Abstract
The macromolecular enzyme complex prothrombinase serves an indispensable role in blood coagulation as it catalyzes the conversion of prothrombin to thrombin, a key regulatory enzyme in the formation of a blood clot. Interestingly, a virtually identical enzyme complex is found in the venom of some Australian elapid snakes, which is composed of a cofactor factor Va-component and a serine protease factor Xa-like subunit. This review will provide an overview of the identification and characterization of the venom prothrombinase complex and will discuss the rationale for its powerful procoagulant nature responsible for the potent hemostatic toxicity of the elapid venom.
Keywords: snake venom, blood coagulation, prothrombinase complex, factor X, factor V, prothrombin activation, serine protease, hemostatic toxin
1. Introduction
Venomous snakes produce an array of toxic compounds, including procoagulant proteins, to defend themselves and incapacitate prey. Using a murine toxicity model to compare 25 snake venoms, Broad et al. found in 1979 that some of the most potent venoms come from the Australian Elapidae family members Oxyuranus microlepidotus (O. microlepidotus; Inland taipan), Pseudonaja textilis (P. textilis; Common brown snake), and Oxyuranus scutellatus (O. scutellatus; Coastal taipan) [1]. A unique feature of their venom is that it contains substantial amounts (~5–40% of total venom protein) of procoagulant proteins with functional features similar to a macromolecular enzyme complex found in blood [2,3,4,5]. This enzyme complex, the prothrombinase complex which consists of the serine protease factor Xa (FXa) and cofactor factor Va (FVa) bound to an anionic membrane surface, plays an important role in blood coagulation as it rapidly converts prothrombin to thrombin, the latter being a key regulatory enzyme in the formation of a blood clot [6].
Prothrombin converting enzymes are commonly found in snake venoms and they are classified into four groups (A, B, C, and D) depending on their cofactor requirements in prothrombin activation [7,8,9]. Group A and B prothrombin activators are metalloproteases that convert prothrombin to the active intermediate meizothrombin; they are found in the venom of several vipers [9]. Group C and D prothrombin activators are serine proteases that are capable of fully activating prothrombin to thrombin and are exclusively found in Australian snakes [8,9]. Whereas group D activators require calcium, phospholipids, and the protein cofactor factor V (FV) for optimal protease activity, group C activators function in the absence of this cofactor. It is these group C activators that have, so far, only been observed in the Elapidae family members and have been the subject of detailed studies. These studies have shown that, remarkably, the group C activator complex consists of a FVa-like subunit and a FXa-like subunit similar to the blood coagulation prothrombinase complex [2,4]. To date, this subset of snakes are the only species identified that have two sources of prothrombinase: one that circulates in plasma and is required for normal hemostasis and one that is present in the venom and likely plays an important role in the envenomation of prey.
This review will focus on the identification and characterization of the venom prothrombinase complex components and will discuss the biochemical rationale for its powerful procoagulant nature that is responsible for the potent hemostatic toxicity of the elapid venom.
2. Identification of the Venom Prothrombinase-like Complex
In 1969, it was first reported that the venoms of the Australian elapids O. scutellatus and P. textilis contain a prothrombin activator which does not require blood coagulation FV as a cofactor for efficient clotting activity [10]. This prothrombin activating complex was initially purified from O. scutellatus venom, and it was shown to convert prothrombin to thrombin in a manner similar to the mammalian prothrombinase complex, independent of the addition of FV [11]. A subsequent study revealed that the purified prothrombin activator is composed of two large subunits [12], which was confirmed by Speijer et al. whose data suggested that the multimeric complex in fact consists of a blood coagulation FXa-like catalytic subunit and a FVa-like cofactor subunit [2]. Given the comparable characteristics of prothrombin conversion for the venoms of O. scutellatus, O. microlepidotus, and P. textilis, it was suggested that each of these prothrombin activators have a similar multisubunit composition [2,13]. These prothrombin activator complexes from O. scutellatus and P. textilis were termed oscutarin C and pseutarin C, respectively [9].
A detailed analysis of the identity of the protein subunits came from a study by Rao and Kini, in which peptide fragments of pseutarin C were purified by HPLC and subsequently subjected to N-terminal sequencing [4]. Their findings confirmed that the catalytic subunit shares a high sequence homology (~60%) with mammalian FXa, whereas the cofactor subunit is highly homologous to the mammalian cofactor FVa (~55%) [4]. Subsequent preparation and sequencing of venom gland cDNA revealed that these venom proteins are encoded by two individual genes [14,15,16,17,18], which are specifically expressed in the venom gland [17,19]. As such, these members of the Elapidae snake family are unique in that they have two gene sets encoding two parallel prothrombin activating systems.
3. Structural Characteristics of the Venom FXa-like Catalytic Subunit
3.1. Blood Coagulation Factor Xa
Blood coagulation FXa is synthesized in the liver and circulates in blood as the inactive zymogen factor X (FX). Factor X consists of two chains which are held together by a disulfide bond. The amino-terminal light chain consists of a γ-carboxyglutamic acid domain (Gla) domain and two epidermal growth factor (EGF) homology domains, and the carboxy-terminal heavy chain contains the serine protease or catalytic domain. All eleven Glu residues present in the Gla domain are posttranslationally γ-carboxylated to Gla in a process that requires vitamin K [20]. The Gla residues interact with Ca2+ ions and induce a conformational change that enables the protein to bind to negatively charged phospholipid membranes [21], which serves to localize the protease to a physiological cell surface [6]. The two EGF domains are involved in protein-protein interactions, while the serine protease domain comprises the active site with the catalytic triad His57 (the chymotrypsin numbering system is used throughout for the FXa residue numbering, unless otherwise noted [22]), Asp102, and Ser195, and several other regions involved in substrate binding and specificity, cofactor interaction, as well as Ca2+ and Na+ ion binding.
