Abstract
Rosai-Dorfman disease (RDD) was formerly known as “sinus histiocytosis with massive lymphadenopathy”, and cases involving the gastrointestinal tract are rare. We present a case of pure extranodal RDD, resected as a polypoid lesion in colonoscopic study. The patient was a 62-year old woman with a history of sigmoidectomy for unexplained peritonitis. Microscopic study of the polypoid lesion showed the submucosal mass with histological and immunological features of RDD. The whole body computed tomography revealed neither lymphadenopathy nor tumor-like mass.
Key words: colon, polypoid lesion, Rosai-Dorfman disease
Introduction
Rosai-Dorfman disease (RDD) is a rare disease defined as a unique histiocytotic proliferative disorder.1 It typically presents as massive, painless bilateral cervical lymphadenopathy in young adults. Extranodal involvement occurs in up to 40% of patients. The skin,2 bone, and head and neck region, and upper respiratory tract3 are commonly involved as extra nodal sites. Involvement of the gastrointestinal (GI) tract is quite rare.
We report a case with a small polypoid lesion found incidentally at colonoscopy in the descending colon.
Clinical summary
The case was a 62-year old woman with previous history of sigmoidectomy for peritonitis of unknown cause three years ago. She complained of lower abdominal pain and came to our hospital. Colonoscopy showed a narrowed sigmoid colon with intact mucosa and abdominal computed tomography revealed adhesive change of sigmoid colon, uterus and right ovary. The pathological diagnosis was acute suppurative inflammation of peritoneum and subserosal tissue of the colon. There were bacterial colonies and the infiltration of neutrophils or histiocytes with fibrosis. There was no significant finding for diverticulitis or gynecological infection.
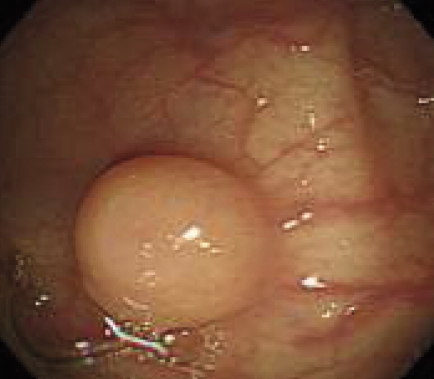
Follow-up colonoscopy showed a normal mucosal colored polypoid lesion of 5 mm in diameter in the descending colon (Figure 1). A snare biopsy of the lesion was performed. Histopathological diagnosis was RDD. To clarify the existence of another lesion, whole body computed tomography was performed. However, there was no remarkable lymphadenopathy and no tumor-like lesion (data not shown). Blood tests showed no inflammatory change. At a post-polypectomy follow-up at nine months, no recurrence was found.
Figure 1.
Endoscopic image of the descending colon showing a normal mucosal colored polypoid lesion.
Pathological findings
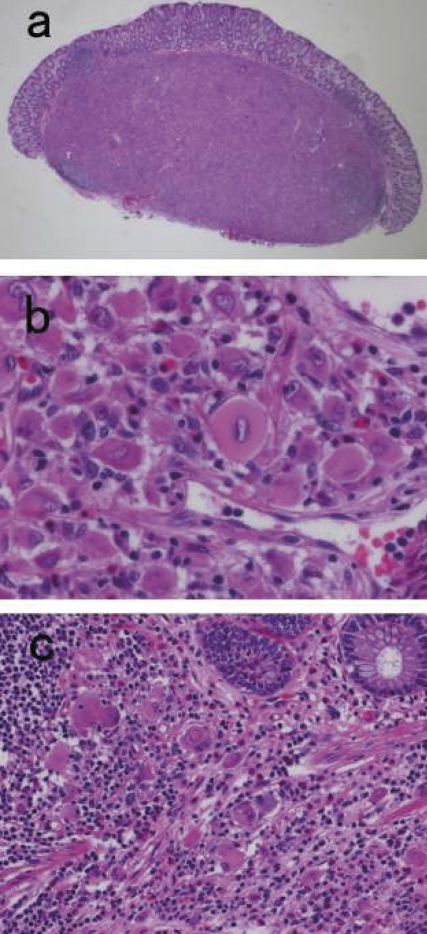
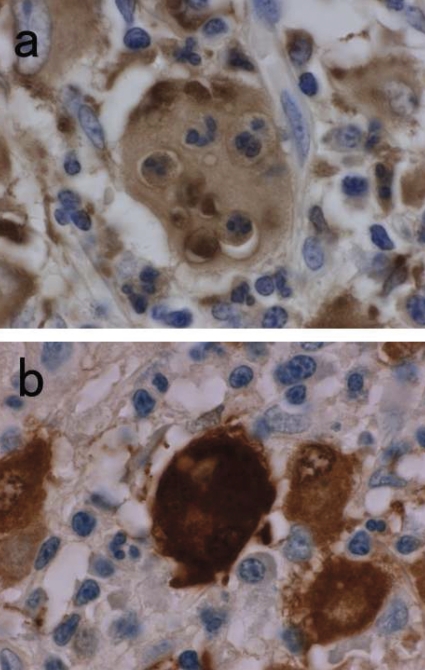
