Abstract
Langerhans cell histiocytosis is a rare group of proliferative disorders. Beside cutaneous involvement, other internal organs can be affected. The treatment of cutaneous lesions is difficult and relies on topical corticosteroids, carmustine, nitrogen mustard, and photochemotherapy. Systemic steroids and vinblastine are used for recalcitrant skin lesions. However, some cases fail to respond. An 18-month old boy presented a CD1a+, S100a+ Langerhans cell histocytosis with cutaneous and severe scalp involvement. Topical corticosteroids and nitrogen mustard failed to improve the skin lesions. Systemic corticosteroids and vinblastine improved the truncal involvement but had no effect on the scalp lesions. Methylaminolevulinate (MAL) based photodynamic therapy (PDT) resulted in a significant regression of the scalp lesions. Control histology revealed an almost complete clearance of the tumor infiltrate. Clinical follow-up after six months showed no recurrence.
Although spontaneous regression of cutaneous Langerhans cell histiocytosis is observed, the rapid effect of photodynamic therapy after several failures of other treatment suggests that photodynamic therapy was successful. As far as we know this is the first report of photodynamic therapy for refractory skin lesions. Larger series are needed to determine whether photodynamic therapy deserves a place in the treatment of multiresistant cutaneous Langerhans cell histiocytosis.
Key words: Langerhans cell histiocytosis, photodynamic therapy, childhood
Introduction
Langerhans cell histiocytosis (LCH) is a rare group of proliferative disorders. The prevalence is estimated between 1 and 2 per 1,000,000. The male:female ratio is 2:1. Usually LCH affects young children, particularly between the ages of one and three years. LCH presents an oligoclonal proliferation of Langerhans cells (LC). The etiology has not been elucidated. LCH affects the skin in 40% of the cases, but almost any other internal organ may be involved.1 The clinical expression and the prognosis principally depend on the clinical type and the number of organs affected.2 First-line treatment for the cutaneous lesions relies on topical corticosteroids. Topical chemotherapy (nitrogen mustard, carmustine) or PUVA-therapy are interesting second-line options. Systemic chemotherapy is reserved for severe and refractory cutaneous involvement.
Notwithstanding, some cases fail to respond and require alternative therapy. Photodynamic therapy (PDT) is an FDA-recognized treatment option for superficial basal cell carcinoma, Bowen's disease and actinic keratosis.3–6 The preferential accumulation of the photosensitizing agents in hypermetabolic cells (i.e. precancerous and cancerous cells) explains the selectiveness of PDT. The spectrum of other cutaneous oncological conditions effectively treated by PDT is continuously increasing.7 The presence of an oligoclonal proliferation of LCs in LCH was the rationale for PDT treatment. This paper reports the first successful PDT treatment of multi-resistant scalp Langerhans cell histiocytosis in a young boy.
Case Report
An 18-month old boy visited the dermatology department for erythematous and squamous lesions of the trunk and the scalp. The lesions appeared at the age of eight months and progressively spread over the trunk and scalp. A pediatrician proposed a diagnosis of atopic dermatitis. The child was otherwise healthy, did not take any medication and presented a normal development. There were no signs of growth retardation. There was no particular family medical history.
