Abstract
Pulmonary surfactant is a lipid-protein complex that promotes alveolar stability by lowering the surface tension at the air-fluid interface in the peripheral air spaces. A group of hydrophobic surfactant-associated proteins has been shown to be essential for rapid surface film formation by surfactant phospholipids. We have purified a hydrophobic surfactant protein of approximately 5 kDa that we term SP5 from bronchopulmonary lavage fluid from a patient with alveolar proteinosis and shown that it promotes rapid surface film formation by simple mixtures of phospholipids. We have derived the full amino acid sequence of human SP5 from the nucleotide sequence of cDNAs identified with oligonucleotide probes based on the NH2-terminal sequence of SP5. SP5 isolated from surfactant is a fragment of a much larger precursor protein (21 kDa). The precursor contains an extremely hydrophobic region of 34 amino acids that comprises most of the mature SP5. This hydrophobicity explains the unusual solubility characteristics of SP5 and the fact that it is lipid-associated when isolated from lung.
Full text
PDF
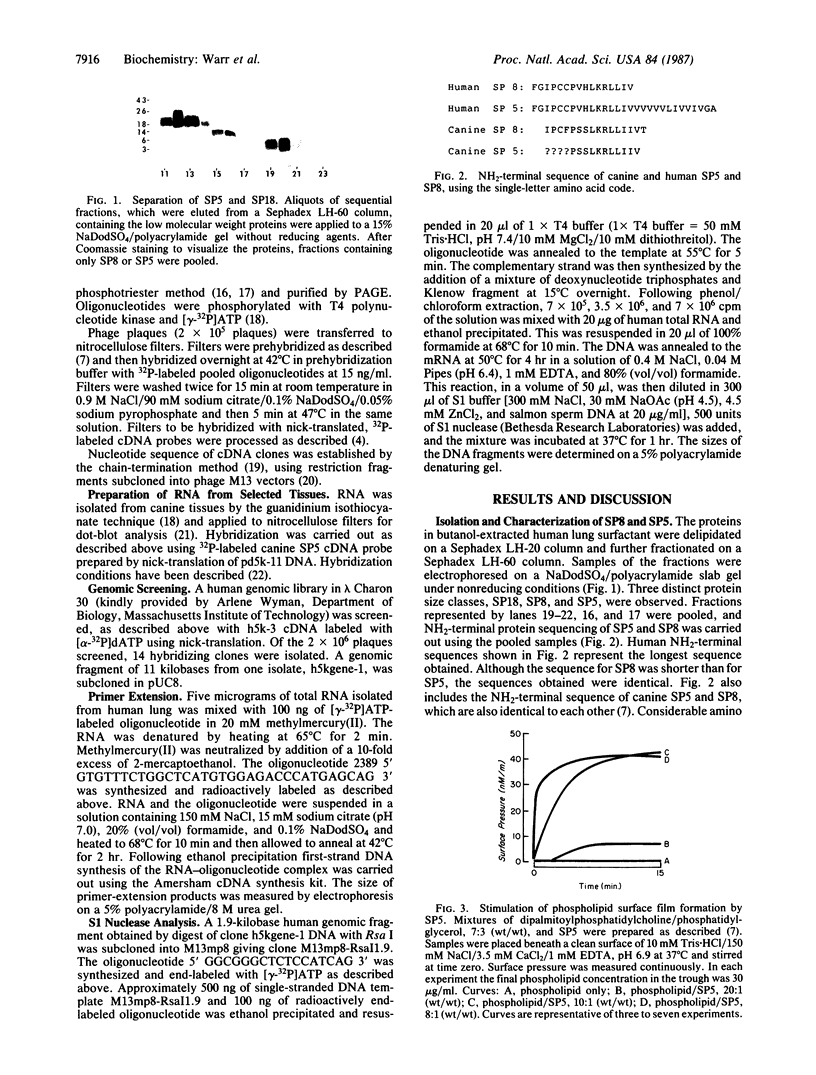

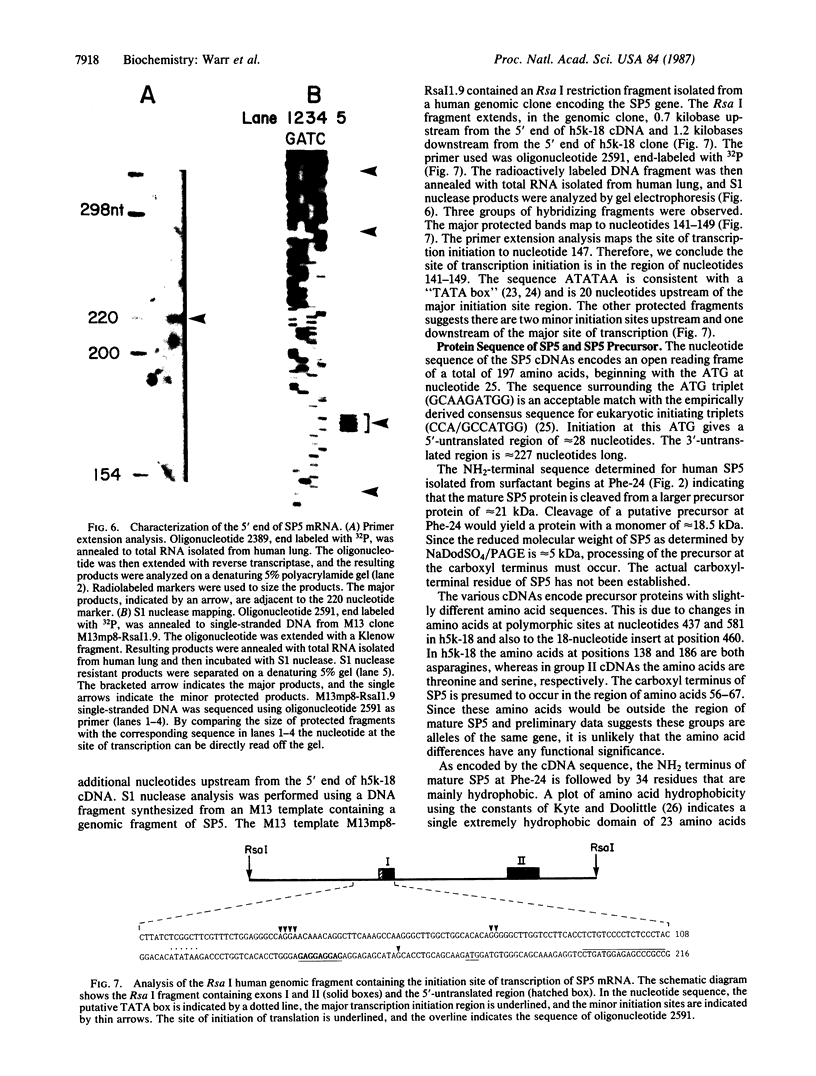

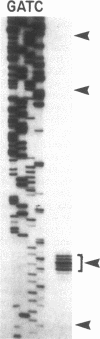
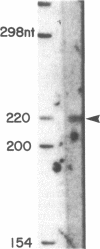
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard P. L., Hawgood S., Liley H., Wellenstein G., Gonzales L. W., Benson B., Cordell B., White R. T. Regulation of pulmonary surfactant apoprotein SP 28-36 gene in fetal human lung. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9527–9531. doi: 10.1073/pnas.83.24.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson B., Hawgood S., Schilling J., Clements J., Damm D., Cordell B., White R. T. Structure of canine pulmonary surfactant apoprotein: cDNA and complete amino acid sequence. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6379–6383. doi: 10.1073/pnas.82.19.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Wright J. R., Hawgood S., Gonzalez R., Venstrom K., Nellenbogen J. Pulmonary surfactant and its components inhibit secretion of phosphatidylcholine from cultured rat alveolar type II cells. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1010–1014. doi: 10.1073/pnas.84.