The polypoid lesion was the sharply-marginated mass centered in the submucosa and was covered by an intact mucosa (Figure 2a). No hemorrhage or necrosis was identified. Microscopic examination revealed sheets of large histiocytes with abundant eosinophilic cytoplasm and vesicular nuclei (Figure 2b). The infiltration of small lymphocytes and plasma cells was presented and was focally dominant, and the amount of fibrosis was minimal. The scant infiltration of histiocytes extended from the submucosa into the lamina propria (Figure 2c). Immunohistochemical studies revealed that the histiocytes were positive for CD68, S-100 protein and vimentin, but no staining for CD1a, α-smooth muscle actin. Emperipolesis with engulfment of intact neutrophils within the cytoplasm of histiocytes was demonstrated with the immunohistochemical stains for CD68 or S-100 (Figure 3a and b). The morphological and immunohistochemical findings were consistent with extranodal RDD. Review of the slides from former peritoneal and sigmoid colon inflammation demonstrated no significant feature of RDD.
Figure 2.
(a) Low magnification view of HE section. The lesion located at the submucosa formed a well-defined round shaped mass (×10). (b) Section of mass exhibiting sheets of large histiocytes with lymphocytes and plasma cells infiltration (×500). (c) Partial infiltration of the histiocytes into the lamina propria was presented. (×250).
Figure 3.
Immunohistochemical study for CD68 (a) and S-100 (b) (×1000). The large histiocytes were showing positive stains for CD68 and S-100. High magnification stains of CD68 or S-100 indicating positive histiocytes with emperipolesis of neutrophils.
Discussion
In 1969, Rosai and Dorfman first established sinus histiocytosis with massive lymphadenopathy (SHML or RDD) as a clinicopathological entry.1 It typically presents with slowly progressive painless massive cervical lymphadenopathy in young adults and resolves spontaneously. Simultaneous involvement of multiple organs is not unusual and is associated with poor prognosis.
The digestive tract is the least affected organ system with RDD and only 9 cases of GI tract involvement have been reported4–8 (Table 1). Including our case, 7 cases of RDD involving GI tract occurred in elderly (over 50 years of age) subjects. Four cases were incidental findings at colonoscopy, at autopsy, or in an appendectomy specimen. The others had symptoms of abdominal mass for extensive growth, abdominal pain, hematochezia. Five cases had other extra nodal involvement. The sites of predilection of RDD were located at the distal portion of the GI tract. Only 2 cases were reported from endoscopic findings.7,8 In these reports, the lesion was described as several or numerous submucosal masses protruding into the colorectal lumen. Nathwani evaluated the lesion with endoscopic ultrasonography and demonstrated well-demarcated hypoechoic submucosal mass with intact muscularis propria and peri-colonic lymph node swelling.8
Table 1. Cases of gastrointestinal Rosai-Dorfman disease.