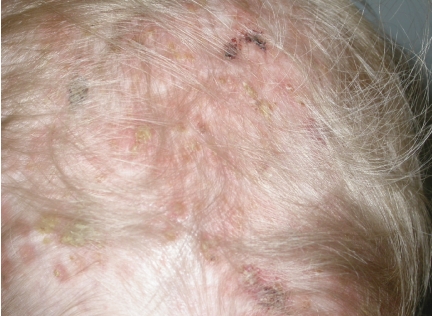
Skin examination revealed infiltrated, sometimes purpuric, papular and slightly squamous, ill-defined lesions on the trunk. These lesions were neither pruritic nor painful. Severe inflammatory and crusting lesions were evidenced on the scalp and behind the ears (Figure 1). These lesions were painful and itchy, and the child used to scratch them. Further clinical examination was unremarkable. No adenopathies were evidenced. A cutaneous biopsy was performed under local anesthesia. Histology suggested LCH. Immunohistochemical staining revealed a CD1a+, S100a+ infiltrate with a proliferative KI67 fraction of 15%, confirming the diagnosis of LCH. Electron microscopy to ascertain the presence of Birbeck granules was not performed. An extensive internal workup, including ultrasound, blood sampling, chest radiography and chest CT scan, was normal. The final diagnosis was a cutaneous LCH (multifocal single organ disease), according to the current classification of histiocytic disorders.2 The clinical score for disease activity was 2 (clinical findings: 2, laboratory evaluation: 0, radiological studies:0).2
Figure 1.
Langerhans cell histiocytosis involving the scalp.
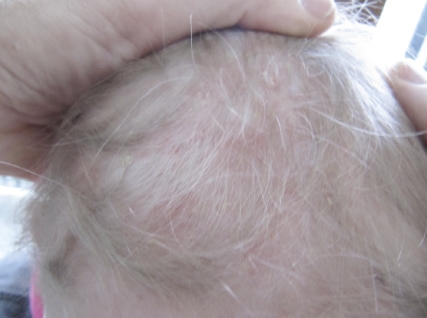
No regression of the skin lesions was observed following topical applications of very potent corticosteroids (clobetasol propionate 0.05% cream, Dermovate©, GSK), but the itching sensations were partially relieved. Subsequent treatment with topical nitrogen mustard (10 mg/50 mL H20, 3 times/week, for three weeks (Mustargen©)) also failed to improve the skin lesions. PUVA therapy was not performed, as efficacy on the hairy scalp is limited. Systemic corticosteroids (40 mg/m2/day) also failed. Finally, following systemic vinblastine (Velbe©), a partial regression of the truncal lesions was observed but the aspect of the scalp lesions did not improve. During the second chemotherapy course, fever and neutropenia with a bilateral interstitial pneumopathy suddenly appeared, necessitating interruption of treatment. The pulmonary lesions resolved and four months later, a subsequent treatment combining vinblastine and corticosteroids (once every two weeks for six months) was started. The truncal lesions progressively disappeared but the scalp and ear lesions resisted. A new skin biopsy of the scalp was performed, revealing S-100a+ and CD1a+ Langerhans cell histiocytosis. The KI67 growth fraction was 5%. Finally, a methyl-aminolevulinate (MAL)-based photodynamic therapy was performed under total anesthesia. Previously, a cream containing 15% urea was applied once daily for 15 days to diminish the hyperkeratotic character of the scalp lesions. MAL (Metvix© cream 5%, Galderma) was applied under occlusion, using a plastic film, for three hours. Red light LED illumination was performed using an Aktilite© light source (634 nm, 74 J/cm2) for eight minutes. Post-treatment crusting was limited using an antibiotic ointment (mupiro cine, Bactroban©) twice daily for one week. The child did not complain of any pain or itching after the treatment. No painkillers were required. Two weeks after PDT treatment, a significant reduction of the inflammation and crusting was noted. Four weeks later, there was an almost complete healing (Figure 2). A control biopsy revealed a complete histological clearing and only some residual CD1a+ LC on immunohistological examination. After a follow-up of six months the scalp was still recurrence free.
Figure 2.
Aspect of the scalp one month after PDT.
