Abstract
Sarcomatoid carcinoma is an extremely rare biphasic tumor characterized by a combination of malignant epithelial and mesenchymal cells. Limited data on sarcomatoid carcinoma showed that most cases occurred with advanced local disease and metastasis, and paraneoplastic syndromes were rare. We present the case of a 63-year-old man with lung sarcomatoid carcinoma associated with jejunum metastasis and leukocytosis, and its clinical, macroscopic, and histopathological features. This case emphasizes the importance of recognizing paraneoplastic syndromes and metastasis of sarcomatoid carcinoma at diagnosis.
Key words: lung cancer, sarcomatoid carcinoma, leukocytosis
Introduction
Sarcomatoid carcinoma is an extremely rare biphasic tumor that is characterized by a combination of malignant epithelial and mesenchymal cells.1–4 Although sarcomatoid carcinoma has been reported in many sites of the body such as the bladder, colon, uterus, ovary, breast, and lung, few pathologists have the opportunity to study very many of them. As for lung cancer, sarcomatoid carcinoma has also been described by a variety of names including spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, blastoma, and others. Fishback et al. designated sarcomatoid carcinomas as pleomorphic (spindle/giant cell) carcinomas of the lung because both spindle and giant cell components often are found in the same tumors.4 The World Health Organization (WHO) histological classification of lung tumors or carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements includes: 1) carcinomas with spindle and/or giant cells (pleomorphic carcinoma, spindle cell carcinoma, and giant cell carcinoma), 2) carcinosarcoma, 3) pulmonary blastoma, and 4) others. Spindle and giant cell carcinoma and carcinosarcoma comprise only 0.4% and 0.1% of all lung malignancies, respectively.3
There is no specific clinical presentation and typical symptoms include chest pain, cough, hemoptysis, dyspnea, fever, and weight loss.5,6 Limited data on sarcomatoid carcinoma showed that most cases were associated with advanced local disease at diagnosis. However, metastasis and paraneoplastic syndromes are rarely seen in the clinic. We present the case of a 63-year-old man with lung sarcomatoid carcinoma with jejunal metastasis and leukocytosis, as well as its clinical, macroscopic, and histopathological features.
Case Report
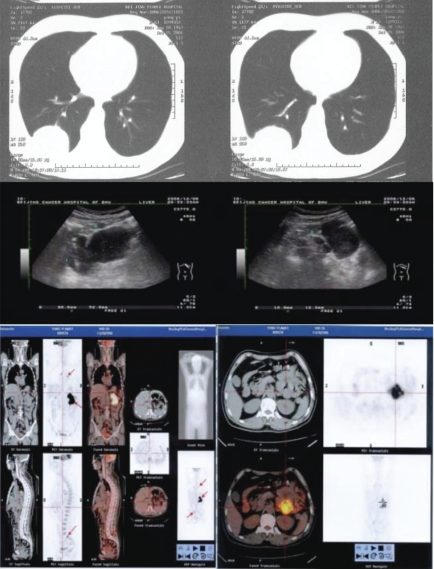
A 63-year-old man was admitted to the hospital because of a diagnosis of lung cancer after a surgical excision of a lung tumor. The patient had been in stable health without any symptoms before a regular health examination two months earlier. A chest X-ray examination showed a mass in the right pulmonary lobe. A computed tomography (CT) scan showed a lesion about 45 mm in diameter in the inferior lobe of the right lung with breaching of the pleurae (Figure 1). Ultrasonographic examination of the abdomen showed swollen lymph nodes, the largest one of which was 32×21mm. A video-assistant thoracoscope was conducted and a biopsy was performed. Frozen pathological sections showed poorly differentiated squamous cell carcinoma. The inferior lobe of the right lung and mediastinal lymph nodes were removed surgically. The resection pathological diagnosis was polymorph sarcomatoid carcinoma with invasion of the pleurae. Mediastinal lymph nodes were negative. A CT scan of the thorax in the supine position without the administration of contrast material revealed a large well-defined soft-tissue mass measuring 5×4×4 cm in the right lung lobe with breaching of the pleura. Abdominal ultrasonography revealed a well-circumscribed mass of 10.4×9.9×5.4 cm with enlarged lymph nodes, the largest one of which was 19×12 mm. A FDG-PET/CT scan with attenuation revealed a well-demarcated elliptical mass in the abdomen with dimensions of 10×9×5 cm and extensive 18FDG uptake.
Figure 1.
Computed tomography scan of the right lung lesion; ultrasonic and PET images of the intestinal lesion.
