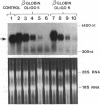
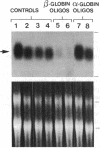
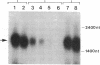
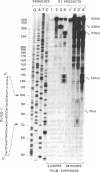
Abstract
Oligodeoxynucleotides lead to translation arrest of complementary mRNAs in the wheat germ translation system by a degradation of the mRNA. In an attempt to develop an effective reverse genetic approach in vivo, we demonstrate that injection of short (15- to 30-nucleotide) oligonucleotides into Xenopus oocytes leads to complete degradation of both injected and endogenous mRNAs by means of an RNase H-like activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass B. L., Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987 Feb 27;48(4):607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
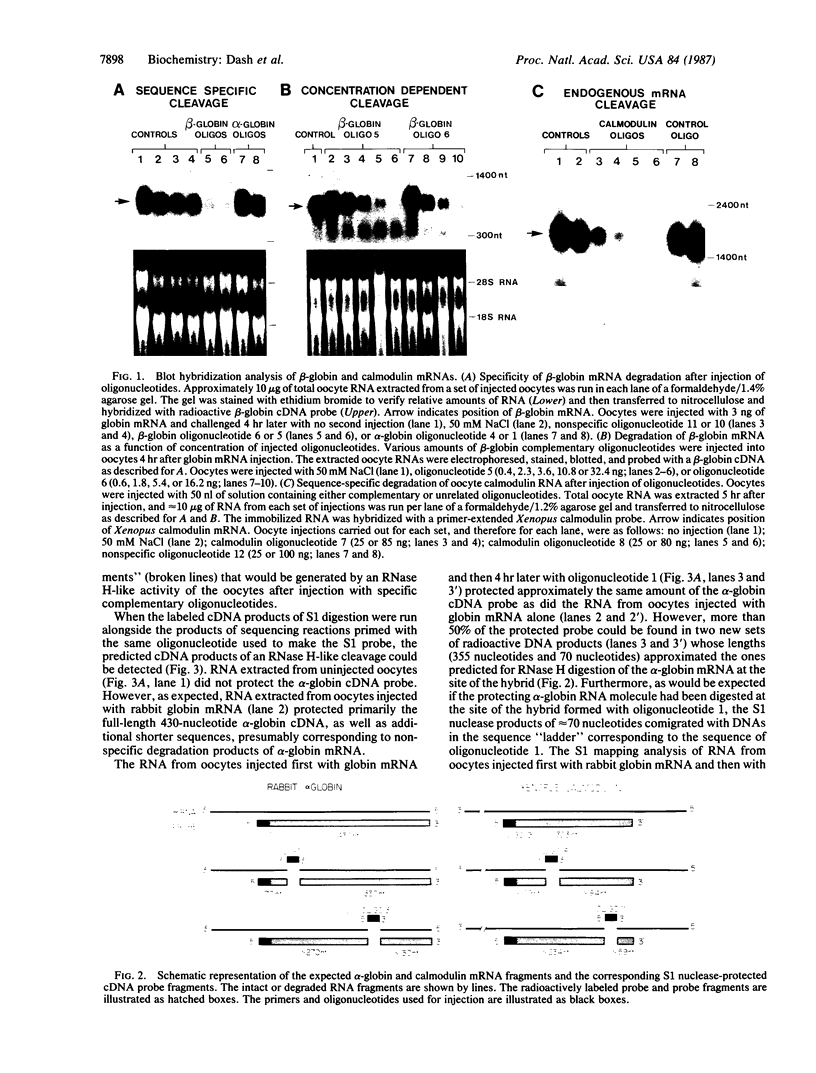
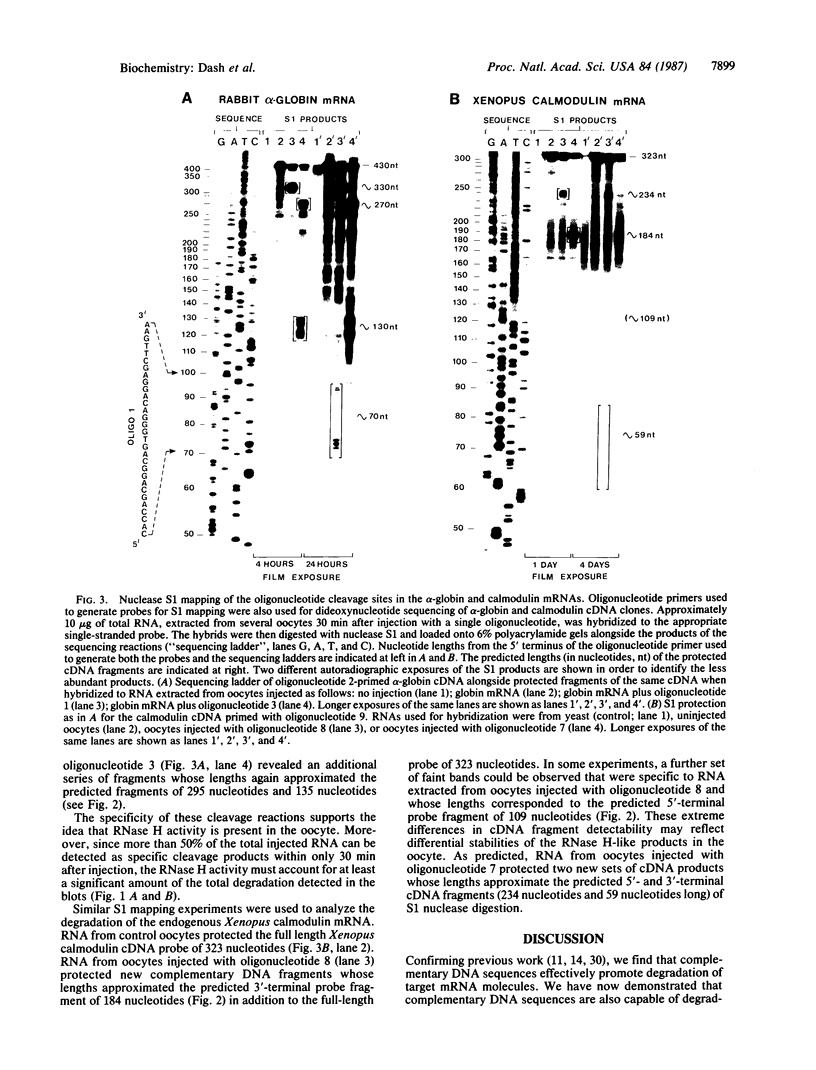
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Thuong N. T., Toulmé J. J., Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987 Jun 25;15(12):4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. F., Raid L., Hardison R. C. Isolation and nucleotide sequence of the rabbit globin gene cluster psi zeta-alpha 1-psi alpha. Absence of a pair of alpha-globin genes evolving in concert. J Biol Chem. 1986 Jan 15;261(2):839–848. [PubMed] [Google Scholar]
- Chien Y. H., Dawid I. B. Isolation and characterization of calmodulin genes from Xenopus laevis. Mol Cell Biol. 1984 Mar;4(3):507–513. doi: 10.1128/mcb.4.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Snutch T. P., Lübbert H., Davidson N., Lester H. A. Expression and modulation of voltage-gated calcium channels after RNA injection in Xenopus oocytes. Science. 