Abstract
Meningeal melanocytoma is a benign lesion arising from leptomeningeal melanocytes that at times can mimic its malignant counterpart, melanoma. Lesions of the spine usually occur in extramedullary locations and present with spinal cord compression symptoms. Because most reported spinal cases occur in the thoracic region, these symptoms usually include lower extremity weakness or numbness. The authors present a case of primary intramedullary spinal meningeal melanocytoma presenting with bilateral lower extremity symptoms in which the patient had no known supratentorial primary lesions. Gross total surgical resection allowed for full recovery, but early recurrence of tumor was detected on close follow-up monitoring, allowing for elective local radiation without loss of neurological function. Case reports of such tumors discuss different treatment strategies, but just as important is the close follow-up monitoring in these patients even after gross total surgical resection, since these tumors can recur.
Key words: intramedullary, spinal tumor, myelotomy, melanocytoma.
Introduction
Melanocytomas (meningeal melanocytoma) are benign, though at times locally aggressive, tumors that arise from melanocytes within the leptomeninges along the neuraxis. Although melanocytomas are most commonly found in the posterior fossa, spinal lesions have also been reported.1–11 The majority of these leptomeningeal tumors are extramedullary, and only a handful of intramedullary tumors have been reported. As with most rare tumors, only case reports are available for reference; thus, no gold standard exists for the definitive treatment and follow-up of patients with these tumors. Most authors favor complete resection whenever possible, with some reports of radiation for incompletely resected lesions.1,5,7,9,12–14 As more reports, with longer follow-up periods of patients with these rare tumors are presented, we may better understand the natural history, aggressive nature, and response of meningeal melanocytomas to various treatments. In this report, the authors describe a very rare patient with intramedullary spinal melanocytoma with no supratentorial primary lesion. After gross total resection, a three-month follow-up magnetic resonance (MR) imaging study demonstrated a small nodule concerning for tumor recurrence; therefore, close follow-up monitoring was undertaken.
This emphasizes the importance of close monitoring in these patients, even in situations of gross total resection.
Case Report
History and presentation
A 45-year-old previously healthy man noticed over a period of nine months an increasing pain, weakness and numbness in both legs, with the right leg being more affected. His symptoms became apparent to him when his daily sporting activities began suffering. Stumbling and feelings of his legs “giving out” prompted him to visit his primary care physician. Lumbar spine MR imaging revealed a focal disc bulge, and the patient was referred to a physical therapy program for conservative management. After approximately seven months of therapy without resolution of symptoms, the patient developed bowel and bladder urgency without frank incontinence, which prompted a return visit to his primary care physician.
Preoperative imaging and evaluation
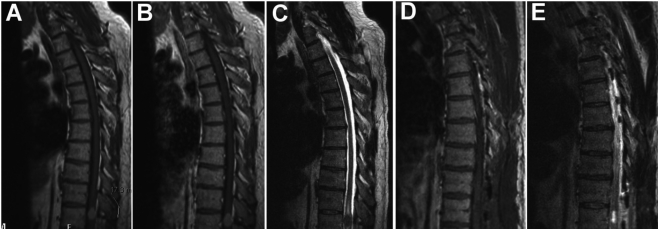
Further evaluation by MR imaging of the thoracic spine revealed a space-occupying, contrast-enhancing mass within the thoracic spinal cord at the level of T11, measuring 17×12×10 mm. The mass was hyperintense on T1-weighted imaging and hypointense on T2-weighted sequences, and full radiographic evaluation supported a diagnosis of intramedullary lesion (Figure 1). The areas superior and inferior to the lesion showed evidence of local edema and early syrinx formation.
Figure 1.
MR imaging showing preoperative (A, B, C) and postoperative (D, E) imaging characteristics of the T11 intradural intramedullary spinal mass. (A) Preoperative T1-weighted image without gadolinium enhancement showing the approximately 1.7 cm lesion. (B) Preoperative T1-weighted image with gadolinium enhancement showing homogeneous enhancement of the lesion, which is not significantly enhancing over the non-contrasted T1 scan. (C) Preoperative T2-weighted image showing syrinx formation both rostral and caudal to the lesion. (D, E) Postoperative T1- and T2-weighted images, respectively, showing near-complete resection of the mass with postoperative intramedullary cavity.