The patient recovered and came to this hospital for further treatment. On physical examination, he appeared healthy with a body temperature of 36.2°C. The lungs, heart, and abdomen were normal. Laboratory studies showed normal liver and renal function. The urine and dejecta were negative. The prothrombin and partial-thromboplastin times were normal. The red-cell count was 2.95×1012/L and the hypochromia was 89 g/L. The white-cell count was 25.42×109/L, with 88.4% neutrophils, 6.3% lymphocytes, 2.6% monocytes, and the platelet count was 268×109/L. An abdominal ultrasonographic examination revealed a well-circumscribed mass of 10.4×9.9×5.4 cm with relatively lower echogenicity but high central echogenicity, which was likely an intestinal structure. Lymph nodes were swollen and the largest one was 19×12 mm (Figure 1). A FDG-PET/CT scan with attenuation showed a significantly increased tracer uptake in the metastatic mass of the small intestine, the seventh thoracic vertebra, and the sacrum. (Figure 1).
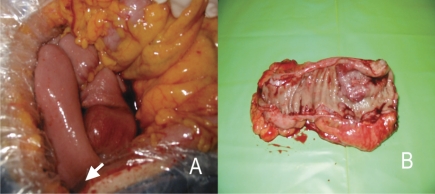
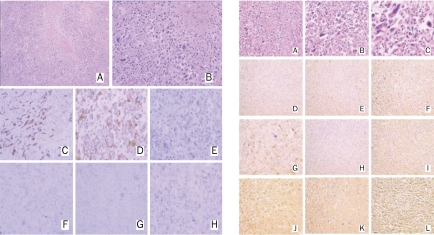
After communication with the patient, and according to his wishes, a second resection of the abdominal mass was performed. The tumor, well coated with membrane, was located at the star part of the jejunum, 3 cm from the transverse mesocolon, encroaching on the mesentery (Figure 2). The whole lesion, with part of the jejunum, was removed. The pathological diagnosis was sarcomatoid carcinoma. The immunohistochemical staining results showed that it was positive for epithelial membrane antigen (EMA), cytokeratin (CK), S-100, vimentin, and TTF-1; CK7 was focally positive; and CD117, CD20, and CDX2 were negative (Figure 3). Surgical removal and immunohistochemical staining of the lung lesion were also done and the results showed that it was positive for CK and vimentin, and focally positive for EMA; but negative for CK7, CD20, and desmin (Figure 3).
Figure 2.
View of the surgery (A) and the lesion that was removed surgically (B). The tumor was located at the star part of the jejunum, 3 cm from the transverse mesocolon, encroaching on the mesentery.
Figure 3.
Immunohistochemical staining of the resected lesion. Left column (right lung lesion): H&E stain of tumor cells; 100× (A) and 200× (B) magnification. Tumor cells were positive for cytokeratin (C) and vimentin (D). Epithelial membrane antigen (E) was focally positive while cytokeratin 7 (F), CD20 (G), and desmin (H) were negative. Right column (intestinal lesion): H&E stain of tumor cells; 100× (A), 200× (B), and 400× (C) magnification. Tumor cells were negative for CD117 (D), CD×2 (E), and CD20 (H). Cytokeratin (F), epithelia/membrane antigen (I), S-100 (J), TTF-1 (K), and vimentin (L) were positive. Cytokeratin 7 (G) was focally positive.
After surgery, our patient received one cycle of chemotherapy with a regimen of docetaxel and cisplatin. He showed no response to the drugs and a CT scan reported new lesions in the pleurae and diaphragm. Considering the expected poor prognosis owing to disseminated disease, the patient declined further treatment.
