Abstract
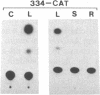
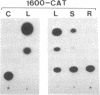
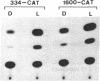
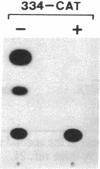
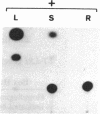
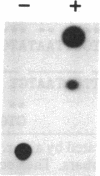
ST-LS1 is a light-inducible, single-copy gene from potato that is expressed only in photosynthetic tissues. Various sequences derived from the 5′-upstream region of this gene were fused to the coding region of the chloramphenicol acetyltransferase (CAT) gene and to the gene 7 termination region of the transfer DNA (T-DNA) from the Agrobacterium Ti plasmid pTiACH5 and transferred to tobacco using Ti-plasmid vectors. After regeneration of whole plants, tissues were assayed for the expression of the CAT gene. Sequences derived from the 5′-upstream region of the ST-LS1 gene comprising positions -334 to +11 were sufficient to confer a leaf/stem-specific as well as a light-inducible expression of the CAT gene. Destruction of chloroplasts by treatment with the herbicide norfluorazon and subsequent exposure to light drastically reduced the expression of the CAT gene indicating that this upstream sequence most likely interacts with a chloroplast-dependent signal. When sequences from position -98 to position +675 were fused to a truncated inactive fragment of the cauliflower mosaic virus 35S promoter in a head-to-head manner, the corresponding chimeric genes were again expressed in photosynthetic tissues only, indicating that these sequences have enhancer-like properties.
Keywords: Agrobacterium, chimeric genes, regulatory sequences, organ-specific expression, photosynthetic tissue
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Z. L., Schuler M. A., Beachy R. N. Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8560–8564. doi: 10.1073/pnas.83.22.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P. The petunia chlorophyll a/b binding protein genes: a comparison of Cab genes from different gene families. Nucleic Acids Res. 1985 Apr 11;13(7):2503–2518. doi: 10.1093/nar/13.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Estrella L., Van den Broeck G., Maenhaut R., Van Montagu M., Schell J., Timko M., Cashmore A. Light-inducible and chloroplast-associated expression of a chimaeric gene introduced into Nicotiana tabacum using a Ti plasmid vector. Nature. 1984 Jul 12;310(5973):115–120. doi: 10.1038/310115a0. [DOI] [PubMed] [Google Scholar]
- Kamalay J. C., Goldberg R. B. Organ-specific nuclear RNAs in tobacco. Proc Natl Acad Sci U S A. 1984 May;81(9):2801–2805. doi: 10.1073/pnas.81.9.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalay J. C., Goldberg R. B. Regulation of structural gene expression in tobacco. Cell. 1980 Apr;19(4):935–946. doi: 10.1016/0092-8674(80)90085-9. [DOI] [PubMed] [Google Scholar]
- Kaulen Hildegard, Schell Jeff, Kreuzaler Fritz. Light-induced expression of the chimeric chalcone synthase-NPTII gene in tobacco cells. EMBO J. 1986 Jan;5(1):1–8. doi: 10.1002/j.1460-2075.1986.tb04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Serrano J. J., Keil M., O'Connor A., Schell J., Willmitzer L. Wound expression of a potato proteinase inhibitor II gene in transgenic tobacco plants. EMBO J. 1987 Feb;6(2):303–306. doi: 10.1002/j.1460-2075.1987.tb04754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J., VAN Montagu M., Herrera-Estrella L. Photosynthesis-associated gene families: differences in response to tissue-specific and environmental factors. Science. 1986 Jul 4;233(4759):34–38. doi: 10.1126/science.233.4759.34. [DOI] [PubMed] [Google Scholar]
- Stockhaus J., Eckes P., Blau A., Schell J., Willmitzer L. Organ-specific and dosage-dependent expression of a leaf/stem specific gene from potato after tagging and transfer into potato and tobacco plants. Nucleic Acids Res. 1987 Apr 24;15(8):3479–3491. doi: 10.1093/nar/15.8.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko M. P., Kausch A. P., Castresana C., Fassler J., Herrera-Estrella L., Van den Broeck G., Van Montagu M., Schell J., Cashmore A. R. Light regulation of plant gene expression by an upstream enhancer-like element. Nature. 1985 Dec 12;318(6046):579–582. doi: 10.1038/318579a0. [DOI] [PubMed] [Google Scholar]
- Velten J., Schell J. Selection-expression plasmid vectors for use in genetic transformation of higher plants. Nucleic Acids Res. 1985 Oct 11;13(19):6981–6998. doi: 10.1093/nar/13.19.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]