Abstract
Mixed tumors are relatively common in the skin and salivary glands, but extremely rare in soft tissues, often resulting in diagnostic problems. The occurrence of these tumors in the hand is especially limited. In this article we report the clinical, radiological, and histological features of a mixed tumor of the hypothenar region of the right hand.
Key words: mixed tumor, soft tissue, hand, immunohistochemistry.
Introduction
Mixed tumors of the soft tissues were recognized as a separate entity only recently, and there is a limited number of case reports to date. Mixed tumors, along with myoepitheliomas, which constitute myoepithelial tumors, are composed of myoepithelial cells predominantly and they are relatively common in major and minor salivary glands. Because fewer than 25 soft tissue mixed tumors have been reported so far, their characterization has been scarce.1,2 To our knowledge, there has not been a report of a mixed tumor of the soft tissue of the hand. In this report, we present a rare case of a soft tissue mixed tumor of the hand.
Case Report
A 79-year old woman presented with a slow-growing mass of the hypothenar region of her right hand with a history of several years. There was no particular incidence of trauma or any relevant medical history. The tumor measured 4×4 cm in size and the overlying skin was smooth and nonadherent with slight redness. The patient did not complain of any pain or tenderness. Physical examination of the cervical and axillary region showed no lymphadenopathy.
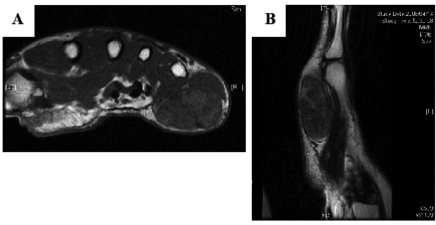
On plain radiography, there was soft tissue enlargement compatible with the physical examination, but there was no calcification or bone erosion. On magnetic resonance imaging (MRI), there was a 4×4×2 cm circumscribed mass in the subcutaneous region. T1- and T2-weighted images depicted a heterogeneous lesion with intermediate intensity, slightly higher than in the muscle (Figure 1A, B).
Figure 1.
(A) Axial T1-weighted magnetic resonance imaging scan revealing a 4×4×2 cm intermediate intensity mass in the subcutaneous region of the right hand. (B) Sagittal T2-weighted image showing a similar heterogeneous finding. The intensity was slightly higher than in the surrounding muscle.
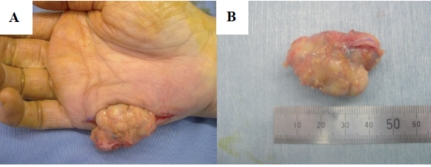
Incisional biopsy was performed followed by marginal resection. The tumor was well encapsulated with slight adhesion to the palmar aponeurosis (Figure 2 A, B).
Figure 2.
(A, B) A 4×4×2 cm soft tissue mixed tumor of the hypothenar region of the hand.
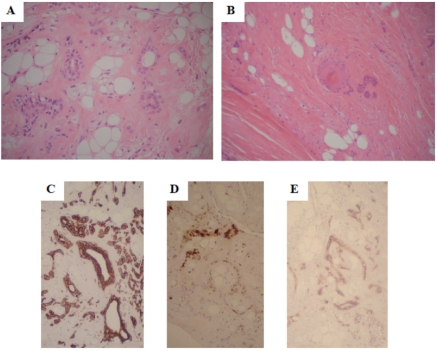
On histopathology, the tumor consisted of a circumscribed lesion with a yellowish-tan appearance grossly. Hematoxylin and eosin staining demonstrated a lobulated architecture, composed of epithelioid cells and myoepithelial elements in the chondromyxoid and collagenous stroma. There was adipocytic differentiation with evidence of ductal differentiation (Figure 3A, B). Nuclear atypia was mild, and although there were sporadic mitoses, atypical mitotic figures were not identified. Immunohistochemical examination demonstrated positivity for cytokeratin, S-100, and CD10. Cytokeratin and CD 10 were positive in both spindle and epithelioid cells. S-100 protein was focally positive in the spindle cells (Figure 3C, D, E).
Figure 3.
(A, B) Photomicrograph of the soft tissue mixed tumor, showing a lobulated architecture with epithelioid cells and myoepithelial elements in the chondromyxoid and collagenous stroma, and evidence of ductal differentiation (H&E stain). Immunohistochemical examination demonstrated positivity for cytokeratin (C) and CD10 (E) in both spindle and epithelioid cells, and S-100 protein in the spindle cells (D).
The postoperative period was uneventful. By her 18-month follow-up, no recurrence or metastasis had developed.
