Abstract
The desmoplastic small round cell tumor (DSRCT) is an uncommon and highly aggressive cancer. The role of 18F-FDG PET in management of DSRCT is little reported. We report a case of metastasized abdominal DSRCT detected in a 43-year old patient whose diagnostic and therapeutic approaches were influenced by 18F-FDG PET-CT. The patient is still alive ten years after diagnosis. 18F-FDG PET-CT seems to be a useful method for assessing therapeutic efficiency and detecting early recurrences even in rare malignancies such as DSRCT.
Introduction
The desmoplastic small round cell tumor (DSRCT) is an extremely rare and highly aggressive neoplasm mainly affecting adolescents and young male adults.1,2 DSRCT originates predominantly in the abdominal or pelvic cavity, spreading along the peritoneum with organ involvement and secondary phenomena.3 Despite multimodality aggressive therapy, patient prognosis is unfavorable, with a median survival of less than 30 months. Only a few long-term survivors are reported.4,5
To our knowledge, no definitive standard recommendations exist concerning DSRCT treatment and patient management during follow-up.3 X-ray Computed Tomography (CT) remains the first choice imaging modality employed in patients affected by DSRCT. 18F-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET) is a functional imaging technique widely used in clinical oncology because of its high diagnostic accuracy.6 However, the role of 18F-FDG PET in the management of DSRCT is little described.
We report a rare case of a metastasized abdominal DSRCT detected in a 43-year old patient whose diagnostic and therapeutic strategies were influenced by the 18F-FDG PET-CT. The patient is still alive at the time of this report, ten years after the diagnosis.
Case Report
A 43-year old man with a history of right kidney cysts was referred to our Institution for further evaluation of a preaortic intraperitoneal mass incidentally detected during abdominal ultrasonography. Clinical examination and routine biological evaluation showed no remarkable findings. Media contrast enhancement (mce) CT of the abdomen and pelvis showed the presence of a vascularized, preaortic mass (7×5 cm) associated to retroperitoneal lymphadenopathy and mesenteric nodules, for which the diagnosis of malignancy was evoked. Chest mce-CT showed neither pathological lymphadenopathy nor lung parenchyma abnormalities. The serum level of common tumoral markers was normal. The results of 24 h urine analysis showed normal concentrations of dopamine, epinephrine and norepinephrine. A whole body (WB) bone scan showed no pathological foci of tracer uptake. An ultrasound guided needle biopsy of preaortic lesion was made for pathological evaluation. Microscopic examination revealed neoplastic proliferation of small cells with abundant and clear cytoplasm. The nuclei were slightly increased in size, oval, hyperchromatic, and with inconspicuous nucleoli. The tumor cells were surrounded by fibro-sclerotic stroma. The immunohistochemical studies revealed that the tumor cells were positive for antibodies against keratin, epithelial membrane antigen, vimentin, desmin and focally neuron specific enolase. Tumoral proliferation index, estimated with antibodies against Ki-67, was elevated (50%). Both the histological aspect and immunohistochemical profile lead to the diagnostic hypothesis of DSRCT. The molecular analysis found no specific gene translocation t(11;22)(p13;q12). In October 1999, the patient underwent surgery including tumoral radical resection, partial sigmoidectomy, and primitive iliac and periaortic lymphadectomy. Microscopic analysis of the excised tumor confirmed biopsy suspicion; thus metastatic DSRCT was retained as the final diagnosis.
Treatment was carried out according to the 99 EURO-EWING protocol.7 From December 1999 to April 2000 the patient received 6 cycles of VIDE combining intravenous (i.v.) vincristine (1.5 mg/m2 on day 1), ifosfamide (3000 mg/m2 on day 1–3), doxorubicin (20 mg/m2 on day 1–3) and etoposide (150 mg/m2 on day 1–3). The sequence was repeated every three weeks. No concomitant radiotherapy was performed. Morphological imaging (CT) performed after 4 and 6 cycles of treatment demonstrated complete remission. No other radiological lesion was described.