| Reference | Race | Age and sex | Site | Presentation | Other extranodal Involvement | Outcome |
|---|---|---|---|---|---|---|
| 4 | W | 13M | Jejunum | Incidental(autopsy) | Bone | DOD |
| 4 | W | 7F | Appendix | Abdominal pain | Eye | AWD, 11y |
| 4 | W | 53F | Colon | Colonic mass | Peritoneal cavity | AWD, 17m |
| 4 | B | 4M | Appendix | RLQ mass | None | AWD, 12y |
| 4 | B | 56F | Rectum | Colonoscopy | None | LFU |
| 5 | N/A | 51F | Rectum | Hematochezia | None | AWD, 15m |
| 6 | B | 60F | Rectum | Hematochezia | Abdominal wall | AWD, 4.5y |
| 7 | W | 79M | S-Colon | Abdominal pain | N/A (relapsed in multiple sites) | DOD, 2y |
| 8 | B | 50F | Rectum | Hematochezia | None | LFU |
| * | EA | 62F | D-colon | Colonoscopy | None | LFU |
Abbreviation: W, White; B, Black; EA, E. Asian; N/A, Not Available; AWD, alive with disease; DOD, dead of disease; LFU, last follow-up.
Current case.
Surgical treatment for RDD of GI tract was undergone in 8 cases. In one case, a local excision for a 4 cm rectal mass was performed.6 In 2 cases, including our own, polypectomy was performed, and in others colorectal resection or appendectomy were performed. As for the clinical course, 2 cases died of disease, 5 cases were alive with disease (range 1.2–11 years), and another 3 cases were being monitored
The morphological and immunophenotypic features among reactive and neoplastic proliferation make diagnosis of RDD problematic. The diagnosis of RDD includes metastatic carcinoma and melanoma, Hodgkin’s lymphoma, malignant histiocytic neoplasm, and various inflammatory or infectious changes. However, a definite diagnosis of an extranodal RDD with inflammatory cells and histiocytes can be made with demonstration of emperipolesis by the histiocytes with immunohistochemical reaction for S-100 and CD68. The distinction between RDD and Langerhans’ cell histiocytosis has also been clarified by the features of the immunoreactivity for CD1a and S-100. RDD is positive for S-100, but negative for CD1a.
The cause of RDD is still unclear. It has been suggested that it expresses immune dysfunction. The histiocytes in RDD express numerous markers including S-100 protein, panmacrophage antigens, antigens associated with phagocytosis. Nguyen demonstrated that histiocytes in RDD express CD163, a hemoglobin scavenger receptor and acute phase-regulated transmembrane protein found on tissue macrophages and monocytes.9 This finding supports the hypothesis that the dominant cell in RDD is activated by macrophages and is distinct from Langerhans’ cells and dendritic cells. Some GI tract RDD cases showed the histopathological features of stromal fibrosis.
In cutaneous RDD, the presence of abundant IgG4-positive plasma cells and stromal fibrosis was suggested to be a link to IgG4-related sclerosing disease.10 Viral infection, such as human herpes virus-6 and parvovirus B19, has also been suggested to play a role in its pathogenesis.11,12
Some previous reports described RDD by the presence of “foamy, pale or clear cytoplasmic” histiocytes with emperipolesis.4–7 To consider differential diagnosis of colorectal polypoid lesion due to infiltration of histiocytes, we note xanthoma. Xanthoma is composed of foamy histiocytes, immunohistchemically expressed CD68. GI xanthoma occurs commonly in the stomach. Xanthoma of the colon and rectum is rarely reported. Nakasono reported 25 cases of rectosigmoid xanthomata with polypoid lesion.13 The histopathological features of xanthoma compared to RDD were that histiocytes were presented in the lamina propria and not presented in the submucosa. The majority of the histiocytes contained neither cell debris nor pigments in the cytoplasm. Lymphocyte and plasma cell infiltration was minimal to mild.
