Abstract
Angiomatoid fibrous histiocytoma (AFH) is a rare disease that is often misdiagnosed initially. Patients can present with a clinical picture concerning for other diseases, and pathologic review is not always revealing. Molecular diagnostics are increasingly being utilized to detect gene fusions characteristic for AFH. Surgery remains the mainstay of management, and can effectively control local recurrences and metastases. Herein we describe a case report of a 25-year-old gentleman whose presentation was concerning for lymphoma. Subsequently we review of the relevant literature.
Key words: angiomatoid fibrous histiocytoma, soft tissue tumor.
Introduction
Angiomatoid fibrous histiocytoma (AFH) is a rare soft tissue tumor that is found primarily in the extremities of children.1 It is normally difficult to make a pre-operative diagnosis of AFH; more and more, molecular diagnostics are being utilized to establish the diagnosis in situations where the pathology is not conclusive. We present a case of a 25-year-old gentleman whose presentation was initially concerning for lymphoma. The diagnosis was established upon pathologic review with immunohistochemistry. Following the case report we provide a review the literature.
Case Report
A 25-year-old gentleman presented to our institution for evaluation of night sweats and fatigue. The night sweats started 18 months prior to presentation and became drenching during the preceding two months. He felt fatigued throughout the day and noticed weight loss despite a normal appetite. He denied fever, chills or pruritus and was not on any medications. He was of Northern and Eastern European decent. His family history was significant for coronary artery disease and breast cancer.
The patient lived with his parents and worked as a chef. He had a 10 pack-year history of smoking but had quit recently. He continued to chew tobacco. Two years prior to presentation he visited many countries in Western Europe. On examination he was afebrile with a normal blood pressure and regular pulse. There was a large, fixed, non-tender mass in his left axilla. The rest of the examination was otherwise normal. Laboratory studies revealed a microcytic anemia with a hemoglobin of 10.6 g/dL (adult males 13.5–17.5 g/dL) and a mean corpuscular volume of 77.9 fL (80–100 fL). His ferritin was 499 μg/L (males 24–336 μg/L) and erythrocyte sedimentation rate 126 mm/hr (0–22 mm in one hour). Peripheral blood smear demonstrated rouleaux formation, anisocytosis, microcytosis and hypochromic red blood cells. Flow cytometry of peripheral blood did not demonstrate any monotypic B-cell population or increase in blast cells. There was no monoclonal protein on serum electrophoresis. Tests for hepatitis A, B and C, HIV 1 and 2 and brucellosis were negative. Fecal occult blood testing was negative.
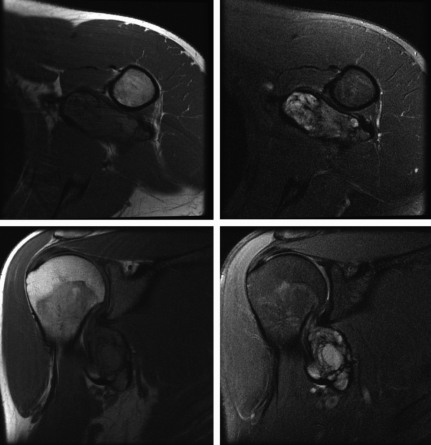
A CT chest showed an indeterminate 4.3×2.7 cm soft tissue mass with multiple prominent left axillary lymph nodes, the largest having an 8 mm diameter. Magnetic resonance imaging (MRI) revealed a 5.3×4.6×3.6 cm solid, heterogenously enhancing intermuscular mass, in the superior portion of the left axilla, inferior to the glenohumeral joint (Figure 1). The mass abutted the left axillary artery and vein, and encased some of the brachial plexus cords. The appearance was suggestive of an extra-abdominal desmoid tumor. An ultrasound-guided fine needle aspiration was pursued. The smears showed atypical spindle cell proliferation suspicious for sarcoma, but they were not diagnostic. Axillary fluid cultures for aerobic and anaerobic bacteria, mycobacteria and fungi demonstrated no growth. An acid fast smear of axillary fluid was negative.
Figure 1.
MRI revealed a 5.3×4.6×3.6 cm solid, heterogenously enhancing mass in the superior portion of the left axilla, inferior to the glenohumeral joint. A cystic component is best seen in the right upper panel.