Discussion
Langerhans cell histiocytosis is rarely encountered and encompasses a group of disorders characterized by the proliferation and infiltration of Langerhans cells, potentially affecting almost every organ.2,8 The diagnosis of cutaneous LCH is suggested by histopathology and confirmed by a positive immunohistochemical signal for the CD1a, S100a and CD207 (Langerin) antigens.1,9 Electron microscopy evidences the pathognomical intracytoplasmic Birbeck granules, but is not routinely performed.1,9 The skin lesions are polymorphous, but macular, papular and vesicular elements covered by slight brown crusts are characteristic clinical features.9,10 The usual localizations include the face, the front and the scalp, the trunk and the large folds. Bone disease usually affects one single bone with pain, swelling, deformation, fracture, etc. Cranial, mandibular, vertebral, and the pelvic bones are the most frequently affected. Lung disease typically presents as a reticulo-micronodular syndrome with dyspnea, a dry cough and fever. Hematologic disease is polymorphous and may evolve to pancytopenia. Other signs, such as diabetes insipidia, hepatosple-nomegalia, sclerosing cholangitis, median otitis may be observed.
Treatment is directed towards decreasing the activity and proliferation of the histiocytes and to relieve symptoms such as itching and stinging, as well as to improve the esthetic aspects and quality of life.11 As the prognosis of isolated cutaneous involvement is favorable and as auto-resolution may be expected, it is recommended to start with the simplest treatment and progress to systemic or interventional therapy only if required. However, treatment is recommended as isolated cutaneous LCH in infants is not always a benign disorder and the evolution is not always predictable.10
Topical corticosteroids (potent or very potent) are recommended as first-line treatment.2 Surgery may be considered for isolated and small lesions. When these fail, topical chemotherapy can be used: daily applications of nitrogen mustard (0.02% mechlorethamine hydrochloride, mustine), an alkylating cytostatic agent12 often bring clinical improvement within ten days.13 After induction treatment, topical chemotherapy can be considered as maintenance therapy.14 Although adverse effects are minimal in the short-term, the possibility of long-term cutaneous carcinogenicity is not excluded.13 Carmustine (bis-chloronitrosourea, BCNU) is another mustard gasrelated α-chloro-nitrosourea compound used as an alkylating agent for cutaneous LCH.15 New treatments include imiquimod, a topical interferon-inducing agent. A case of LCH was effectively treated with topical imiquimod, resulting in clinical and histological clearance.16 Oral 8-methoxypsoralen (8-MOP) plus ultraviolet A (PUVA) therapy (3 times per week for two months, 1–2 times per week maintenance PUVA) is an interesting treatment option, in particular offering treatment to patients presenting extensive cutaneous disease.17–19 Low-dose methotrexate (20 mg weekly) successfully treated multiresistant cutaneous LCH.20,21 In some patients with multiresistant cutaneous LCH, subcutaneous interferon- α2b achieved long remission periods.15 Oral thalidomide (N-phtalimidoglutarimide, 200 mg/d) was effective for mucocutaneous LCH after four weeks of treatment with total clearing after three months. Maintenance therapy using 100 mg/d can be recommended to prevent recurrent disease. Hence, thalidomide monotherapy represents an effective, safe and well-tolerated treatment option that may be considered as first-line therapy for mucocutaneous LCH.21–23 Systemic chemotherapy including mercaptopurine and vinblastine, with or without prednisone and/or methotrexate, may be used in case of extensive internal disease.19 Effective therapies for cases unresponsive to the above include cytosine arabinoside and cladribine. Intravenous cladribine (2-chlorodeoxyadenosine, 2CDA), a purine analog, has also been proven effective.15 Emerging therapies include monoclonal antibodies against the CD1a or CD52 epitopes found on Langerhans cells. Although normal epidermal Langerhans cells do not express CD52, pathological LCH cells express CD52, suggesting that alemtuzumab may represent a new, targeted therapy for LCH.24
PDT permits selective destruction of cells accumulating the topical photosensitizer MAL and subsequently activated by a light source.7 Through a non-elucidated selectivity mechanism, a ten-fold higher intracellular concentration of the photosensitizer is achieved in metabolically more active cells, such as the keratinocytes of actinic keratosis. This selectivity is not only restricted to epithelial cells, as endothelial cells, lymphocytes, as well as glandular cells may also be effectively targeted, hence expanding the therapeutic potential of PDT.7 This case furthermore suggests that the LCH cells are capable of concentrating the photosensitizer, whereas the normal epidermal LC are not affected. The rationale behind PDT for LCH was the selective accumulation of the photosensitizer in metabolically more active cells. In our patient, the large area involved, the pain inherent in the illumination phase and the young age of the child, prompted us to perform PDT under total anesthesia. As no previous experience is available on PDT in LCH, a standard treatment procedure was chosen. It is clear that specific PDT protocols for LCH should be developed according to future clinical experience. The child did not experience any post-procedure pain and did not ask for pain reducing medication. The time course of the crusting was similar to superficial basal cell carcinoma treatments.