During the initiation of blood coagulation, FX is proteolytically activated by either the factor VIIIa (FVIIIa)-factor IXa (FIXa) (intrinsic tenase) or the factor VIIa (FVIIa)-tissue factor (extrinsic tenase) enzyme complexes following cleavage between Arg15–Ile16 in the heavy chain [6]. This results in the release of a large activation peptide and insertion of the newly formed heavy chain N-terminus Ile16 into the interior of the protease domain where it forms a salt bridge with Asp194. The subsequent conformational changes in specific functional regions of the protease domain are essential to protease maturation, resulting in a conformation optimal for substrate recognition and cofactor binding [23]. Following activation, FXa reversibly associates with its cofactor FVa on an anionic membrane surface in the presence of Ca2+ ions to form prothrombinase, the physiological activator of prothrombin [6].
3.2. The Venom-Derived Factor Xa-like Catalytic Subunit
Two groups have published the cDNA sequences of the FXa-like subunits expressed in the venom glands of P. textilis, O. scutellatus, and O. microlepidotus [15,17,18]. These venom FX sequences are very similar (91–95% sequence identity) and also share high homology with the group D prothrombin activators found in other Australian snakes (81–85% sequence identity) [17,23]; by comparison, the overall sequence identity with human FX is 49%. An alignment of the amino acid sequences of human FX, venom P. textilis FX, and liver-expressed P. textilis FX is given in Figure 1. In the next few sections, some of the structural properties of the FXa-like venom component will be discussed in more detail.
Figure 1.
Alignment of the amino acid sequences from human FX (h-FX), P. textilis liver-expressed FX (pt-lFX), and P. textilis venom gland-expressed FX (pt-vFX) (AlignX Module; Invitrogen Carlsbad, CA, USA). Residues fully conserved between all three FX derivatives are shown in green; amino acids partially conserved between the three variants but maintained in most vertebrates are indicated in yellow. Shown in red are: the cysteines connecting the heavy and the light chains, the catalytic triad His57, Asp102, and Ser195, as well as Asp194. The start of the light chain, activation peptide, and serine protease or catalytic domain is indicated. Putative glycosylation sites are underlined, and γ-carboxylated Glu residues are indicated by * (the last Gla residue does not align between human and snake FX). The signal peptide is numbered from -40 to -1 and the mature sequence starts at +1 (regular numbering of venom FX).
3.2.1. The Activation Peptide of Venom FX
The most striking difference between the sequences of the Australian elapid venom FXa-like proteins and blood coagulation FX is the length of the activation peptide. Even though the length of the activation peptide varies throughout evolution from 43 residues in the rat to 52 in human and 65 in zebrafish, the activation peptide of snake venom FX is considerably shorter, being 27 residues [15,17,18]. This is also considerably shorter than the FX expressed in the liver of P. textilis, which has an activation peptide of 56 residues (Figure 1) [24]. It is unclear if the FXa-like venom component comprises an intact activation peptide, as it was not detected upon N-terminal sequencing [4]. This would suggest that the activation peptide is either removed upon intracellular proteolysis of FX, or following its secretion into the venom. There is some evidence that glycosylation of the human FX activation peptide may contribute to substrate recognition by the intrinsic or extrinsic FX activating complex [25,26]. Although the activation peptide comprises several putative N-glycosylation sites (Figure 1), whether or not it plays a role in the proteolytic activation of venom FX similar to mammalian FX remains to be determined.
3.2.2. The Light Chain of Venom FXa
The light chain of the venom FXa-like component is very similar to that of blood coagulation FX. The Gla domain of venom FXa comprises eleven conserved Glu residues, which have been shown by chemical Gla analysis to be fully γ-carboxylated in P. textilis FXa (Figure 1) [27]. Furthermore, the structural elements that mediate anionic phospholipid binding are also present in the FXa-like subunit [21], which implies that, similar to mammalian FXa, venom FXa is capable of membrane binding. The structure of the EGF domains seems to be maintained as well, given that the six cysteines that form disulfide bridges characterizing the individual EGF domains are conserved. In addition, the cysteines that connect the heavy and light chains are also present in venom FXa (Figure 1).
3.2.3. The Heavy Chain of Venom FXa
Other important regions of FXa that are well conserved in the snake venom FXa-like subunit include the N-terminal heavy chain sequence Ile16–Val17, which is essential to protease maturation [23,28], and the catalytic triad His57, Asp102, and Ser195 (Figure 1). Some of the regions that undergo a conformational change upon activation are highly conserved in venom FXa, such as active site loop Ala183–Asp194, which includes Asp194 that forms a salt bridge with Ile16, and the Na+ binding site Glu216–Glu226. In contrast, most of the residues in the catalytic domain important for Ca2+ coordination are lost. More intriguingly, the Ca2+-interactive region is followed by a 13 residue insert, Pro251–Leu263 (regular numbering of venom FX), that has been found in the venom FXa of all three elapids and, to some extent, in the group D prothrombin activators [17,24]. However, this sequence is not present in liver-expressed P. textilis FX, nor in other serine proteases involved in blood coagulation, such as FVIIa, FIXa, or activated protein C (APC). The functional role of this insertion is unclear.