Discussion
Sarcomatoid carcinoma of the lung is a rare histological subtype and has been reported as having a relatively poorer prognosis.1,5–9 It is difficult to distinguish sarcomatoid carcinomas from true sarcomas, such as malignant fibrous histiocytoma and fibrosarcoma, if no carcinomatous areas are recognized on the hematoxylin-and-eosin stained sections. Immunocytochemistry seems to be the gold standard for diagnosis of sarcomatoid carcinoma, because CT, MR, and PET scans appear to be less efficient, and standard light microscopy is not always adequate. However, carcinoembryonic antigen, EMA, CK, pancreatin, chromogranin A, CD56, and synaptophysin are highly specific markers for the carcinomatous components, while desmin, vimentin, and smooth muscle/sarcomeric actin show an affinity for the sarcomatous elements.7,9,10
With respect to the histogenesis of sarcomatoid carcinoma, some authors have suggested that sarcomatoid carcinoma is derived from a single totipotent stem cell that has the ability to pursue both epithelial and mesenchymal differentiation.1,2,4 Halachmi et al. analyzed the loss of heterozygosity (LOH) in both the carcinomatous and sarcomatous components of six bladder tumors by microsatellite analysis of 40 markers from 19 human chromosomes. The results showed that all six cases revealed identical LOH on chromosomal arms 9p, 9q, 8p, and 8q, corresponding to relatively early events in bladder carcinogenesis.11 Armstrong et al. Analyzed the TP53 mutation status of a series of 17 sarcomatoid urothelial carcinomas using single-strand conformation polymorphism, DNA sequencing, and p53 immunohistochemistry. The results showed that both the sarcomatoid and epithelial tumor components in all 17 cases demonstrated concordant p53 expression patterns and five carcinomas were with identical TP53 point mutations in both the epithelial and sarcomatoid components.12 These results suggest that despite their conspicuous divergence at the phenotypic level, the sarcomatoid and carcinomatoid elements of this uncommon tumor might share a common clonal origin or be derived from a common stem cell.
The typical symptoms of pulmonary sarcomatoid carcinoma are the same as for most lung cancers and include chest pain, cough, hemoptysis, dyspnea, fever, and weight loss. Paraneoplastic syndromes such as dermatomyositis and Trousseau’s syndrome were reported in uterine sarcomatoid carcinoma and ovarian carcinosarcoma.13–15 However, no paraneoplastic syndromes were reported in lung sarcomatoid carcinoma although they occurred with about 15–20% of small cell lung cancers and 5–8% of non-small cell lung cancers.16
The most common metastases to invade the small intestine are of gastrointestinal or gynecological origin, such as those arising from the colon, uterine cervix, or ovaries.17 Small intestinal metastases from extra-abdominal malignancies have been reported previously, mainly in cases of melanoma18 and lung cancer.17,19,20 Berger et al.20 reported that only six of 1399 (0.5%) patients with primary lung cancer developed clinically apparent small intestinal metastases, and McNeill et al.17 found a lower frequency of six among 6006 hospital admissions for lung cancer. In lung cancer patients, all cell types may develop small intestinal metastases.17,20,21 It was reported that large cell and small cell carcinoma lead to gastrointestinal metastases more often than other histological types.21,22 This is the first documented case of lung sarcomatoid carcinoma invading the small intestine and accompanied by leukocytosis. The morphology of sarcomatoid carcinoma cells from both lung and jejunal lesions was similar and the immunohistochemical stains for CK, EMA, and vimentin were positive while that for CD20 was negative. Furthermore, TTF-1 was positive and CK7 was focally positive in the jejunal lesion, which supports the diagnosis of a lung derivation. Rossi et al.23 reported that in 18 lung cancers with intestinal metastasis (10 large-cell undifferentiated carcinomas and eight adenocarcinomas), all cases were stained positively for CK7 and 89% for TTF-1, whereas CK20 and CDX2 were completely negative. They suggested that as for the differential diagnosis, immunohistochemical staining with TTF-1, CDX2, CK7, and CK20 is helpful in highlighting the lung primary.
In conclusion, sarcomatoid carcinoma of the lung is a rare tumor with a high malignant potential, and might derive from a stem cell. At present, no clinical tests are available for its early diagnosis. Attention should be pay to the importance of paraneoplastic syndromes and metastasis at diagnosis. The possibilities for therapy are confined to radical resection, although novel effective treatment was expected in the clinic.
Acknowledgments
Acknowledgement: we acknowledge our patient for his consent for publication, in print and electronically.