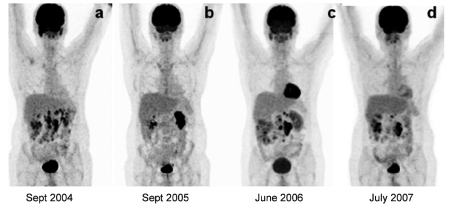
In September 2003, retroperitoneal lymphadenophathy was detected on CT study and biopsied. Microscopic examination revealed lymphatic DSRCT relapse. Radiological partial remission was obtained after 3 cycles of VIDE, thus 12 cycles i.v. of irinotecan (180 mg/m2 on day 1, every two weeks) were administered as a second-line chemotherapy. In an attempt to determine the response to therapy, the patient underwent whole-body (WB) 18F-FDG PET examination (Figure 1a). A combined PET-CT scanner was employed (Discovery ST, GE Medical System; Milwaukee, USA). PET-CT acquisitions started 60 min after tracer injection (5.5 MBq/kg), including a head to thighs CT followed by a 2D PET. CT, PET (attenuation corrected) and fusioned PET-CT images were displayed and visually interpreted. For quantitative analysis of 18F-FDG tumoral uptake, we use the maximum standardized uptake value (mSUV) per focus. In all of the PET studies performed, the mSUV was measured in the foci of highest 18F-FDG uptake within the tumor. Those values were then considered for tumor residual viability assessment. 18F-FDG PET-CT showed several retroperitoneal lymphadenopathy with pathological glucidic hypermetabolism, inducing modification of chemotherapeutic strategy. Three cycles of actinomicine (2.5 mg/m2 on day 1, every three weeks) were performed without any considerable effect on tumoral progression. Thereafter, the patient was treated by VIP 500, combining i.v. etoposide (100 mg/m2 on day 1–5), ifosfamide (1200 mg/m2 on day 1–5) and cisplatine (20 mg/m2 on day 1–5). The sequence was repeated every three weeks. WB 18F-FDG PET-CT study was performed after the completion of the first 2 of 6 cycles of VIP 500, to assess early response to therapy. A limited area of residual abnormal glucidic hypermetabolism within the reduced tumoral mass was recorded on PET-CT images. No other abnormal hypermetabolic spots suggestive of metastatic localizations were detected. The patient was considered to be a positive responder to treatment; thus chemotherapy was completed and followed by oral administration of etoposide 50 mg daily. A follow-up 18F-FDG PET-CT performed in September 2005 demonstrated complete regression of the tracer uptake abnormalities above retroperitoneal lymphadenopathy detected on previous examination and morphologically unchanged, so the treatment was stopped (Figure 1b). In December, the PET-CT study showed no active disease, and the patient was considered to be in remission.
Figure 1.
Whole-body maximum intensity projection 18F-FDG PET-CT images in 43-year old patient with DSRCT showing: (a) first relapse, (b) complete temporary remission after chemotherapy, (c) second relapse, (d) with moderate response to medical treatment.
In June 2006, a follow-up PET-CT study showed pathological 18F-FDG uptake within several retroperitoneal lymphadenopathy of one centimeter of maximal diameter, consistent with second tumoral relapse (Figure 1c). Thus, the patient was treated by oral etoposide 50 mg daily and corticoids 100 mg, day 1–5 monthly. Follow-up scintigraphic examination was performed in July 2007, demonstrating the persistence of hypermetabolic retroperitoneal lymphadenopathy (Figure 1d). The same chemotherapy was continued including oral etoposide 50 mg daily, 3–4 days weekly according to the hematologic results, associated to corticoids 100 mg, day 1–5 monthly. The patient is still alive at the time of this report, ten years after the diagnosis.
Discussion
DSRCT is a rare and highly aggressive malignancy of still unknown etiopathogenesis, first described in 1989 by Gerald and Rosai.8 In 1991, it was proposed as a distinct entity with well-recognized histological features, karyotype and immunochemical characteristics with coexpression of epithelial, mesenchymal and neural markers.1,4,9 DSRCT has a predilection for adolescent and young adult males, with a median age of 22 years.10 The majority of DSCRT occurs in the abdominal cavity, including retroperitoneum, pelvis, omentum and mesentery as primary origin site. Some cases had presentation sites in the scrotum, pleura, lung, mediastenum, chest wall, thigh, intracranial, soft tissue, sino-nasal regions, submental, parotid gland and kidney.11–19
DSCRT are characterized by multinodular growth on the serosal surface.11,20 These tumors have a tendency to spread along the peritoneum and mesothelial-lined surfaces with organ involvement and secondary phenomena.3 The most frequent sites of metastasis are the liver, lymph nodes, lung and bone marrow.3,9,21 This pattern of disease spread reveals the predilection of these tumors to metastasize through both the lymphatic and hematogenous routes.3 In our clinical observation, metastatic proliferation was essentially lymphatic, without soft tissue or bone involvement.
DSCRT are frequently characterized by a recurrent specific translocation t(11;22) (p13;q12), resulting in a fusion gene between Ewing's sarcoma gene and Wilm's tumor gene.5 Nevertheless, according to several reports,19,22 no specific gene translocation was found in our patient.