The results of the neurological examination revealed the patient had full strength with decreased sensation and 3+ reflexes in both legs. He did not exhibit any clonus, Hoffmann or Babinski reflexes. The remainder of his neurological and general physical examination was unremarkable.
Operative treatment
The differential diagnosis for the lesion included ependymoma, astrocytoma and the rarer glioma and metastasis. The patient underwent decompression and removal of the mass to obtain a tissue biopsy. Preoperative motor and sensory evoked potentials were measured to be used as baseline comparisons.
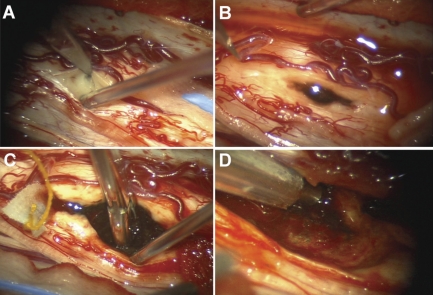
An incision was planned to span T10-T12. Intraoperative fluoroscopy was used to confirm each level. A complete laminectomy of T10 and T11 was completed. Ultrasonography confirmed the tumor to be under the laminectomy site. Using microsurgical technique, the dura and arachnoid were opened, exposing a reddish mass medially near the myelotomy. A micro-cottonoid was used to find a plane above and below the lesion. A combination of careful dissection and a Cavitron ultrasonic surgical aspirator was used to remove nearly the entire tumor. Most of the highly pigmented soft tumor was resected (Figure 2), and intraoperative specimens were sent for histopathological analysis. Unfortunately, the intraoperative frozen samples were inconclusive, and the fresh diagnosis included metastatic melanoma or meningioma. Because of safety concerns, aggressive resection of abnormally stained (possibly hemosiderin) tissue visualized deep within the cavity was not attempted, after the soft tumor bulk was removed.
Figure 2.
Intraoperative photographs showing the pigmented lesion coming near the surface of the spinal cord (A); myelotomy was required to expose the tumor surface (B). Tissue biopsy was taken with micropituitary forceps (C), and the remaining mass was aspirated (D).
Histopathology
Permanent tissue was analyzed as three separate aspirates from the soft pigmented tumor bulk. Pathology specimens were immunohistochemically stained with MIB-1 for proliferating cells and an antibody directed against collagen IV. The MIB-1 stain failed to reveal significant numbers of proliferating neoplastic cells. Pericellular collagen IV, which is usually associated with the neoplastic cells, was also noticeably absent in this specimen; this may be indicative of artifactual loss of detectable epitope.
Postoperative care and follow-up
Postoperatively, the patient experienced loss of motor ability in his left hamstring and quadriceps femoris muscle with decreased motor strength in other left leg muscle groups with normal sensation. The patient quickly regained motor function in his left leg and did not have any problems with his right leg postoperatively. He began walking with physical therapy on a daily basis with increased strength and ambulatory ability throughout his hospital stay. He was discharged to the inpatient rehabilitation unit on postoperative day four in stable condition. He continued to improve and was discharged four days after starting rehabilitation.