References
- 1.Terzi A, Gorla A, Piubello Q, et al. Biphasic sarcomatoid carcinoma of the lung: report of five cases and review of the literature. Eur J Surg Oncol. 1997;23:457. doi: 10.1016/s0748-7983(97)93733-1. [DOI] [PubMed] [Google Scholar]
- 2.Nappi O, Glasner SD, Swanson PE, et al. Biphasic and monophasic sarcomatoid carcinomas of the lung. A reappraisal of “carcinosarcomas” and “spindle-cell carcinomas”. Am J Clin Pathol. 1994;102:331–40. doi: 10.1093/ajcp/102.3.331. [DOI] [PubMed] [Google Scholar]
- 3.Brambilla E, Travis WD, Colby TV, et al. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–68. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 4.Fishback NF, Travis WD, Moran CA, et al. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer. 1994;73:2936–45. doi: 10.1002/1097-0142(19940615)73:12<2936::aid-cncr2820731210>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Yoshino N, Kubokura H, Yamauchi S, et al. A true pulmonary carcinosarcoma that required diagnostic differentiation from a pleomorphic adenoma: a case report. Ann Thorac Cardiovasc Surg. 2009;15:42–5. [PubMed] [Google Scholar]
- 6.Kim HM, Shin BS, Song YW, et al. A case of pulmonary carcinosarcoma with persistent mild fever. Korean J Intern Med. 2002;17:78–82. doi: 10.3904/kjim.2002.17.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koss MN, Hochholzer L, Frommelt RA. Carcinosarcomas of the lung: a clinicopathologic study of 66 patients. Am J Surg Pathol. 1999;23:1514–26. doi: 10.1097/00000478-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Takeda S, Nanjo S, Nakamoto K, et al. Carcinosarcoma of the lung. Report of a case and review of the literature. Respiration. 1994;61:113–6. doi: 10.1159/000196319. [DOI] [PubMed] [Google Scholar]
- 9.Wick MR, Ritter JH, Humphrey PA. Sarcomatoid carcinoma of the lung. Am J Clin Pathol. 1997;108:40–53. [PubMed] [Google Scholar]
- 10.Nakajima M, Kasai T, Hashimoto H, et al. Sarcomatoid carcinoma of the lung: a clinicopathologic study of 37 cases. Cancer. 1999;86:608–16. [PubMed] [Google Scholar]
- 11.Halachmi S, DeMarzo AM, Chow NH, et al. Genetic alterations in urinary bladder carcinosarcoma: evidence of a common clonal origin. Eur Urol. 2000;37:350–7. doi: 10.1159/000052369. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong AB, Wang M, Eble JN, et al. TP53 mutational analysis supports monoclonal origin of biphasic sarcomatoid urothelial carcinoma (carcinosarcoma) of the urinary bladder. Mod Pathol. 2009;22:113–8. doi: 10.1038/modpathol.2008.176. [DOI] [PubMed] [Google Scholar]
- 13.Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol) 2006;18:641–8. doi: 10.1016/j.clon.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama M, Iha T, Kanazawa K. Unusual Trousseau’s syndrome in ovarian carcinosarcoma: multiple systemic thromboembolic events. J Obstet Gynaecol. 2002;22:699–700. doi: 10.1080/014436102762062475. [DOI] [PubMed] [Google Scholar]
- 15.Kimura T, Chino M, Ogasawara N, et al. Trousseau’s syndrome with brachiocephalic vein thrombosis in a patient with uterine carcinosarcoma. A case report. Angiology. 1999;50:515–8. doi: 10.1177/000331979905000611. [DOI] [PubMed] [Google Scholar]
- 16.Hansen M. Paraneoplastic syndromes and tumor markers for small cell and non-small cell lung cancer. Curr Opin Oncol. 1990;2:345–51. doi: 10.1097/00001622-199004000-00017. [DOI] [PubMed] [Google Scholar]
- 17.McNeill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the lung. Cancer. 1987;59:1486–9. doi: 10.1002/1097-0142(19870415)59:8<1486::aid-cncr2820590815>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal S, Yao TJ, Coit DG. Surgery for melanoma metastatic to the gastrointestinal tract. Ann Surg Oncol. 1999;6:336–44. doi: 10.1007/s10434-999-0336-5. [DOI] [PubMed] [Google Scholar]
- 19.Stenbygaard LE, Sorensen JB. Small bowel metastases in non-small cell lung cancer. Lung cancer. 1999;26:95–101. doi: 10.1016/s0169-5002(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 20.Berger A, Cellier C, Daniel C, et al. Small bowel metastases from primary carcinoma of the lung: clinical findings and outcome. Am J Gastroenterology. 1999;94:1884–7. doi: 10.1111/j.1572-0241.1999.01224.x. [DOI] [PubMed] [Google Scholar]
- 21.Antler AS, Ough Y, Pitchumoni CS, et al. Gastrointestinal metastasis from malignant tumors of the lung. Cancer. 1982;49:170–2. doi: 10.1002/1097-0142(19820101)49:1<170::aid-cncr2820490134>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 22.Kanemoto K, Kurishima K, Ishikawa H, et al. Small intestinal metastasis from small cell lung cancer. Intern Med. 2006;45:967–70. doi: 10.2169/internalmedicine.45.1651. [DOI] [PubMed] [Google Scholar]
- 23.Rossi G, Marchioni A, Romagnani E, et al. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol. 2007;2:115–20. [PubMed] [Google Scholar]