4. Structural Characteristics of the Venom FVa-like Cofactor Subunit
4.1. Blood Coagulation Factor V
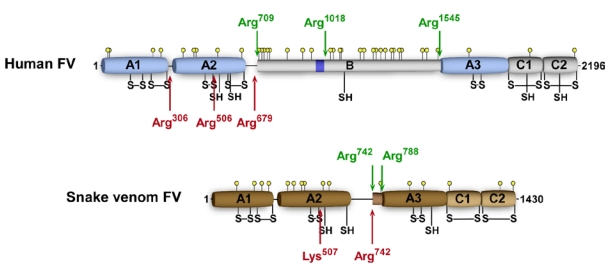
Blood coagulation FV is a large (330 kD), heavily glycosylated, single chain protein that circulates in blood as an inactive procofactor [29]. It has an A1-A2-B-A3-C1-C2 domain structure (Figure 2), and only following removal of the large, central B domain can FV assemble or function in the prothrombinase complex [29,30]. Thrombin is considered the key physiological activator of FV and cleaves the three peptide bonds Arg709, Arg1018, and Arg1545 in the B domain, thereby facilitating B domain removal. The resulting cofactor, FVa, is a heterodimer composed of a heavy chain (105 kD) and a light chain (71/74 kD) that are associated through Ca2+ ions. Assembly of FVa with FXa in the prothrombinase complex on the cellular surface at the site of vascular injury is an essential step during blood coagulation as it dramatically enhances the rate of thrombin generation by FXa [6]. Factor Va is inactivated by APC, which cleaves at Arg306, Arg506, and Arg679, resulting in A2 domain dissociation and loss of FXa binding and cofactor function [29].
4.2. The Factor Va-like Cofactor Subunit
Similar to venom FXa, the available cDNA sequences make clear that the FVa-like cofactor subunits expressed in the venom of P. textilis, O. scutellatus, and O. microlepidotus are very similar (97% sequence identity) [14,16,17], whereas the sequence identity with human FV is 54%. The deduced protein sequences indicate that some of the structural features involved in FV function appear to differ significantly; these elements will be discussed in more detail in the following sections.
Figure 2.
Schematic A1-A2-B-A3-C1-C2 domain representation of human and snake venom (P. textilis) FV. Thrombin cleavage sites are indicated by green arrows and APC cleavage sites by red arrows. Yellow circles represent potential N-linked glycosylation sites and the dark blue box corresponds to the basic sequence 963–1008 implicated in preserving the FV procofactor state. Disulfide bonds are indicated by S-S and free cysteines by SH.
4.2.1. The B Domain of Venom FV
A remarkable feature of the FV homologs expressed in the elapid venom is that their B domain is extraordinarily short: 46 versus ~600–800 residues in mammals (the FV B domain is defined as the region removed following thrombin cleavage to generate the heavy and light chains; Figure 2) [30]. Our laboratory has previously shown that the B domain plays an important function by interfering with functional binding interactions of FV through imposing steric and/or conformational constraints, thereby preventing constitutive cofactor function [31,32]. In addition, we have identified a discrete region of the B domain that plays a critical role in stabilizing the procofactor state [32]. Part of this region (residues 963–1008 of human FV) is unusually basic with 18 of 46 residues being Arg or Lys, and is well conserved across the vertebrate lineage [30]. Given that venom FV lacks the majority of the B domain sequence, it is not surprising that the so called basic region is absent as well. This intriguing observation prompted us to assess the cofactor capacity of purified recombinant venom-derived P. textilis FV (pt-FV) [27]. Consistent with our previous observations, we were able to show that the absence of the basic region correlates with the expression of procoagulant FV activity, indicating that venom FV is expressed as a constitutionally active FV variant [27]. As such, this is the first FV species observed thus far that exists as a constitutively active cofactor. Interestingly, liver-expressed P. textilis FV has a similar short B domain that lacks the basic region [19], which would suggest that the FV circulating in the elapid’s plasma is constitutionally active as well; however, biochemical studies are needed to confirm this possibility.
4.2.2. The Disulfide Bond Connecting the Heavy and Light Chains of Venom FV
Sequence alignment reveals that in comparison to human FV, venom FV has lost six cysteine residues: three in the A2 domain (Cys575–Cys656, Cys585; human residue numbering), one in the B domain (Cys1085), and two free cysteines in the C1-C2 domains, of which Cys1960 is nonconserved throughout vertebrate evolution, whereas Cys2113 is only present in mammals. Venom FV has two newly introduced cysteines that are unique to Australian elapids: Cys642 in A2 and Cys1002 in A3 (snake residue numbering; Figure 2). Recent data from our laboratory show that either Cys540 or Cys642 in the A2 domain is involved in a disulfide bond that connects the heavy and light chains of pt-FV [27]. This is consistent with previous observations made by Speijer et al., who obtained evidence suggesting the existence of such a linker between the heavy and light chains of O. scutellatus FV [2]. This disulfide bond is a unique feature of snake venom FV, since it has not been observed in any other FV species to date. Based on homology with the cysteines in human FV, Cys1002 could be the only free cysteine in the venom FV light chain; therefore, this residue may be part of this unique disulfide bond linking the heavy and light chains. This assumption would also imply that plasma P. textilis FV cannot form a similar disulfide bond, as it lacks Cys1002 [19].