In summary, as far as we know, this case presents the first successful PDT treatment of multiresistant cutaneous LHC. More experience should be gathered to determine the appropriate role of PDT in the management of cutaneous LHC.
References
- 1.Hussein MR. Skin-limited Langerhans' cell histiocytosis in children. Cancer Invest. 2009;27:504–11. doi: 10.1080/07357900802216452. [DOI] [PubMed] [Google Scholar]
- 2.Satter EK, High WA. Langerhans Cell Histiocytosis. A review of the current recommendations of the Histiocyte Society. Ped Dermatol. 2008;25:291–5. doi: 10.1111/j.1525-1470.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 3.Zeitouni NC, Oseroff AR, Shieh S. Photodynamic therapy for nonmelanoma skin cancers. Current review and update. Mol Immunol. 2003;39:1133–6. doi: 10.1016/s0161-5890(03)00083-x. [DOI] [PubMed] [Google Scholar]
- 4.Nikkels AF, Piérard-Franchimont C, Nikkels-Tassoudji N, et al. Photodynamic therapy and imiquimod immunotherapy for basal cell carcinomas. Acta Clinica Belgica. 2005;60:227–34. doi: 10.1179/acb.2005.039. [DOI] [PubMed] [Google Scholar]
- 5.Annemans L, Roelandts R, Boonen H, et al. Real-life practice study of the clinical outcome and cost-effectiveness of photodynamic therapy using methyl aminolevulinate (MAL-PDT) in the management of actinic keratosis and basal cell carcinoma. Eur J Dermatol. 2008;18:539–46. doi: 10.1684/ejd.2008.0469. [DOI] [PubMed] [Google Scholar]
- 6.Caekelbergh K, Roelandts R, Nikkels A, et al. Photodynamic therapy using methyl aminolevulinate in the management of primary superficial basal cell carcinoma: clinical and health economic outcomes from a real-life study. J Drugs Dermatol. 2009 In press. [PubMed] [Google Scholar]
- 7.Wauters O, Caucanas M, Richert B, et al. The clinical relevance of off-lable photodynamic therapy in onco-dermatology. J Clin Dermatol. In press. [Google Scholar]
- 8.Broadbent V, Gadner H, Komp DM, et al. Histiocytosis syndromes in children. II. Approach to the clinical and laboratory evaluation of children with Langerhans cell histiocytosis. Clinical Writing Group of the Histiocyte Society. Med Pediatr Oncol. 1898;17:492–5. doi: 10.1002/mpo.2950170527. [DOI] [PubMed] [Google Scholar]
- 9.Querings K, Starz H, Balda BR. Clinical spectrum of cutaneous Langerhans' cell histiocytosis mimicking various diseases. Acta Derm Venereol. 2006;86:39–43. doi: 10.2340/00015555-0003. [DOI] [PubMed] [Google Scholar]
- 10.Lau L, Krafchik B, Trebo MM, Weitzman S. Cutaneous Langerhans cell histiocytosis in children under one year. Pediatr Blood Cancer. 2006;46:66–71. doi: 10.1002/pbc.20479. [DOI] [PubMed] [Google Scholar]
- 11.Allen CE, McClain KL. Langerhans cell histiocytosis: a review of past, current and future therapies. Drugs Today (Barc) 2007;43:627–43. doi: 10.1358/dot.2007.43.9.1088823. [DOI] [PubMed] [Google Scholar]