Discussion
Mixed tumors and myoepitheliomas are well characterized in salivary glands, but were recognized to occur in soft tissue only recently.2,3 Histological patterns are analogous to that of the salivary gland counterpart. The tumors show a spectrum of cellular and architectural morphologies. Myoepithelial cells may be spindled, plasmacytoid, ovoid, or epithelioid, and can express a wide variety of cytoplasmic filaments. The growth pattern may be solid, myxoid, or reticular, and each individual tumor includes areas with more than one growth pattern or cell subtype.4,5
Myoepithelial tumors of soft tissue occur equally in male and female patients. They have been reported in a wide age range with a peak in the third to fifth decades, most commonly occurring in the limbs and the limb girdles.2,5 The vast majority of cases arise in the subcutaneous or deep subfascial soft tissue. Most patients present with painless swelling ranging in duration from a few weeks to several years.5 MRI appearance of soft tissue mixed tumors varies in accordance with the heterogeneity of the tumor. Depending on the amount of hemorrhage, chondromyxoid and fibrous stroma, it is possible for the tumor to present various findings. Preoperative diagnosis based on the MRI should be performed prudently, and incisional biopsy should be performed at all times.
There is a debate still as to whether mixed tumors and myoepithelioma should be distinguished as separate entities or considered as the same spectrum of tumors with overlapping histological appearances and similar clinical behavior. Those tumors either lacking or with very limited ductal differentiation generally are classified as myoepitheliomas. Current classification simply separates those tumors with ductal differentiation into the mixed tumor categories.6,7 Whereas some investigators allow up to five percent or ten percent ductal differentiation in myoepitheliomas,4,8–10 others classify tumors with any ducts as mixed tumors. In the present case, the tumor showed distinct duct formations; therefore we diagnosed it in accordance with the strict criteria.
The absence of clear-cut histopathological clues for the diagnosis of myoepithelial tumors is hampered further by the wide variability in their immunohistochemical characteristics. Immunoreactivity for the S-100 protein and muscle actins seems to be the most constant immunophenotype, whereas immunoexpression for epithelial markers such as cytokeratins and EMA, or neural markers such as GFAP, is somewhat erratic and variable from case to case. The same immunophenotype has been described in salivary gland myoepithelial tumors, which usually coexpresses immunore-activity for S-100 protein, muscle actins, and GFAP, with variable immunoexpression for EMA and cytokeratins.8,11–16
Although the majority of morphologically benign-looking mixed tumors of soft tissue behave in a benign fashion, there is approximately a twenty percent risk for local recurrence. The malignant potency of myoepithelial tumors varies, and it is difficult to differentiate myoepithelial tumors into benign and malignant categories on histological grounds only.17,18 For the diagnosis of malignant myoepithelioma in salivary glands, an invasive growth pattern has been considered as the most important feature, because the immunohistochemical features are similar for the benign and malignant forms.18 In addition, cytological atypia and mitotic rate have been reported to be useful.19,20 However, in the case of soft tissue myoepithelial tumors, there has been no association between the degree of nuclear pleomorphism or mitotic activity and clinical behavior.21 Recurrent chromosome rearrangements, particularly reciprocal translocations, with breakpoints on 8q12, 3p21, and 12q14–15 have been described in myoepithelial tumors of the salivary gland.22 It is interesting to note that the malignant myoepithelial tumor has been reported to show different chromosomal abnormalities such as gains of 1p31∼p34, 1q21∼q23, and 16q22, and loss of 15q.23 Nevertheless, it is difficult to establish prognostic indicators for soft tissue myoepithelial tumors until further reports are available.
Because there is considerable morphologic heterogeneity of soft tissue myoepithelial tumors, the differential diagnosis should be based on the dominant histological pattern. If the tumor displays a reticular architecture with chondromyxoid or hyalinized stroma, extraskeletal myxoid chondrosarcoma and ossifying fibromyxoid tumor should be considered as differential diagnoses. Solid spindle cell myoepithelial tumors can resemble leiomyomas and schwannomas. Furthermore, if the tumor shows significant cytological atypia, metastatic carcinomas, metastatic melanomas, and epithelioid sarcomas should be considered as well.
The mixed tumor should be considered as one of the differential diagnoses of soft tissue tumors of the hand. The rarity of this tumor has not enabled prediction of the possible outcome after resection. Because there are reports of local recurrence and malignant transformation, complete resection with appropriate follow-up of the patients should be warranted.