4.2.3. Activated Protein C Cleavage Sites in Venom FV
We have recently shown that P. textilis venom-derived FV is functionally resistant to inactivation by APC, as no loss of cofactor activity was observed upon incubation with very high concentrations of APC [27]; a similar observation was made using the venom FV-FXa complex, pseutarin C [14]. However, while the FVa functional activity was unaffected following APC treatment, we did observe proteolytic degradation of pt-FV, indicative of cleavage by APC [27]. N-terminal sequencing of the degradation products indicated that human APC cleaves pt-FV at Lys507 and Arg742. These sites are homologous to the APC cleavage site Arg506 in human FV and to Arg709, the thrombin cleavage site. Furthermore, our data suggested that APC cleaves pt-FV at some additional sites in the A2 region somewhere within Glu508–Arg742; however, we were unable to make the exact determination. Both the venom FV from O. scutellatus and O. microlepidotus as well as the FV expressed in the liver of P. textilis have similar residues at these sites, suggesting that they would be cleaved by APC in a comparable manner.
4.2.4. Interdomain Connections in Venom FV
The mammalian FVa structure is stabilized by several interdomain contacts linking the C1-C2, A3-C1, and A3-C2 domains [33]. Metal ion binding further contributes to these interdomain interactions by providing stability to the local structure [33]. Analysis of the A3-C1-C2 residues involved in direct interdomain contacts indicates that all of the residues connecting the C1-C2 and A3-C2 domains are conserved in venom FV, while the hydrophobic interactions linking the A3-C1 domains are at least partially preserved. Of the residues implied in Cu2+ binding, the majority are found in venom FV, as well as several residues involved in essential interactions between A1 and A3. Furthermore, the high affinity Ca2+ binding site is completely conserved. This implies that heavy and light chains of venom FV are linked in a manner similar to mammalian FV.
4.2.5. Posttranslational Modifications of Venom FV
Factor V undergoes multiple posttranslational modifications, including N-glycosylation, phosphorylation, and sulfation at multiple sites [29], which are not fully conserved in venom FV. Mammalian FV is heavily N-glycosylated, and 25 out of the 37 putative N-glycosylation sites are located within the B domain (Figure 1). Venom FV, on the other hand, has 16 potential N-glycosylation sites of which one is in the B domain (Figure 1) [34]. Some of these N-glysocylation sites are similar to human FV, whereas others are unique to snake FV. Interestingly, liver-expressed P. textilis FV has an additional putative N-glycosylation site in the B domain at Asn573 [19], which is not preserved in any of the venom FV species. Phosphorylation of the human FVa heavy chain at Ser692 has been implied to enhance the rate of APC-dependent inactivation [35]. Although this phosphorylation site is absent in the venom FVa-like subunit, venom-derived P. textilis FV could be proteolyzed by human APC as discussed above [27]. This suggests that phosphorylation of the FVa heavy chain may not be critical to inactivation by APC per se. Sulfation of FV has been speculated to be of importance for the recognition of FV by thrombin and for full FVa cofactor activity [36]. However, out of the six sulfation sites present in human FV, the one homologous to human Tyr1593 is conserved in snake venom FV. Furthermore, our data on the thrombin-mediated pt-FV activation indicate a minor role for sulfation in the recognition of venom FV by thrombin [27].
5. Evolutionary Adaptation of Prothrombinase to a Powerful Hemostatic Toxin
One of the major clinical effects of envenomation by the Australian Elapidae genera Pseudonaja and Oxyuranus is significant consumptive coagulopathy, causing early hypotension, spontaneous bleeding, and severe fibrinogen depletion [37,38,39,40]. It is thought that the injected prothrombin activating complex is responsible for these symptoms, as it rapidly converts the prey’s prothrombin to thrombin, which subsequently leads to the formation of microthrombi. This process can be life threatening in several ways: the microthrombi could get lodged in the microvasculature of the lungs, thereby resulting in a pulmonary embolism, and the formation of microthrombi severely exhausts the blood coagulation system (coagulopathy), giving rise to fibrinogen depletion and spontaneous bleedings.
This powerful procoagulant effect of the venom prothrombinase-like complex cannot be attributed to an enhanced intrinsic prothrombin converting capacity, as we have recently shown that the kinetic parameters for the conversion of human prothrombin are similar between venom-derived P. textilis prothrombinase and human prothrombinase [27]. Based on this, it seems likely that a combination of modifications in the protein structure as well as modulations at the level of gene transcription and subsequent protein expression have transformed the blood coagulation prothrombinase complex into a venom toxin. Several of these adaptations will be discussed in the following sections.