1986 Mar 7;231(4742):1147–1150. doi: 10.1126/science.2418503. [DOI] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Harland R., Weintraub H. Translation of mRNA injected into Xenopus oocytes is specifically inhibited by antisense RNA. J Cell Biol. 1985 Sep;101(3):1094–1099. doi: 10.1083/jcb.101.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S. Quantitative hybridization-arrest of mRNA in Xenopus oocytes using single-stranded complementary DNA or oligonucleotide probes. Nucleic Acids Res. 1985 Jul 11;13(13):4991–5004. doi: 10.1093/nar/13.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati M. R., Melton D. A. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987 Feb 27;48(4):599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh M. L., Johnson J. E., 3rd, James M. D., Hardison R. C. Transcription unit of the rabbit beta 1 globin gene. Mol Cell Biol. 1985 Jan;5(1):147–160. doi: 10.1128/mcb.5.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg U. B., Preiss A., Seifert E., Jäckle H., Knipple D. C. Production of phenocopies by Krüppel antisense RNA injection into Drosophila embryos. Nature. 1985 Feb 21;313(6004):703–706. doi: 10.1038/313703a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H., Hausen P. Enzyme from calf thymus degrading the RNA moiety of DNA-RNA Hybrids: effect on DNA-dependent RNA polymerase. Science. 1969 Oct 17;166(3903):393–395. doi: 10.1126/science.166.3903.393. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]