References
- 1.Klijanienko J, Servois V, Jammet P, et al. Pleomorphic adenoma. Am J Surg Pathol. 1998;22:772–3. doi: 10.1097/00000478-199806000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183–96. doi: 10.1097/00000478-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Go JH. A case of soft tissue myoepithelial tumor arising in masticator space. Yonsei Med J. 2005;46:710–4. doi: 10.3349/ymj.2005.46.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson RHW, Jones H, Beasley P. Benign myoepithelioma of the salivary glands: a true entity? Histopathology. 1995;27:1–9. doi: 10.1111/j.1365-2559.1995.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick SE, Limon J. World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. Mixed tumour/ myoepithelioma/parachordoma; pp. 198–9. [Google Scholar]
- 6.Ellis GL, Auclair PL. Atlas of Tumor Pathology: Tumors of the Salivary Glands, 3rd series, fascicle 17. Washington, DC: Armed Forces Institute of Pathology; 1996. [Google Scholar]
- 7.Seifert G, Sobin LH. World Health Organization International Histological Classification of Tumours: Histological Typing of Salivary Gland Tumours. Berlin, Germany: Springer-Verlag; 1991. [Google Scholar]
- 8.Alos L, Cardesa A, Bombi JA, et al. Myoepithelial tumors of salivary glands: a clinicopathologic, immunohistochemical, ultrastructural, and flow-cytometric study. Semin Diagn Pathol. 1996;13:138–47. [PubMed] [Google Scholar]
- 9.Barnes L, Appel BN, Perez H, et al. Myoepitheliomas of the head and neck: case report and review. J Surg Oncol. 1985;28:21–8. doi: 10.1002/jso.2930280107. [DOI] [PubMed] [Google Scholar]
- 10.Dardick I. Myoepithelioma: definitions and diagnostic criteria. Ultrastruct Pathol. 1995;19:335–45. doi: 10.3109/01913129509021906. [DOI] [PubMed] [Google Scholar]
- 11.Anderson C, Knibbs DR, Abbott SJ, et al. Glial fibrillary acidic protein expression in pleomorphic adenoma of salivary gland: an immunoelectron microscopic study. Ultrastruct Pathol. 1990;14:263–71. doi: 10.3109/01913129009076130. [DOI] [PubMed] [Google Scholar]
- 12.Dardick I, Cavell S, Boivin M, et al. Salivary gland myoepithelioma variants: histological, ultrastructural, and immunocytological features. Virchows Arch A Pathol Anat Histopathol. 1989;416:25–42. doi: 10.1007/BF01606467. [DOI] [PubMed] [Google Scholar]
- 13.Domagala W, Halczy-Kowalik L, Weber K, et al. Coexpression of glial fibrillary acid protein, keratin and vimentin: a unique feature useful in the diagnosis of pleomorphic adenoma of the salivary gland in fine needle aspiration biopsy smears. Acta Cytol. 1988;32:403–8. [PubMed] [Google Scholar]
- 14.Gupta RK, Naran S, Dowle C, et al. Co-expression of vimentin, cytokeratin and S-100 in monomorphic adenoma of salivary gland: value of marker studies in the differential diagnosis of salivary gland tumours. Cytopathology. 1992;3:303–9. doi: 10.1111/j.1365-2303.1992.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura T, Furukawa M, Kawahara E, et al. Differential diagnosis of pleomorphic adenoma by immunohistochemical means. J Laryngol Otol. 1991;105:1057–60. doi: 10.1017/s0022215100118183. [DOI] [PubMed] [Google Scholar]
- 16.Wittchow R, Landas SK. Glial fibrillary acidic protein expression in pleomorphic adenoma, chordoma, and astrocytoma: A comparison of the three antibodies. Arch Pathol Lab Med. 1991;115:1030–3. [PubMed] [Google Scholar]
- 17.Bombi JA, Alos L, Rey MJ, et al. Myoepithelial carcinoma arising in a benign myoepithelioma: Immunohisto-chemical, ultrastructural, and flow-cytometrical study. Ultrastruct Pathol. 1996;20:145–54. doi: 10.3109/01913129609016309. [DOI] [PubMed] [Google Scholar]
- 18.Palma SD, Guzzo M. Malignant myoepithelioma of salivary glands: clinicopathological features of ten cases. Virchows Archiv A Pathol Anat Histopathol. 1993;423:389–96. doi: 10.1007/BF01607152. [DOI] [PubMed] [Google Scholar]
- 19.Nagao T, Sugano I, Ishida Y, et al. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998;83:1292–9. doi: 10.1002/(sici)1097-0142(19981001)83:7<1292::aid-cncr4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761–74. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Tanimoto A, Sasaguri T, Arima T, et al. Endometrial stromal sarcoma of the uterus with rhabdoid features. Pathol Int. 1996;46:231–7. doi: 10.1111/j.1440-1827.1996.tb03604.x. [DOI] [PubMed] [Google Scholar]
- 22.Balogh Z, Deák L, Sápi Z. Malignant myoepithelioma of soft tissue: a case report with cytogenetic findings. Cancer Genet Cytogenet. 2008;183:121–4. doi: 10.1016/j.cancergencyto.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Bullerdiek J, Wobst G, Meyer-Bolte K, et al. Cytogenetic subtyping of 220 salivary gland pleomorphic adenomas: correlation to occurrence, histological subtype, and in vitro cellular behavior. Cancer Genet Cytogenet. 1993;65:27–31. doi: 10.1016/0165-4608(93)90054-p. [DOI] [PubMed] [Google Scholar]