5.1. Transcriptional Regulation and Protein Expression of the Venom Prothrombinase-like Complex
Recently, Kini and coworkers have investigated the transcriptional regulation and protein expression of venom FVa and FXa. They were able to demonstrate that the expression of venom P. textilis FV and FX is almost 300-fold and 80-fold higher, respectively, as compared to their liver counterparts [19]. Further gene analysis suggested that this increase in expression is due to a so-called ~270 bp VERSE (Venom Recruitment/Switch Element) region in the promoter, which is thus far only found in the genes encoding venom FX of P. textilis and T. carinatus [41,42]. This indicates that the recruitment of FX to the venom gland is accompanied by one or more specific gene modulations, resulting in elevated levels of protein expression. No evidence has yet been obtained in support of a similar modification of the venom gland FV gene.
High expression of the venom prothrombinase-like components largely contributes to the hemostatic toxicity of the venom, as it allows for the rapid generation of extremely high levels of thrombin upon envenomation. The amount of venom produced by a snake when it bites (e.g., venom yield) has been found to vary from 8 to 146 mg for the Pseudonaja and Oxyuranus snakes [43]. Considering that the prothrombinase-like complex makes up 5–40% of the yield [2,3,4,5], 0.4–58 mg or 2–260 nmols (FXa-FVa ~220 kD) of the enzyme complex could be injected into the prey. Assuming that the kinetic parameters for mammalian prothrombin conversion are similar to that of human prothrombin, the amount of thrombin generated in 30 minutes can go from 20 µmols up to 2 mmols (kcat ~ 300 min−1) [27]. This exceeds the amount of thrombin required for clot formation at the site of injury by several orders of magnitude, which is generally assumed to be in the nanomolar range [44]. Based on these values, it is not surprising that the first symptoms of consumptive coagulopathy can be observed within half an hour following envenomation [38,40].
5.2. Gain-of-Function Adaptations in the P. textilis Venom-Derived FVa-like Subunit
We have previously found that venom FV from P. textilis circumvents the paradigms of normal hemostasis through a variety of changes transforming it into a potent procoagulant [27]. One of these gain-of-function elements is that pt-FV is synthesized in an active state and does not require proteolytic removal of the B domain to express procoagulant activity. As such, pt-FV is a naturally occurring example of a protein that has acquired a new functional state through loss of inhibitory sequences [30].
Another remarkable feature of pt-FV is that it binds P. textilis venom FXa (pt-FXa) with high affinity both in the presence and absence of anionic membranes [27]. Once assembled in solution, the FVa-FXa complex functions equivalently to the membrane-bound complex [27]. Thus, pt-FV has bypassed the restraints observed for the mammalian prothrombinase complex, which, at physiological concentrations, only assembles on a phospholipid surface such as that of platelets or damaged vascular cells. The exact mechanism underlying this requirement is generally considered to stem from a limitation in reaction dimensions due to assembly on the membrane surface [6,45]. Recently, however, it was proposed that a conformational change in the FV molecule following phospholipid binding enhances the interaction with FXa [46,47]. Our findings with the soluble P. textilis FVa-FXa complex would seem to support this conformational model. In the case of the venom-derived proteins, these conformational transitions are probably induced through a variety of changes to their primary structure, which mimics the structural configuration of membrane-bound FVa-FXa.
As a further procoagulant enhancement, pt-FV is functionally resistant to APC despite APC-dependent cleavage in the heavy chain at Lys507 and Arg742 [27]. We speculate that the disulfide bond between the A2 and A3 domains contributes to stabilizing pt-FV and prevents dissociation of the A2 domain from the rest of the molecule [27]. This unique disulfide bond that connects the heavy and light chains of pt-FV may result from a newly introduced cysteine. Interestingly, although quite rare, some of the phospholipase A2 enzyme variants expressed in the venom of P. textilis and O. scutellatus have also been observed to have additional, nonconserved cysteines that are presumed to form intramolecular disulfide bonds [48,49]. Whether or not this unique structural feature is limited to the venom toxins of the Australian elapids P. textilis and O. scutellatus remains to be determined.
6. Final Remarks
Recent studies from several laboratories indicate that the prothrombinase-like complex found in the venom of a specific subset of Australian elapids has undergone several regulatory and structural modifications that account for its potent hemostatic toxicity. Uncoupling the preservation of the procofactor state of the cofactor component (FV) and bypassing the need for membrane binding to function for the P. textilis FVa-FXa complex are central underlying modifications allowing for the enzyme complex to rapidly activate prothrombin in solution and contribute to disseminated clotting.
The similarities between venom and plasma FV present in these snakes raise some interesting questions concerning the constitutive activity of plasma FV. Based on our data, we speculate that the liver-expressed P. textilis FV is already active in the absence of proteolytic activation by thrombin [27]. The implications for normal hemostasis in the snake are unclear at this point, but considering FV can only function in the presence of the active serine protease FXa, we would anticipate that the presence of an active cofactor contributes minimally to unregulated clotting.
Another interesting aspect of the presence of two almost identical enzyme systems with different functions is that the venom prothrombinase-like complex can induce coagulation in the snake’s own plasma, as has been shown previously [50]. In some cases, it has been acknowledged that snake plasma contains inhibitors that neutralize venom toxins, including those toxins affecting blood coagulation [51]. Another mechanism that protects the snake against its own venom toxins is through structural modulation of the target molecule. One of these examples can be found in the Egyptian cobra (N. haje) that has a unique N-glycosylation site in the ligand binding domain of the nicotinic acetylcholine receptor, which has been demonstrated to obstruct binding by a venom neurotoxin, but allows binding by its natural ligand [52]. We can only speculate as to whether elapid prothrombin has undergone a similar structural modification, since its sequence is not yet available.