An open biopsy was performed and a 3.0×1.7×0.8 cm aggregate of hemorrhagic soft tissue was removed. The frozen section was read as an undifferentiated neoplasm, and metastatic melanoma and rhabdomyosarcoma were on the pathologic differential diagnosis. Immunoperoxidase studies performed on paraffin sections demonstrated that the neoplastic cells were positive for vimentin, EMA (focal) and CD68. These cells were negative for S100, melan A, HMB45, myogenin, desmin, smooth muscle actin and keratin. The diagnosis of angiomatoid fibrous histiocytoma was made based on these studies.
Treatment options were discussed with the patient. He elected to pursue preoperative radiation followed by definitive resection. He received 50 Gy in 25 fractions to the tumor. One month later, the superior aspect of the tumor was dissected from the brachial plexus and the axillary artery and vein, but the thoracodorsal artery and vein were sacrificed. The margins were negative on visual inspection, but the deep margin was negative only by 1 mm. It was elected to boost the tumor bed with intraoperative radiation therapy and he received 1000 cGy with 6 MeV electrons calculated at the 90% isodose depth to the tumor bed.
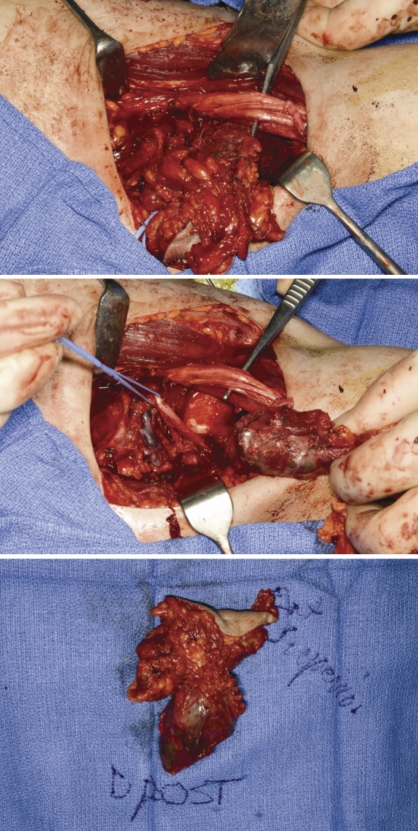
A white-tan, lobulated, hemorrhagic, firm mass measuring 6.5×4.3×3.2 cm was resected (Figure 2). Pathology revealed an angiomatoid fibrous histiocytoma. Minimal treatment effect was present. Eight regional axillary lymph nodes were negative for tumor. All surgical resection margins were negative for tumor and the minimum tumor free margin was less than 0.1 cm.
Figure 2.
At surgery a white-tan, lobulated, hemorrhagic and focally necrotic firm mass measuring 6.5×4.3×3.2 cm was removed for pathologic review.
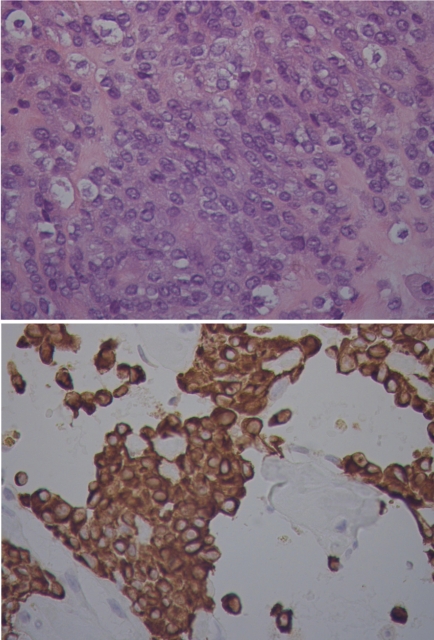
The neoplastic cells were positive for desmin and negative for smooth muscle actin, myogenin, EMA and S100 (Figure 3). Chromosome analysis revealed several nonclonal structural anomalies in each metaphase, likely due to preoperative radiation therapy.
Figure 3.
Pathologic review revealed multiple bland, vesicular nuclei (upper image). There was also a thick fibrous pseudocapsule and a lymphoplasmacytic infiltrate (not shown). The neoplastic cells were positive for desmin (lower image).
An MRI obtained three months postoperatively revealed a fluid collection within the axilla but no discrete enhancing mass to suggest recurrence.
