tRNA ligases, EC 6.1.1.-) catalyze the aminoacylation of specific amino acids onto their cognate tRNAs with extraordinary accuracy. Recent reports, however, indicate that this class of enzymes may play other roles in cellular metabolism. Several aminoacyl-tRNA synthetases are herein shown to catalyze the AMP----ADP and ADP----ATP exchange reactions (in the absence of tRNAs) by utilizing a transfer of the gamma-phosphate of ATP to reactive AMP and ADP intermediates that are probably the mixed anhydrides of the nucleotide and the corresponding amino acid. AMP and ADP produce active intermediates with amino acids by entering the back-reaction of amino acid activation, reacting with labile covalent amino acid-enzyme intermediates. Gramicidin synthetases 1 and 2, which are known to activate certain amino acids through the formation of intermediate thiol-esters of the amino acids and the enzymes, catalyze the same set of reactions with similar characteristics. Several lines of evidence suggest that these activities are an inherent part of the enzymatic reactions catalyzed by the aminoacyl-tRNA synthetases and gramicidin synthetases and are not due to impurities of adenylate kinase, NDP kinase, or low levels of tRNAs bound to the enzymes. The covalent amino acid-enzyme adducts are likely intermediates in the aminoacylation of their cognate tRNAs. The use of gramicidin synthetases has thus helped to illuminate mechanistic details of amino acid activation catalyzed by the aminoacyl-tRNA synthetases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERG P. Acyl adenylates; the interaction of adenosine triphosphate and L-methionine. J Biol Chem. 1956 Oct;222(2):1025–1034. [PubMed] [Google Scholar]
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Bone R., Cheng Y. C., Wolfenden R. Inhibition of adenosine and thymidylate kinases by bisubstrate analogs. J Biol Chem. 1986 Dec 15;261(35):16410–16413. [PubMed] [Google Scholar]
- Castroviejo M., Fournier M., Gatius M., Gandar J. C., Labouesse B., Litvak S. Tryptophanyl-tRNA synthetase is found closely associated with an stimulates DNA polymerase alpha-like activity from wheat embryos. Biochem Biophys Res Commun. 1982 Jul 16;107(1):294–301. doi: 10.1016/0006-291x(82)91703-x. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Dang C. V. Higher eukaryotic aminoacyl-tRNA synthetases in physiologic and pathologic states. Mol Cell Biochem. 1986 Aug;71(2):107–120. doi: 10.1007/BF00214769. [DOI] [PubMed] [Google Scholar]
- Fasiolo F., Ebel J. P. Yeast phenylalanyl-tRNA synthetase. Stoichiometry of the phenylalanyl adenylate-enzyme complex and transfer of phenylalanine from this complex to tRNA-PHE. Eur J Biochem. 1974 Nov 1;49(1):257–263. doi: 10.1111/j.1432-1033.1974.tb03830.x. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Gangloff J., Dirheimer G. Reaction pathway and rate-determining step in the aminoacylation of tRNAArg catalyzed by the arginyl-tRNA synthetase from yeast. Biochemistry. 1978 Sep 5;17(18):3740–3746. doi: 10.1021/bi00611a011. [DOI] [PubMed] [Google Scholar]
- Gabius H. J., Graupner G., Cramer F. Activity patterns of aminoacyl-tRNA synthetases, tRNA methylases, arginyltransferase and tubulin: tyrosine ligase during development and ageing of Caenorhabditis elegans. Eur J Biochem. 1983 Mar 1;131(1):231–234. doi: 10.1111/j.1432-1033.1983.tb07254.x. [DOI] [PubMed] [Google Scholar]
- Gangloff J., Dirheimer G. Studies on aspartyl-tRNA synthetase from Baker's yeast. I. Purification and properties of the enzyme. Biochim Biophys Acta. 1973 Jan 19;294(1):263–272. [PubMed] [Google Scholar]
- Gangloff J., Schutz A., Dirheimer G. Arginyl-tRNA synthetase from baker's yeast. Purification and some properties. Eur J Biochem. 1976 May 17;65(1):177–182. doi: 10.1111/j.1432-1033.1976.tb10403.x. [DOI] [PubMed] [Google Scholar]