Taken together, the prothrombinase complex represents an exceptional example of an enzyme system that has evolved into a potent biological weapon for host defense and envenomation of prey.
Acknowledgements
We would like to acknowledge funding from the National Institutes of Health (HL-88010 and HL-74124, Project 2; to RMC) and the Judith Graham Pool Postdoctoral Research Fellowship from the National Hemophilia Foundation (to MHAB).
Conflicts of Interest
RMC receives research support and royalties from Pfizer Pharmaceuticals for technology related to FXa. MHAB declares no conflicts of interest.
References
- 1.Broad A.J., Sutherland S.K., Coulter A.R. The lethality in mice of dangerous Australian and other snake venom. Toxicon. 1979;17:661–664. doi: 10.1016/0041-0101(79)90245-9. [DOI] [PubMed] [Google Scholar]
- 2.Speijer H., Govers-Riemslag J.W., Zwaal R.F., Rosing J. Prothrombin activation by an activator from the venom of Oxyuranus scutellatus (Taipan snake) J. Biol. Chem. 1986;261:13258–13267. [PubMed] [Google Scholar]
- 3.Masci P.P., Whitaker A.N., de Jersey J. Purification and characterization of a prothrombin activator from the venom of the Australian brown snake, Pseudonaja textilis textilis. Biochem. Int. 1988;17:825–835. [PubMed] [Google Scholar]
- 4.Rao V.S., Kini R.M. Pseutarin C, a prothrombin activator from Pseudonaja textilis venom: its structural and functional similarity to mammalian coagulation factor Xa-Va complex. Thromb. Haemost. 2002;88:611–619. [PubMed] [Google Scholar]
- 5.Lavin M.F., Masci P.P. Prothrombinase complexes with different physiological roles. Thromb. Haemost. 2009;102:421–423. doi: 10.1160/TH09-08-0050. [DOI] [PubMed] [Google Scholar]
- 6.Mann K.G., Nesheim M.E., Church W.R., Haley P.E., Krishnaswamy S. Surface dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76:1–16. [PubMed] [Google Scholar]
- 7.Chester A., Crawford G.P. In vitro coagulant properties of venoms from Australian snakes. Toxicon. 1982;20:501–504. doi: 10.1016/0041-0101(82)90014-9. [DOI] [PubMed] [Google Scholar]
- 8.Rosing J., Tans G. Structural and functional properties of snake venom prothrombin activators. Toxicon. 1992;30:1515–1527. doi: 10.1016/0041-0101(92)90023-x. [DOI] [PubMed] [Google Scholar]
- 9.Kini R.M. The intriguing world of prothrombin activators from snake venom. Toxicon. 2005;45:1133–1145. doi: 10.1016/j.toxicon.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Denson K.W. Coagulant and anticoagulant action of snake venoms. Toxicon. 1969;7:5–11. doi: 10.1016/0041-0101(69)90154-8. [DOI] [PubMed] [Google Scholar]
- 11.Owen W.G., Jackson C.M. Activation of prothrombin with Oxyuranus scutellatus scutellatus (taipan snake) venom. Thromb. Res. 1973;3:705–714. doi: 10.1016/0049-3848(73)90017-0. [DOI] [Google Scholar]
- 12.Walker F.J., Owen W.G., Esmon C.T. Characterization of the prothrombin activator from the venom of Oxyuranus scutellatus scutellatus (taipan venom) Biochemistry. 1980;19:1020–1023. doi: 10.1021/bi00546a029. [DOI] [PubMed] [Google Scholar]
- 13.Chen L., Rezaie A.R. Proexosite-1-dependent recognition and activation of prothrombin by taipan venom. J. Biol. Chem. 2004;279:17869–17874. doi: 10.1074/jbc.M314285200. [DOI] [PubMed] [Google Scholar]
- 14.Rao V.S., Swarup S., Kini R.M. The nonenzymatic subunit of pseutarin C, a prothrombin activator from eastern brown snake (Pseudonaja textilis) venom, shows structural similarity to mammalian coagulation factor V. Blood. 2003;102:1347–1354. doi: 10.1182/blood-2002-12-3839. [DOI] [PubMed] [Google Scholar]
- 15.Rao V.S., Swarup S., Kini R.M. The catalytic subunit of pseutarin C, a group C prothrombin activator from the venom of Pseudonaja textilis, is structrually similar to mammalian blood coagulation factor Xa. Thromb. Haemost. 2004;92:509–521. doi: 10.1160/TH04-03-0144. [DOI] [PubMed] [Google Scholar]
- 16.Welton R.E., Burnell J.N. Full length nucleotide sequence of a factor V-like subunit of oscutarin from Oxyuranus scutellatus scutellatus (coastal Taipan) Toxicon. 2005;46:328–336. doi: 10.1016/j.toxicon.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 17.St. Pierre L., Masci P.P., Filippovich I., Sorokina N., Marsh N., Miller D.J., Lavin M.F. Comparative analysis of prothrombin activators from the venom of Australian elapids. Mol. Biol. Evol. 2005;22:1853–1864. doi: 10.1093/molbev/msi181. [DOI] [PubMed] [Google Scholar]