Immediate postoperative MR imaging scans demonstrated a gross total resection (Figure 1), and after discussion with a multifocal tumor board, it was decided to monitor this patient carefully with serial MR imaging to watch for recurrence. At the three-month follow-up appointment, MR imaging showed a very small nodule that was concerning for recurrent tumor. Since the patient remained asymptomatic, this was monitored with frequent MR imaging studies. Continued growth of this nodule was observed on subsequent clinic visits and imaging studies, although approximately one year after surgery, the patient remained neurologically intact with full strength and sensation. He did report some mild neuropathic pain in his legs, which was controlled with pregabalin. At the two-year post-operative follow-up examination, MR imaging showed evidence that the nodule, although about half the size of the original tumor, had doubled in size since originally noticed post-operatively. Since the patient was noticing more pain in his legs and back, discussion of treatment options ensued. A multidisciplinary treatment Tumor Board Committee decided to initiate radiation therapy with close monitoring instead of surgical re-resection. The patient underwent 25–30 treatments of intensity-modulated fractionated radiation therapy, and completed a total of 5040cGy to the recurrent nodule. The patient is now three years post-surgical resection and neurologically stable with minimal back pain, leg weakness and numbness. He undergoes surveillance MR imaging at six-month intervals and has no signs of tumor recurrence.
Discussion
Intramedullary melanocytomas of the spine are an exceedingly rare subclass within the spectrum of primary melanocytic neoplasms of the central nervous system (CNS), for which very little data exist to guide clinicians in management of patients post-surgical resection. Our current case illustrates the locally aggressive nature of this benign tumor and reiterates the importance of a multimodal approach to less well-described tumor entities of the CNS. Large case series or clinical studies may never be possible because of the rarity of this tumor entity; however, reportable long-term case reports carefully correlating intraoperative observation, histopathologic findings and radiographic information with tumor recurrence and post-operative management will continue to improve our understanding of these tumors and allow us providing the most appropriate care for patients with this condition.
These tumors were first described as meningeal melanocytoma by Limas and Tio in 197215; electron microscopy of the biopsied mass in their case showed numerous melanosomes within spindle-shaped cells. Clinical histopathological studies have since shown that the spectrum of CNS melanocytic tumors ranges from well-differentiated melanocytes on one end to extremely malignant melanoma on the other. Histopathological analysis also reveals immunoreactivity for HBM-45 and S-100 protein but not for epithelial membrane antigen. These tumors usually have low mitotic indices and regular, small eosinophilic nuclei,16as in our patient, who had a notable lack of MIB-1 staining in the tumor specimen. The cell of origin is the leptomeningeal melanocyte, and tumors are almost exclusively found in the posterior fossa with spinal cord lesions being most commonly extramedullary. However, these tumors can occur anywhere along the neural axis. Intraoperatively, surgeons usually encounter a grossly localized, soft but solid, encapsulated pigmented mass, usually with meningeal attachment.5,11,16 Diagnosis at the time of surgery is often difficult and misleading, as distinct cell types are not obvious until frozen pathological analysis is completed. Crush preparations obtained in one case showed multiple homogeneous-looking cells with intracellular inclusions of pigment.17 The diagnosis of meningeal melanocytoma was then confirmed using conventional frozen pathological evaluation.
About 50 to 60 diagnosed cases have been reported in the literature since 1912, approximately one third in spine and two thirds intracranially.5,7 A total of only a dozen patients with intramedullary spinal cord meningeal melanocytomas have been reported, only a fraction of whom underwent resection via myelotomy.1,5 Spinal lesions are more common in women, typically in their fifth decade, with multiyear history of myelopathy with or without radiculopathy. Surgical goals are to decompress the spinal cord and obtain a complete resection with a specimen for diagnosis. Local control rates have been shown to be four times higher if complete resection is achieved, and morbidity and mortality rates for incomplete resection now mandate the use of adjuvant radiation therapy.1,5,12–14 Although they are considered to be a benign tumor, melanocytomas have been reported to recur, even after gross total resection, as in the present case.5 Enough clinical evidence exists, based on multiple small case reports and series, to show that gross total resection is the initial treatment of choice for the management of these tumors;1,5,12–14 however, the question remains about the best possible follow-up method for post-surgical patients and how residual tumor or recurrences should be managed. Rades and Schild13 have proposed high-dose local radiation, even in cases of complete tumor resection, to prevent local recurrence. More aggressive approaches have also been advocated, including re-resection of recurring tumor and reservation of adjuvant radiotherapy for those lesions that cannot be completely resected.2,3 Very few long-term follow-up studies have been presented, especially in those patients with recurrence after radiographic gross total resection, to show which treatment provides the best survival benefit.