Discussion
Angiomatoid fibrous histiocytoma (AFH) was first described in 1979 in a case series of 41 patients.1 It typically occurs in the extremities of children and young adults, but patients aged even 71 years have also been reported.2
Most of the time, AFH presents as a superficial subcutaneous mass and is thought to represent lymphadenopathy, or a benign cyst on clinical examination.1,3 Patients may also present with fever and weight loss while anemia and polyclonal hypergammaglobulinemia are common laboratory findings.1,3,4 Imaging studies alone, cannot differentiate AFH from malignant fibrous histiocytoma, sarcoma or other tumors.5 Cystic components seen on magnetic resonance imaging were correlated with hemorrhage in a case report.6
Pathologically, AFH usually demonstrates four features: a fibrous pseudocapsule, a round or spindle fibrohistiocytic cell proliferation, a pseudoangiomatous pattern and a plasmalymphocytic infiltrate.7 Sometimes the round cells and CD99 or desmin positivity raise concern for Ewing's sarcoma or rhabdomyosarcoma, respectively. In one immunohistochemical review, all cases of AFH were positive for vimentin, most were positive for calponin, and more than half were positive for desmin. There were also some samples that were positive for CD99, SMA or MSA, but no samples were positive for myoglobin, S100, CD21, or CD35.2 Fluorescent in situ hybridization (FISH) and reverse transcription-polymerase chain reaction have been used more recently to demonstrate that EWSR1-CREB1 is the predominant fusion gene in AFH, but EWSR1-ATF1 and FUS-ATF1 gene rearrangements have also been described.8-13 Sometimes FISH has been used to confirm cases of AFH with pleomorphic features that were difficult to diagnose with optical morphology and immunohistochemistry.14 The EWSR1-ATF1 gene arrangement is also described in another clinically distinct entity,clear cell sarcoma.15
In the initial series of 41 patients, 24 were available for follow-up. Among these 24 patients, 11 had local recurrences and 5 had metastatic disease. The name angiomatoid malignant fibrous histiocytoma (AMFH) was given based on the observed metastatic potential.1 Eleven years later, another series of 108 patients was presented, of whom 94 were available for follow-up. Of those patients, 11 had recurred locally and were adequately treated with wide local excisions. Five patients were found or suspected to have metastatic disease on follow-up.3 AMFH was considered to be a fibrohistiocytic malignancy of intermediate malignancy based on this larger series and was renamed AFH. Recurrence in patients with AFH has been associated with location in the head or neck, ostensibly due to the difficulty with complete excision in this area, and with an irregular tumor border3 that makes complete resection also difficult.
Surgery alone with a wide local excision can be utilized to establish local control; however, in cases where a marginal excision is planned, radiation therapy should be utilized to maximize control. In this setting, pre-operative radiation is preferred to post-operative radiation, as the latter implies greater toxicity.16
It is important to distinguish AFH from malignant fibrous histiocytoma,17 Ewing's sarcoma and rhabdomyosarcoma, due to the differences in the potential for local recurrence and metastasis and management of these diseases.
In the case we describe, the initial presentation was concerning for lymphoma due to the palpable axillary mass, anemia and history of weight loss and night sweats. This presentation is not unusual for patients with AFH.1–4 The imaging studies we obtained were not diagnostic, but pathologic review with immunohistochemistry led to the diagnosis of AFH. Although chromosomal analysis was performed, neither FISH nor RT-PCR was used to detect the fusion transcripts seen in AFH. Although surgery is often curative, radiation therapy was utilized to maximize control due to the narrow surgical margins.
Conclusions
AFH is a rare disease that is often misdiagnosed initially. Patients can present with a clinical picture suggestive of other diseases, such as lymphoma as in our patient. Additionally, pathologic review can be concerning for a sarcoma with more malignant potential. Although thorough pathologic review is critical for the diagnosis, techniques such as FISH have been used to confirm cases with pleomorphic features. AFH can recur locally, but most of these patients do well with wide local excisions alone, if a wide surgical margin is possible. Radiotherapy may be utilized when wide excision margins are not feasible. AFH does have low potential for metastasis, but surgery can be effective in these cases as well. Our patient did well following his procedure without evidence of recurrence at follow-up.
Acknowledgments
Acknowledgements: the authors would like to thank Eric Hostetter for his assistance in the preparation of the figures.
Footnotes
Conflict of interest: the authors report no conflicts of interest.
Contributions: AM and DD were both responsible for the concept and drafting of this manuscript. All authors revised and provided final approval of the final version of this manuscript.