- 18.Filippovich I., Sorokina N., St Pierre L., Flight S., de Jersey J., Perry N., Masci P.P., Lavin M.F. Cloning and functional expression of venom prothrombin activator protease from Pseudonaja textilis with whole blood procoagulant activity. Br. J. Haematol. 2005;131:237–246. doi: 10.1111/j.1365-2141.2005.05744.x. [DOI] [PubMed] [Google Scholar]
- 19.Minh Le T.N., Reza M.A., Swarup S., Kini R.M. Gene duplication of coagulation factor V and origin of venom prothrombin activator in Pseudonaja textilis snake. Thromb. Haemost. 2005;93:420–429. doi: 10.1160/TH04-11-0707. [DOI] [PubMed] [Google Scholar]
- 20.Furie B., Bouchard B.A., Furie B.C. Vitamin K-dependent biosynthesis of γ-carboxyglutamic acid. Blood. 1999;93:1798–1808. [PubMed] [Google Scholar]
- 21.Sunnerhagen M., Forsén S., Hoffrén A.-M., Drakenberg T., Teleman O., Stenflo J. Structure of the Ca2+-free Gla domain sheds light on membrane binding of blood coagulation proteins. Nat. Struct. Biol. 1995;2:504–509. doi: 10.1038/nsb0695-504. [DOI] [PubMed] [Google Scholar]
- 22.Bode W., Mayr I., Bauman Y., Huber R., Stone S.R., Hofsteenge J. The refined 1.9 Å crystal structure of human α-thrombin: Interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Trp insertion segment. EMBO J. 1989;8:3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber R., Bode W. Structural basis of the activation and action of trypsin. Acc. Chem. Res. 1978;11:114–122. [Google Scholar]
- 24.Reza M.A., Minh Le T.N., Swarup S., Manjunatha K.R. Molecular evolution caught in action: gene duplication and evolution of molecular isoforms of prothrombin activators in Pseudonaja textilis (brown snake) J. Thromb. Haemost. 2006;4:1346–1353. doi: 10.1111/j.1538-7836.2006.01969.x. [DOI] [PubMed] [Google Scholar]
- 25.Rudolph A.E., Mullane M.P., Porche-Sorbet R., Daust H.A., Miletich J.P. The role of the factor X activation peptide: a deletion mutagenesis approach. Thromb. Haemost. 2002;88:756–762. [PubMed] [Google Scholar]
- 26.Yang L., Manithody C., Rezaie A.R. Functional role of O-linked and N-linked glycosylation sites present on the activation peptide of factor X. J. Thromb. Haemost. 2009;7:1696–1702. doi: 10.1111/j.1538-7836.2009.03578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bos M.H.A., Boltz M., St. Pierre L., Masci P.P., de Jersey J., Lavin M.F., Camire R.M. Venom factor V from the common brown snake escapes hemostatic regulation through procoagulant adaptations. Blood. 2009;114:686–692. doi: 10.1182/blood-2009-02-202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toso R., Zhu H., Camire R.M. The conformational switch from the factor X zymogen to protease state mediates exosite expression and prothrombinase assembly. J. Biol. Chem. 2008;283:18627–18635. doi: 10.1074/jbc.M802205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann K.G., Kalafatis M. Factor V: A combination of Dr. Jekyll and Mr. Hyde. Blood. 2002;101:20–30. doi: 10.1182/blood-2002-01-0290. [DOI] [PubMed] [Google Scholar]
- 30.Camire R.M., Bos M.H.A. The molecular basis of factor V and VIII procofactor activation. J. Thromb. Haemost. 2009;7:1951–1961. doi: 10.1111/j.1538-7836.2009.03622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toso R., Camire R.M. Removal of B-domain sequences from factor V rather than specific proteolysis underlies the mechanism by which cofactor function is realized. J. Biol. Chem. 2004;279:21643–21650. doi: 10.1074/jbc.M402107200. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H., Toso R., Camire R.M. Inhibitory sequences within the B-domain stabilize circulating factor V in an inactive state. J. Biol. Chem. 2007;282:15033–15039. doi: 10.1074/jbc.M701315200. [DOI] [PubMed] [Google Scholar]
- 33.Adams T.E., Hockin M.F., Mann K.G., Everse S.J. The crystal structure of activated protein C-inactivated bovine factor Va: Implications for cofactor function. Proc. Natl. Acad. Sci. USA. 2004;101:8918–8923. doi: 10.1073/pnas.0403072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birrell G.W., Earl S., Masci P.P., de Jersey J., Wallis T.P., Gorman J.J., Lavin M.F. Molecular diversity in venom from the Australian Brown snake, Pseudonaja textilis. Mol. Cell. Proteomics. 2006;5:379–389. doi: 10.1074/mcp.M500270-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Kalafatis M. Identification and partial characterization of factor Va heavy-chain kinase from human platelets. J. Biol. Chem. 1998;273:8459–8466. doi: 10.1074/jbc.273.14.8459. [DOI] [PubMed] [Google Scholar]