- 12.Hoeger PH, Nanduri VR, Harper JI, Atherton DA, Pritchard J. Long term follow up of topical mustine treatment for cutaneousLangerhans cell histiocytosis. Arch Dis Child. 2000;82:483–7. doi: 10.1136/adc.82.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheehan MP, Atherton DJ, Broadbent V, Pritchard J. Topical nitrogen mustard: an effective treatment for cutaneous Langerhans cell histiocytosis. J Pediatr. 1991;119:317–21. doi: 10.1016/s0022-3476(05)80752-8. [DOI] [PubMed] [Google Scholar]
- 14.Berman B, Chang DL, Shupack JL. Histiocytosis X: treatment with topical nitrogen mustard. J Am Acad Dermatol. 1980;3:23–9. doi: 10.1016/s0190-9622(80)80220-9. [DOI] [PubMed] [Google Scholar]
- 15.Chang SE, Koh GJ, Choi JH, et al. Widespread skin-limited adult Langerhans cell histiocytosis: long-term follow-up with good response to interferon alpha. Clin Exp Dermatol. 2002;27:135–7. doi: 10.1046/j.1365-2230.2002.00989.x. [DOI] [PubMed] [Google Scholar]
- 16.O'Kane D, Jenkinson H, Carson J. Langerhans cell histiocytosis associated with breast carcinoma successfully treated with topical imiquimod. Clin Exp Dermatol. 2009;34:e829–32. doi: 10.1111/j.1365-2230.2009.03569.x. [DOI] [PubMed] [Google Scholar]
- 17.Kwon OS, Cho KH, Song KY. Primary cutaneous Langerhans cell histiocytosis treated with photochemotherapy. J Dermatol. 1997;24:54–6. doi: 10.1111/j.1346-8138.1997.tb02740.x. [DOI] [PubMed] [Google Scholar]
- 18.Neumann C, Kolde G, Bonsmann G. Histiocytosis X in an elderly patient. Ultrastructure and immunocytochemistry after PUVA photochemotherapy. Br J Dermatol. 1988;119:385–91. doi: 10.1111/j.1365-2133.1988.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 19.von Stebut E, Schadmand-Fischer S, Bräuninger W, et al. Successful treatment of adult multisystemic Langerhans cell histiocytosis with psoralen-UV-A, prednisolone, mercaptopurine, and vinblastine. Arch Dermatol. 2008;144:649–53. doi: 10.1001/archderm.144.5.649. [DOI] [PubMed] [Google Scholar]
- 20.Steen AE, Steen KH, Bauer R, Bieber T. Successful treatment of cutaneous Langerhans cell histiocytosis with lowdose methotrexate. Br J Dermatol. 2001;145:137–40. doi: 10.1046/j.1365-2133.2001.04298.x. [DOI] [PubMed] [Google Scholar]
- 21.McClain KL. Drug therapy for the treatment of Langerhans cell histiocytosis. Expert Opin Pharmacother. 2005;6:2435–41. doi: 10.1517/14656566.6.14.2435. [DOI] [PubMed] [Google Scholar]
- 22.Sander CS, Kaatz M, Elsner P. Successful treatment of cutaneous langerhans cell histiocytosis with thalidomide. Dermatology. 2004;208:149–52. doi: 10.1159/000076491. [DOI] [PubMed] [Google Scholar]
- 23.Broekaert SM, Metzler G, Burgdorf W, et al. Multisystem Langerhans cell histiocytosis: successful treatment with thalidomide. Am J Clin Dermatol. 2007;8:311–4. doi: 10.2165/00128071-200708050-00007. [DOI] [PubMed] [Google Scholar]
- 24.Jordan MB, McClain KL, Yan X, et al. Anti- CD52 antibody, alemtuzumab, binds to Langerhans cells in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2005;44:251–4. doi: 10.1002/pbc.20181. [DOI] [PubMed] [Google Scholar]