- HOAGLAND M. B. An enzymic mechanism for amino acid activation in animal tissues. Biochim Biophys Acta. 1955 Feb;16(2):288–289. doi: 10.1016/0006-3002(55)90218-3. [DOI] [PubMed] [Google Scholar]
- HOAGLAND M. B., KELLER E. B., ZAMECNIK P. C. Enzymatic carboxyl activation of amino acids. J Biol Chem. 1956 Jan;218(1):345–358. [PubMed] [Google Scholar]
- Holler E., Rainey P., Orme A., Bennett E. L., Calvin M. On the active site topography of isoleucyl transfer ribonucleic acid synthetase of Escherichia coli B. Biochemistry. 1973 Mar 13;12(6):1150–1159. doi: 10.1021/bi00730a021. [DOI] [PubMed] [Google Scholar]
- Kern D., Giegé R., Ebel J. P. Valyl-tRNA synthetase from baker's yeast. Ligand binding properties and stability of the enzyme-bound adenylate. Biochemistry. 1981 Sep 1;20(18):5156–5169. doi: 10.1021/bi00521a009. [DOI] [PubMed] [Google Scholar]
- Kern D., Giegé R., Robre-Saul S., Boulanger Y., Ebel J. P. Complete purification and studies on the structural and kinetic properties of two forms of yeast valyl-tRNA synthetase. Biochimie. 1975;57(10):1167–1176. doi: 10.1016/s0300-9084(76)80579-2. [DOI] [PubMed] [Google Scholar]
- Kleinkauf H., Gevers W., Lipmann F. Interrelation between activation and polymerization in gramicidin S biosynthesis. Proc Natl Acad Sci U S A. 1969 Jan;62(1):226–233. doi: 10.1073/pnas.62.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I. P 1 ,P 5 -Di(adenosine-5')pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J Biol Chem. 1973 Feb 10;248(3):1121–1123. [PubMed] [Google Scholar]
- Loftfield R. B., Eigner E. A. Mechanism of action of amino acid transfer ribonucleic acid ligases. J Biol Chem. 1969 Apr 10;244(7):1746–1754. [PubMed] [Google Scholar]
- Mitra S. K., Mehler A. H. The arginyl transfer ribonucleic acid synthetase of Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5490–5494. [PubMed] [Google Scholar]
- Murayama A., Raffin J. P., Remy P., Ebel J. P. Yeast phenylalanyl-tRNA synthetase: properties of the sulfhydryl groups; evidence for -SH requirement in tRNA acylation. FEBS Lett. 1975 Apr 15;53(1):15–22. doi: 10.1016/0014-5793(75)80671-5. [DOI] [PubMed] [Google Scholar]
- Plateau P., Mayaux J. F., Blanquet S. Zinc(II)-dependent synthesis of diadenosine 5', 5"' -P(1) ,P(4) -tetraphosphate by Escherichia coli and yeast phenylalanyl transfer ribonucleic acid synthetases. Biochemistry. 1981 Aug 4;20(16):4654–4662. doi: 10.1021/bi00519a021. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Yogeeswaran G., Zamecnik P. C., Remy P. Covalent modification of phenylalanyl-tRNA synthetase with phenylalanine during the amino acid activation reaction catalyzed by the enzyme. J Biol Chem. 1985 Aug 15;260(17):9509–9512. [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C., Baril E. F. HeLa cell DNA polymerase alpha is tightly associated with tryptophanyl-tRNA synthetase and diadenosine 5',5"'-P1,P4-tetraphosphate binding activities. Proc Natl Acad Sci U S A. 1981 Feb;78(2):838–842. doi: 10.1073/pnas.78.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy P., Ebel J. P. Yeast phenylalanyl-tRNA synthetase: evidence for the triggering of an AMP--ATP exchange by tRNA. FEBS Lett. 1976 Jan 1;61(1):28–31. doi: 10.1016/0014-5793(76)80163-9. [DOI] [PubMed] [Google Scholar]
- Sallafranque M. L., Garret M., Benedetto J. P., Fournier M., Labouesse B., Bonnet J. Tryptophanyl-tRNA synthetase is a major soluble protein species in bovine pancreas. Biochim Biophys Acta. 1986 Jun 19;882(2):192–199. doi: 10.1016/0304-4165(86)90155-8. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Thiebe R., Hirsch R. A test for the active intermediate in the aminoacylation of tRNAPhe from yeast. FEBS Lett. 1975 Dec 15;60(2):338–341. doi: 10.1016/0014-5793(75)80744-7. [DOI] [PubMed] [Google Scholar]