Because of the rarity of this tumor, only case reports and small series of patients are documented, thus no consensus exists concerning standard methods for its diagnosis, treatment and management.3
Although magnetic resonance imaging has been reported to be more useful than other radiological techniques in delineating the extent of the abdominal and pelvic disease,19 CT remains the radiological investigation of first choice. DSCRT appear as single, or multiple, lobulated soft tissue masses within the omentum, mesentery, perisplenic space, paravesical region, retroperitoneum and in the scrotum.4,21
The usefulness of 18F-FDG PET-CT in the management of patients affected by DSRCT is scarcely reported. Dimitrakopoulou-Strauss et al.,23 recently suggested 18F-FDG PET-CT as a valuable diagnostic tool for monitoring therapeutic effect in patients with metastasized DSRCT treated by inhibitors of mammalian target of rapamycin (mTOR). In the report of Rosoff et al.,24 18F-FDG PET-CT was explored with the aim of assessing the therapeutic effect of irinotecan in a patient with DSRCT showing residual mass, morphologically unchanged on CT images. PET-CT showed no evidence of active disease, especially above pelvic tumor, demonstrating complete treatment efficacy. 18 F-FDG PET accuracy is higher than that of CT in the assessment of a viable tumor after chemoradiation treatment, in particular in lymphomas.25 Moreover, the sensitivity of a sole morphological approach may be reduced in normal-sized metastatic lymph node detection, considering size as the principal diagnostic criteria for tumor involvement. Our results are in accordance with those found by Rosoff et al.,24 showing no pathological 18F-FDG uptake in PET images above stable lymphadenopathy on CT slices, after the completion of chemotherapy. The non-invasive nature of PET and its ability to detect early responses to therapy make it an ideal modality to evaluate the effectiveness of chemotherapy.
Prognosis of patients affected by DSRCT is extremely poor, with a median survival of less than 30 months and a rate of 3- and 5-year survival of 29% and 15%, respectively.5,21 The long survival observed in our patient is largely superior to the survival reported in the literature. 18F-FDG PET-CT played an important role in patient management, allowing detection of early recurrence of disease and consequent change in chemotherapeutic strategy. The relatively long disease free interval of 42 months may be due to complete tumoral resection followed by adjuvant chemotherapy.3,5 The invasive and disseminated nature of the disease makes radical resection difficult to achieve3,9 with consequent worse patient prognosis. Furthermore, chemotherapeutic agents are only temporarily effective in treating DSRCT and no consistent response to chemotherapy has been described.9 Patients who present extra-abdominal tumors that are amenable to complete surgical resection appear to have a better prognosis.19 In the series reported by Saab et al.,19 2 patients with extra-abdominal tumors had no evidence of disease at eight and ten years, respectively.
Conclusions
The optimal management for DSRCT remains to be determined. The usefulness of non-invasive metabolic imaging modality, such as 18F-FDG PET-CT, may improve the prognosis of DSRCT patients by detecting tumoral relapse earlier than conventional anatomic imaging. Additional prospective studies are needed to confirm these results, assessing the validity of a morpho-functional approach in DSRCT management.
References
- 1.Bellah R, Suzuki-Bordalo L, Brecher E, et al. Desmoplastic small round cell tumor in the abdomen and pelvis: report of CT findings in 11 affected children and young adults. Am J Roentgenol. 2005;184:1910–4. doi: 10.2214/ajr.184.6.01841910. [DOI] [PubMed] [Google Scholar]
- 2.Jellouli M, Mekki M, Krichene I, et al. Intra-abdominal desmoplastic small round cell tumor in childhood: case report and review of the literature. Bull Cancer. 2003;90:1063–6. [PubMed] [Google Scholar]
- 3.Hassan I, Shyyan R, Donohue JH, et al. Intraabdominal desmoplastic small round cell tumors. Cancer. 2005;104:1264–70. doi: 10.1002/cncr.21282. [DOI] [PubMed] [Google Scholar]
- 4.Hiralal Gamanagatti S, Thulkar S, Rao SK. Desmoplastic round cell tumour of the abdomen. Singapore Med J. 2007;48:19–21. [PubMed] [Google Scholar]
- 5.Mingo L, Seguel F, Rollan V. Intra-abdominal desmoplastic small round cell tumour. Pediatr Surg Int. 2005;21:279–81. doi: 10.1007/s00383-005-1401-1. [DOI] [PubMed] [Google Scholar]