- 36.Pittman D.D., Tomkinson K.N., Michnick D., Seligsohn U., Kaufman R.J. Posttranslational sulfation of factor V is required for efficient thrombin cleavage and activation and for full procoagulant activity. Biochemistry. 1994;33:6952–6959. doi: 10.1021/bi00188a026. [DOI] [PubMed] [Google Scholar]
- 37.Masci P.P., Rowe E.A., Whitaker A.N., de Jersey J. Fibrinolysis as a feature of disseminated intravascular coagulation (DIC) after Pseudonaja textilis textilis envenomation. Thromb. Res. 1990;59:859–870. doi: 10.1016/0049-3848(90)90399-W. [DOI] [PubMed] [Google Scholar]
- 38.Lalloo D.G., Trevett A.J., Korinhona A., Nwokolo N., Laurenson I.F., Paul M., Black J., Naraqi S., Mavo B., Saweri A., et al. Snake bites by the Papuan taipan (Oxyuranus scutellatus canni): paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am. J. Trop. Med. Hyg. 1995;52:525–531. doi: 10.4269/ajtmh.1995.52.525. [DOI] [PubMed] [Google Scholar]
- 39.Barrett R., Little M. Five years of snake envenoming in far north Queensland. Emerg. Med. (Fremantle) 2003;15:500–510. doi: 10.1046/j.1442-2026.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- 40.Currie B.J. Snakebite in tropical Australia: a prospective study in the "Top End" of the Northern Territory. Med. J. Aust. 2004;181:693–697. doi: 10.5694/j.1326-5377.2004.tb06526.x. [DOI] [PubMed] [Google Scholar]
- 41.Reza M.A., Swarup S., Kini R.M. Structure of two genes encoding parallel prothrombin activators in Tropidechis carinatus snake: gene duplication and recruitment of factor X gene to the venom gland. J. Thromb. Haemost. 2007;5:117–126. doi: 10.1111/j.1538-7836.2006.02266.x. [DOI] [PubMed] [Google Scholar]
- 42.Kwong S., Woods A.E., Mirtschin P.J., Ge R., Kini R.M. The recruitment of blood coagulation factor X into snake venom gland as a toxin: the role of promoter cis-elements in its expression. Thromb. Haemost. 2009;102:469–478. doi: 10.1160/TH09-03-0162. [DOI] [PubMed] [Google Scholar]
- 43.Mirtschin P.J., Dunstan N., Hough B., Hamilton E., Klein S., Lucas J., Millar D., Madaras F., Nias T. Venom yields from Australian and some other species of snakes. Ecotoxicology. 2006;15:531–538. doi: 10.1007/s10646-006-0089-x. [DOI] [PubMed] [Google Scholar]
- 44.Mann K.G., Brummel K., Butenas S. What is all that throm bin for? J. Thromb. Haemost. 2003;1:1504–1514. doi: 10.1046/j.1538-7836.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 45.Krishnaswamy S., Jones K.C., Mann K.G. Prothrombinase complex assembly: Kinetic mechanism of enzyme assembly on phospholipid vesicles. J. Biol. Chem. 1988;263:3823–3834. [PubMed] [Google Scholar]
- 46.Majumder R., Quinn-Allen M.A., Kane W.H., Lentz B.R. A phosphatidylserine binding site in factor Va C1 domain regulates both assembly and activity of the prothrombinase complex. Blood. 2008;112:2795–2802. doi: 10.1182/blood-2008-02-138941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qureshi S.H., Yang L., Manithody C., Rezaie A.R. Membrane-dependent interaction of factor Xa and prothrombin with factor Va in the prothrombinase complex. Biochemistry. 2009;48:5034–5041. doi: 10.1021/bi900240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fohlman J., Lind P., Eaker D. Taipoxin, an extremely potent presynaptic snake venom neurotoxin. Elucidation of the primary structure of the acidic carbohydrate-containing taipoxin-subunit, a prophospholipase homolog. FEBS Lett. 1977;84:367–371. doi: 10.1016/0014-5793(77)80726-6. [DOI] [PubMed] [Google Scholar]
- 49.Pearson J.A., Tyler M.I., Retson K.V., Howden M.E. Studies on the subunit structure of textilotoxin, a potent presynaptic neurotoxin from the venom of the Australian common brown snake (Pseudonaja textilis). 3. The complete amino-acid sequences of all the subunits. Biochim. Biophys. Acta. 1993;1161:223–229. doi: 10.1016/0167-4838(93)90217-F. [DOI] [PubMed] [Google Scholar]
- 50.Joseph J.S., Chung M.C., Mirtschin P.J., Kini R.M. Effect of snake venom procoagulants on snake plasma: implications for the coagulation cascade of snakes. Toxicon. 2002;40:175–183. doi: 10.1016/s0041-0101(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 51.Thwin M.M., Gopalakrishnakone P. Snake envenomation and protective natural endogenous proteins: a mini review of the recent developments (1991–1997) Toxicon. 1998;36:1471–1482. doi: 10.1016/s0041-0101(98)00137-8. [DOI] [PubMed] [Google Scholar]
- 52.Takacs Z., Wilhelmsen K.C., Sorota S. Cobra (Naja spp.) nicotinic acetylcholine receptor exhibits resistance to Erabu sea snake (Laticauda semifasciata) short-chain alpha-neurotoxin. J. Mol. Evol. 2004;58:516–526. doi: 10.1007/s00239-003-2573-8. [DOI] [PubMed] [Google Scholar]