The preferable treatment for RDD has not yet been defined. Patients with RDD that does not involve or compress major organs are followed-up with observation. Surgical resection or radiation therapy may be used in cases in which major organs are involved. In some cases, the therapeutic advantage of oral prednisolone compared to harmful antineoplastic therapy has been reported.14,15 In our case, an unexplained peritonitis with no meaningful infiltration of histiocytes might be antecedent pathology. There was no significant inflammatory change when colonic RDD was diagnosed and no additional treatment has been given so far. Therefore, continuous monitoring will be needed.
Acknowledgments
Acknowledgments: we would like to thank Ms. Michiko Takagi, Fujioka General Hospital for her skillful editorial assistance.
References
- 1.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entry. Arch Pathol. 1969;87:63–70. [PubMed] [Google Scholar]
- 2.Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281–90. doi: 10.2310/7750.2006.00067. [DOI] [PubMed] [Google Scholar]
- 3.Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19–73. [PubMed] [Google Scholar]
- 4.Lauwers GY, Perez-Atayde A, Dorfman RF, Rosai J, et al. The digestive system manifestations of Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy): review of 11 cases. Hum Pathol. 2000;31:380–5. doi: 10.1016/s0046-8177(00)80254-3. [DOI] [PubMed] [Google Scholar]
- 5.Anders RA, Keith JN, Hart J. Rosai-Dorfman disease presenting in the gastrointestinal tract. Arch Pathol Lab Med. 2003;127:E74–5. doi: 10.5858/2003-127-e74-RDDPIT. [DOI] [PubMed] [Google Scholar]
- 6.Long E, Lassalle S, Cheikh-Rouhou R, et al. Intestinal occlusion caused by Rosai-Dorfman disease mimicking colonic diverticulitis. Pathol Res Pract. 2007;203:233–7. doi: 10.1016/j.prp.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Alatassi H, Ray MB, Galandiuk S, Sahoo S. Rosai-Dorfman disease of the gastrointestinal tract: report of a case and review of the literature. Int J Surg Pathol. 2006;14:95–9. doi: 10.1177/106689690601400119. [DOI] [PubMed] [Google Scholar]
- 8.Nathwani RA, Kenyon L, Kowalski T. Rosai-Dorfman disease of the colon. Gastrointest Endosc. 2008;68:194–6. doi: 10.1016/j.gie.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TT, Schwartz EJ, West RB, et al. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol. 2005;29:617–24. doi: 10.1097/01.pas.0000157940.80538.ec. [DOI] [PubMed] [Google Scholar]
- 10.Kuo TT, Chen TC, Lee LY, Lu PH. IgG4-positive plasma cells in cutaneous Rosai-Dorfman disease: an additional immunohistochemical feature and possible relationship to IgG4-related sclerosing disease. J Cutan Pathol. 2009;36:1069–73. doi: 10.1111/j.1600-0560.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- 11.Levine PH, Jahan N, Murari P, et al. Detection of human herpesvirus 6 in tissues involved by sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) J Infect Dis. 1992;166:291–5. doi: 10.1093/infdis/166.2.291. [DOI] [PubMed] [Google Scholar]
- 12.Mehraein Y, Wagner M, Remberger K, et al. Parvovirus B19 detected in Rosai-Dorfman disease in nodal and extranodal manifestations. J Clin Pathol. 2006;59:1320–6. doi: 10.1136/jcp.2005.029850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakasono M, Hirokawa M, Muguruma N, et al. Colorectal xanthomas with polypoid lesion: report of 25 cases. APMIS. 2004;112:3–10. doi: 10.1111/j.1600-0463.2004.apm1120102.x. [DOI] [PubMed] [Google Scholar]
- 14.Antonius JI, Farid SM, Baez-Giangreco A. Steroid-responsive Rosai-Dorfman disease. Pediatr Hematol Oncol. 1996;13:563–70. doi: 10.3109/08880019609030873. [DOI] [PubMed] [Google Scholar]
- 15.Ocheni S, Ibegbulam OG, Okafor OC, et al. Usefulness of oral corticosteroid in Rosai-Dorfman disease. Eur J Cancer Care (Engl) 2007;16:286–8. doi: 10.1111/j.1365-2354.2006.00746.x. [DOI] [PubMed] [Google Scholar]