References
- 1.Enzinger FM. Angiomatoid malignant fibrous histiocytoma: a distinct fibrohistiocytic tumor of children and young adults simulating a vascular neoplasm. Cancer. 1979;44:2147–57. doi: 10.1002/1097-0142(197912)44:6<2147::aid-cncr2820440627>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Fanburg-Smith JC, Miettinen M. Angiomatoid “malignant” fibrous histiocytoma: a clinicopathologic study of 158 cases and further exploration of the myoid phenotype. Hum Pathol. 1999;30:1336–43. doi: 10.1016/s0046-8177(99)90065-5. [DOI] [PubMed] [Google Scholar]
- 3.Costa MJ, Weiss SW. Angiomatoid malignant fibrous histiocytoma. A follow-up study of 108 cases with evaluation of possible histologic predictors of outcome. Am J Surg Pathol. 1990;14:1126–32. [PubMed] [Google Scholar]
- 4.Hasegawa T, Seki K, Ono K, Hirohashi S. Angiomatoid (malignant) fibrous histiocytoma: a peculiar low-grade tumor showing immunophenotypic heterogeneity and ultrastructural variations. Pathol Int. 2000;50:731–8. doi: 10.1046/j.1440-1827.2000.01112.x. [DOI] [PubMed] [Google Scholar]
- 5.De Beuckeleer L. Fibrohistiocytic tumors. In: De Schepper AM, Parizel PM, De Beuckeleer L, Vanhoenacker F, editors. Imaging of soft tissue tumors. Berlin, Heidelberg, New York: Springer; 2001. pp. 181–93. [Google Scholar]
- 6.Li CS, Chan WP, Chen WT, et al. MRI of angiomatoid fibrous histiocytoma. Skeletal Radiol. 2004;33:604–8. doi: 10.1007/s00256-004-0769-7. [DOI] [PubMed] [Google Scholar]
- 7.Grossman LD, White RR, 4th, Arber DA. Angiomatoid fibrous histiocytoma. Ann Plast Surg. 1996;36:649–51. doi: 10.1097/00000637-199606000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Antonescu CR, Dal Cin P, Nafa K, et al. EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer. 2007;46:1051–60. doi: 10.1002/gcc.20491. [DOI] [PubMed] [Google Scholar]
- 9.Hallor KH, Mertens F, Jin Y, et al. Fusion of the EWSR1 and ATF1 genes without expression of the MITF-M transcript in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer. 2005;44:97–102. doi: 10.1002/gcc.20201. [DOI] [PubMed] [Google Scholar]
- 10.Hallor KH, Micci F, Meis-Kindblom JM, et al. Fusion genes in angiomatoid fibrous histiocytoma. Cancer Lett. 2007;251:158–63. doi: 10.1016/j.canlet.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Rossi S, Szuhai K, Ijszenga M, et al. EWSR1-CREB1 and EWSR1-ATF1 fusion genes in angiomatoid fibrous histiocytoma. Clin Cancer Res. 2007;13:7322–8. doi: 10.1158/1078-0432.CCR-07-1744. [DOI] [PubMed] [Google Scholar]
- 12.Raddaoui E, Donner LR, Panagopoulos I. Fusion of the FUS and ATF1 genes in a large, deep-seated angiomatoid fibrous histiocytoma. Diagn Mol Pathol. 2002;11:157–62. doi: 10.1097/00019606-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Waters BL, Panagopoulos I, Allen EF. Genetic characterization of angiomatoid fibrous histiocytoma identifies fusion of the FUS and ATF-1 genes induced by a chromosomal translocation involving bands 12q13 and 16p11. Cancer Genet Cytogenet. 2000;121:109–16. doi: 10.1016/s0165-4608(00)00237-5. [DOI] [PubMed] [Google Scholar]
- 14.Weinreb I, Rubin BP, Goldblum JR. Pleomorphic angiomatoid fibrous histiocytoma: a case confirmed by fluorescence in situ hybridization analysis for EWSR1 rearrangement. J Cutan Pathol. 2008;35:855–60. doi: 10.1111/j.1600-0560.2007.00908.x. [DOI] [PubMed] [Google Scholar]
- 15.Panagopoulos I, Mertens F, Debiec-Rychter M, et al. Molecular genetic characterization of the EWS/ATF1 fusion gene in clear cell sarcoma of tendons and aponeuroses. Int J Cancer. 2002;99:560–7. doi: 10.1002/ijc.10404. [DOI] [PubMed] [Google Scholar]
- 16.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Daw NC, Billups CA, Pappo AS, et al. Malignant fibrous histiocytoma and other fibrohistiocytic tumors in pediatric patients: the St. Jude Children's Research Hospital experience. Cancer. 2003;97:2839–47. doi: 10.1002/cncr.11384. [DOI] [PubMed] [Google Scholar]