- 6.Gambhir SS, Czernin J, Schwimmer J, et al. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- 7.Juergens C, Weston C, Lewis I, et al. Safety assessment of intensive induction with Vincristine, Ifosfamide, Doxorubicin, and Etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G 99 clinical trial. Pediatr Blood Cancer. 2006;47:22–9. doi: 10.1002/pbc.20820. [DOI] [PubMed] [Google Scholar]
- 8.Gerald WL, Rosai J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol. 1989;9:177–83. doi: 10.3109/15513818909022347. [DOI] [PubMed] [Google Scholar]
- 9.Chang CC, Hsu JT, Tseng J, et al. Combined resection and multi-agent adjuvant chemotherapy for desmoplastic small round cell tumor arising in the abdominal cavity: Report of a case. World J Gastro-enterol. 2006;12:800–3. doi: 10.3748/wjg.v12.i5.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battisti S, Renaudin K, Rigaud J, et al. Small round cell desmoplastic tumour presenting as a testicular mass. Prog Urol. 2005;15:333–6. [PubMed] [Google Scholar]
- 11.Su MC, Jeng YM, Chu YC. Desmoplastic small round cell tumor of the Kidney. Am J Surg Pathol. 2004;28:1379–83. doi: 10.1097/01.pas.0000128676.64288.0f. [DOI] [PubMed] [Google Scholar]
- 12.Cummings OW, Ulbright TM, Young RH, et al. Desmoplastic small round cell tumors of the paratesticular region. A report of six cases. Am J Surg Pathol. 1997;21:219–25. doi: 10.1097/00000478-199702000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Parkash V, Gerald WL, Parma A, et al. Desmoplastic small round cell tumor of the pleura. Am J Surg Pathol. 1995;19:659–65. doi: 10.1097/00000478-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Karavitakis EM, Moschovi M, Stefanaki K, et al. Desmoplastic small round cell tumor of the pleura. Pediatr Blood Cancer. 2007;49:335–8. doi: 10.1002/pbc.20762. [DOI] [PubMed] [Google Scholar]
- 15.Syed S, Haque AK, Hawkins HK, et al. Desmoplastic small round cell tumor of the lung. Arch Pathol Lab Med. 2002;126:1226–8. doi: 10.5858/2002-126-1226-DSRCTO. [DOI] [PubMed] [Google Scholar]
- 16.Biswas G, Laskar S, Banavali SD, et al. Desmoplastic small round cell tumor: extraabdominal and abdominal presentations and the results of treatment. Indian J Cancer. 2005;42:78–84. doi: 10.4103/0019-509x.16696. [DOI] [PubMed] [Google Scholar]
- 17.Adsay V, Cheng J, Athanasian E, et al. Primary desmoplastic small cell tumor of soft tissues and bone of the hand. Am J Surg Pathol. 1999;23:1408–13. doi: 10.1097/00000478-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Finke NM, Lae ME, Lloyd RV, et al. Sinosal desmoplastic small round cell tumor: A case report. Am J Surg Pathol. 2002;26:799–803. doi: 10.1097/00000478-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Saab R, Khoury JD, Krasin M, et al. Desmoplastic small round cell tumor in childhood: the St Jude Children's Hospital experience. Pediatr Blood Cancer. 2007;49:274–9. doi: 10.1002/pbc.20893. [DOI] [PubMed] [Google Scholar]
- 20.Granja NM, Begnami MD, Bortolan J, et al. Desmoplastic small round cell tumor: Cytological and immunocytochemical features. CytoJournal. 2005;2:6–6. doi: 10.1186/1742-6413-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Church DN, Bailey J, Hughes J, Williams CJ. Desmoplastic small round cell tumour: Obstetric and gynecological presentations. Gynecol Oncol. 2006;102:583–6. doi: 10.1016/j.ygyno.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Gil A, Gomez Portilla A, Brun EA, Sugar-baker PH. Clinical perspective on desmoplastic small round cell tumor. Oncology. 2004;67:231–42. doi: 10.1159/000081323. [DOI] [PubMed] [Google Scholar]
- 23.Dimitrakopoulou-Strauss A, Hohenberger P, Ströbel P, et al. A recent application of fluoro-18-deoxyglucose positron emission tomography, treatment monitoring with a mammalian target of rapamycin inhibitor: an example of a patient with a desmoplastic small round cell tumor. Hell J Nucl Med. 2007;10:77–9. [PubMed] [Google Scholar]
- 24.Rosoff PM, Bayliff S. Successful clinical response to Irinotecan in desmoplastic round blue cell tumor. Med Pediatr Oncol. 1999;33:500–3. doi: 10.1002/(sici)1096-911x(199911)33:5<500::aid-mpo12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Coleman M, Kostakoglu L. Early 18F-labeled fluoro-2-deoxy-D-glucose positron emission tomography scanning in the lymphoma. Cancer. 2006;107:1425–8. doi: 10.1002/cncr.22178. [DOI] [PubMed] [Google Scholar]