We emphasize the importance of very close post-operative monitoring and diligent follow-up, even in the case of radiographically gross total resection. Although they are histopathologically benign tumors, spinal intramedullary melanocytomas do have an aggressive clinical course secondary to their location and the mass effect they impose within the spinal cord. It is therefore crucial that recurrence post-surgical resection be caught early, as this allows for early adjuvant therapy or re-resection. As in our patient, in whom gross total resection was achieved, local recurrence is possible as early as 3 months post-operatively. Clinicians should be aware of the recurrence risks of these tumors, because of their aggressive nature, even after a surgical resection. Although no large series exists to conclusively determine why such benign tumors may recur locally, we propose a possible explanation. As noted in intraoperative reports from our case as well as others,3 pigmented tissues may remain in the tumor bed after removal of the soft, pigmented tumor mass. These tissues may be thought to be structurally normal with possible hemosiderin staining, and therefore not in need of aggressive resection; however, it is unknown whether these pigmented so-called normal regions actually have tumor cells coating them or even incorporated within them. The aggressive nature of melanocytomas has been documented by Chacko and Rajshekhar,3 who observed what they referred to as an infiltrative residue of the spinal cord substance during intraoperative inspection of the resection cavity of the tumor at the poles. This exact type of tissue is the unknown aspect of the surgical resection, and even though these authors advocated a radical excision procedure, they did not report aggressive resection of the infiltrative portions from the spinal cord. Although their patient showed no radiographic recurrence at eight years post-surgery, the infiltrative nature as well as aggressive and seemingly inconsistent behavior of this tumor must be taken into account intraoperatively as well as radiographically at follow-up.
The risks of recurrence or residual tumor progression are quite serious, and several reports describe malignant transformation as well as leptomeningeal seeding even years after surgical resection.2,18,19 Spread beyond the scope of surgical resection dramatically reduces treatment options and places the patient at significant risk of neurological deficits, while malignant transformation reduces effectiveness of treatments. To minimize these risks, we employed a multidisciplinary tumor board at our institution, to help with decision-making. We believe that presenting these rare tumors at such a venue allows for a broader sense of understanding and will hopefully inform the larger oncological community as to the nature of these benign but aggressive lesions. Although our patient was treated with intensity-modulated radiation therapy for local recurrence, operative resection of locally recurrent tumor is also a reasonable method of treatment. Considering the location of the tumor in our patient, close follow-up with regular surveillance MR imaging has been crucial, since re-resection has substantial associated morbidity. Hopefully, more reports and longer follow-up periods after surgical resection, recurrence and subsequent treatment will enable clinicians to render more accurate information as to the best treatment for melanocytomas as well as the best method for dealing with their recurrence. This may include altering treatment decisions based on intraoperative findings, such as normal-appearing neural tissue stained with pigmentation, even after gross total resection. The question remains whether more aggressive early adjuvant therapy should play a role in treating patients with these benign, yet locally aggressive intramedullary tumors.
Acknowledgments
Acknowledgments: the authors thank Kristin Kraus, M.Sc., for her editorial assistance in preparing this paper.