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser S. W., Korfhagen T. R., Weaver T., Pilot-Matias T., Fox J. L., Whitsett J. A. cDNA and deduced amino acid sequence of human pulmonary surfactant-associated proteolipid SPL(Phe). Proc Natl Acad Sci U S A. 1987 Jun;84(12):4007–4011. doi: 10.1073/pnas.84.12.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nussinov R. Nucleotide quartets in the vicinity of eukaryotic transcriptional initiation sites: some DNA and chromatin structural implications. DNA. 1987 Feb;6(1):13–22. doi: 10.1089/dna.1987.6.13. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., Fujiwara T. Proteolipid in bovine lung surfactant: its role in surfactant function. Biochem Biophys Res Commun. 1986 Mar 13;135(2):527–532. doi: 10.1016/0006-291x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Takei T., Aiba T., Masuda K., Kiuchi A., Fujiwara T. Development of synthetic lung surfactants. J Lipid Res. 1986 May;27(5):475–485. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Golde L. M. Metabolism of phospholipids in the lung. Am Rev Respir Dis. 1976 Nov;114(5):977–1000. doi: 10.1164/arrd.1976.114.5.977. [DOI] [PubMed] [Google Scholar]
- White R. T., Damm D., Miller J., Spratt K., Schilling J., Hawgood S., Benson B., Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. 1985 Sep 26-Oct 2Nature. 317(6035):361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Ohning B. L., Ross G., Meuth J., Weaver T., Holm B. A., Shapiro D. L., Notter R. H. Hydrophobic surfactant-associated protein in whole lung surfactant and its importance for biophysical activity in lung surfactant extracts used for replacement therapy. Pediatr Res. 1986 May;20(5):460–467. doi: 10.1203/00006450-198605000-00016. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Wager R. E., Hawgood S., Dobbs L., Clements J. A. Surfactant apoprotein Mr = 26,000-36,000 enhances uptake of liposomes by type II cells. J Biol Chem. 1987 Feb 25;262(6):2888–2894. [PubMed] [Google Scholar]
- Yu S. H., Possmayer F. Reconstitution of surfactant activity by using the 6 kDa apoprotein associated with pulmonary surfactant. Biochem J. 1986 May 15;236(1):85–89. doi: 10.1042/bj2360085. [DOI] [PMC free article] [PubMed] [Google Scholar]