References
- 1.Ahluwalia S, Ashkan K, Casey AT. Meningeal melanocytoma: clinical features and review of the literature. Br J Neurosurg. 2003;17:347–51. doi: 10.1080/02688690310001601243. [DOI] [PubMed] [Google Scholar]
- 2.Bydon A, Gutierrez JA, Mahmood A. Meningeal melanocytoma: an aggressive course for a benign tumor. J Neurooncol. 2003;64:259–63. doi: 10.1023/a:1025628802228. [DOI] [PubMed] [Google Scholar]
- 3.Chacko G, Rajshekhar V. Thoracic intramedullary melanocytoma with long-term follow-up. J Neurosurg Spine. 2008;9:589–92. doi: 10.3171/SPI.2008.9.08323. [DOI] [PubMed] [Google Scholar]
- 4.Goyal A, Sinha S, Singh AK, et al. Lumbar spinal meningeal melanocytoma of the L3 nerve root with paraspinal extension: a case report. Spine. 2003;28:E140–2. doi: 10.1097/01.BRS.0000051879.20360.8A. [DOI] [PubMed] [Google Scholar]
- 5.Horn EM, Nakaji P, Coons SW, Dickman CA. Surgical treatment for intramedullary spinal cord melanocytomas. J Neurosurg Spine. 2008;9:48–54. doi: 10.3171/SPI/2008/9/7/048. [DOI] [PubMed] [Google Scholar]
- 6.Karikari IO, Powers CJ, Bagley CA, et al. Primary intramedullary melanocytoma of the spinal cord: case report. Neurosurgery. 2009;64:E777–8. doi: 10.1227/01.NEU.0000341516.22126.AA. [DOI] [PubMed] [Google Scholar]
- 7.Khadilkar U, Agarwal N, Deeshma B. Primary meningeal melanocytoma. Kathmandu Univ Med J. 2008;6:245–7. [PubMed] [Google Scholar]
- 8.Mangels KJ, Johnson MD, Weil RJ. 35-year-old woman with progressive bilateral leg weakness. Brain Pathol. 2006;16:183–4. doi: 10.1111/j.1750-3639.2006.00003_2.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shownkeen HN, Harmath C, Thomas C. Multiform cervical melanocytoma: a case report. Neuroradiology. 2002;44:1008–10. doi: 10.1007/s00234-002-0875-y. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg JM, Gillespie JJ, MacKay B, et al. Meningeal melanocytoma with invasion of the thoracic spinal cord Case report. J Neurosurg. 1978;48:818–24. doi: 10.3171/jns.1978.48.5.0818. [DOI] [PubMed] [Google Scholar]
- 11.Turhan T, Oner K, Yurtseven T, et al. Spinal meningeal melanocytoma. Report of two cases and review of the literature. J Neurosurg. 2004;100(3 Suppl Spine):287–90. [PubMed] [Google Scholar]
- 12.Rades D, Heidenreich F, Tatagiba M, et al. Therapeutic options for meningeal melanocytoma Case report. J Neurosurg. 2001;95(2 Suppl Spine:):225–31. doi: 10.3171/spi.2001.95.2.0225. [DOI] [PubMed] [Google Scholar]
- 13.Rades D, Schild SE. Dose-response relationship for fractionated irradiation in the treatment of spinal meningeal melanocytomas: a review of the literature. J Neurooncol. 2006;77:311–4. doi: 10.1007/s11060-005-9048-2. [DOI] [PubMed] [Google Scholar]
- 14.Rades D, Schild SE, Tatagiba M, et al. Therapy of meningeal melanocytomas. Cancer. 2004;100:2442–7. doi: 10.1002/cncr.20296. [DOI] [PubMed] [Google Scholar]
- 15.Limas C, Tio FO. Meningeal melanocytoma ("melanotic meningioma") Its melanocytic origin as revealed by electron microscopy. Cancer. 1972;30:1286–94. doi: 10.1002/1097-0142(197211)30:5<1286::aid-cncr2820300522>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol. 1999;23:745–54. doi: 10.1097/00000478-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Chen KT. Crush cytology of melanocytoma of the spinal cord A case report. Acta Cytol. 2003;47:1091–4. doi: 10.1159/000326654. [DOI] [PubMed] [Google Scholar]
- 18.Ali Y, Rahme R, Moussa R, et al. Multifocal meningeal melanocytoma: a new pathological entity or the result of leptomeningeal seeding? J Neurosurg. 2009;111:488–91. doi: 10.3171/2009.3.JNS081096. [DOI] [PubMed] [Google Scholar]
- 19.Uozumi Y, Kawano T, Kawaguchi T, et al. Malignant transformation of meningeal melanocytoma: a case report. Brain Tumor Pathol. 2003;20:21–5. doi: 10.1007/BF02478943. [DOI] [PubMed] [Google Scholar]