Abstract
Tumors of the matrix of rigid structures include matrical tumors of the hairs, nails, and teeth. These tumors share similar phenotypical and signaling features. Although benign matrical hair tumors are among the most common of these tumors, hair matrix tumors containing pigmented melanocytes are very rare. The malignant variant called melanocytic pilomatrix carcinoma contains benign colonizing dendritic melanocytes admixed with the carcinomatous follicular matrical cells.
We studied the expression of cadherins and β-catenin in melanocytic pilomatrix carcinoma because cadherin/catenin-dependent cell-cell adhesion and signals play a critical role in the development of hair and hair tumors. We examined the expression of E- and P-cadherin and the multifunctional protein β-catenin in two cases of melanocytic pilomatrix carcinoma by immunohistochemistry. E- and P-cadherin are expressed at the cell membrane. In contrast, β-catenin is distributed uniformly in the nucleus and cytoplasm of all tumor cells. The diffuse nuclear and cytoplasmic β-catenin expression found in melanocytic pilomatrix carcinomas is indicative of transcriptional activation and β-catenin-induced cell transformation.
This is the first report of cadherin/catenin expression in melanocytic pilomatrix carcinoma. Although the study is limited by the number of these rare tumors, the data add information for the understanding of disease mechanisms in hair matrical tumors. Matrical tumors of the hairs share phenotypical features with other matrical tumors and show nuclear translocation of β-catenin, suggesting a transcriptional activating rather than a cellcell adhesion function.
Key words: cadherins, β-catenin, melanocytic pilomatrix carcinoma
Introduction
Matrical tumors of rigid structures include tumors of the hair, nail, and teeth matrices. Tumors of the hair matrix constitute a spectrum of neoplasms that includes the common benign pilomatricoma,1,2 pigmented matricoma,3 and the uncommon melanocytic matricoma, which contain colonizing dendritic melanocytes admixed with the matrical epithelial cell.4 The malignant variants include the proliferating pilomatricoma,5 pilomatrix carcinoma,6 pigmented pilomatrix carcinoma,7 and melanocytic pilomatrix carcinoma.8 Melanocytic pilomatrix carcinoma is the rarest of this group and is composed of highly atypical epithelial matrix cells exhibiting atypical mitoses admixed with benign dendritic melanocytes.9
Cadherins are a large family of calciumdependent cell-cell adhesion proteins linked within the cytoplasm to a group of cytoskeletal proteins called catenins.10 Cadherin/catenindependent cell-cell adhesion is involved in cell sorting and tissue morphogenesis. The best characterized are the type I or classical cadherins, a group that includes E (epithelial)-, P (placental)-, and N (nerve)-cadherin.11 One of the catenins, β-catenin, is a multifunctional protein with both cell-cell adhesion and signaling functions. In the skin, cadherin/catenindependent cell-cell adhesion and signals play a critical role in the development of hair and hair tumors.12–14 Here we report the expression of E-cadherin, P-cadherin, and β-catenin in two cases of melanocytic pilomatrix carcinoma.
Materials and Methods
The patients were an 84-year-old Caucasian male with a 0.5-cm left postauricular dark nodule, clinically diagnosed as a basal cell carcinoma, and an 83-year-old Caucasian male with a 6-month duration, dark nodule on the left midback, approximately 0.6 cm in size. After routine histological evaluation, immunohistochemistry was done either manually or with the Ventana (Tucson, AZ) and Bond Max (Leica Microsystems, Bannockburn IL, USA) automated systems using alkaline phosphatase and horseradish peroxidase detection systems. Mouse monoclonal antibodies included anti-E-cadherin, clone NCH-38 IgG1 (Dako, Carpinteria CA) used at 1:100; anti-P-cadherin, clone 56 IgG1 (BD Biosciences, San Jose, CA) used at 1:50, and anti-β-catenin, clone β-catenin-1 IgG1 (Dako) used at 1:200. Melanocytic markers included Melan-A mouse monoclonal IgG1, (Dako) used at 1:100; HMB- 45 mouse monoclonal IgG1 (Dako) used at 1:100, and S-100 rabbit polyclonal antibody (Dako, Carpinteria CA) used at 1:1200 without antigen retrieval pretreatment. Four micronthick sections were placed on positively charged slides and air dried. After paraffin removal and quenching of endogenous peroxidase, sections were hydrated and heatinduced epitope retrieval was performed. For E-cadherin manual staining, 10 mM Tris and 1 mM EDTA pH 9.0 cocktail was used in a pressure cooker for 5 min. For P-cadherin, β-catenin, and HMB-45 manual staining, 1x Target Retrieval citrate buffer solution pH 6.1 (Dako) was used for 20 min in a 100°C water bath. For manual staining, after cooling and rinsing, primary antibodies diluted in 1% bovine albumin in TBS were incubated for 1 hr at room temperature. Mouse IgG (Southern Biotech) or rabbit Ig (Dako) were used as negative controls. The primary antibodies were detected with alkaline phosphatase labeled anti-rabbit or anti-mouse detection systems (Biocare Medical, Concord CA, USA). Color end product was obtained with either Vulcan Fast Red or 3,3'diaminobenzidine chromogens (Biocare Medical) and counterstaining with Harris hematoxylin. Automated staining was done following the manufacturers’ programs. Overlying epidermis in both cases served as the internal positive control. Tissue sections from a melanocytic lesion were used as a positive control for the melanocytic markers. A block containing various carcinomas expressing E-cadherin, P-cadherin, and β-catenin was used as an additional positive control.
Results
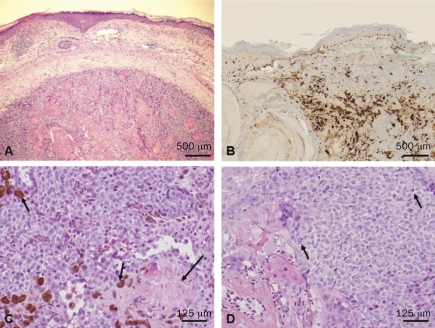
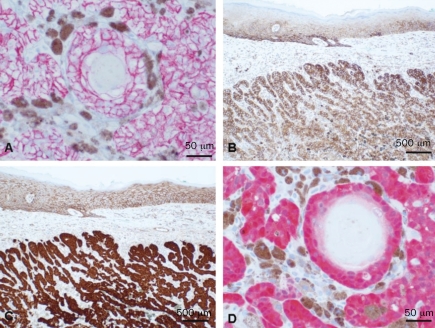
Routine hematoxylin and eosin sections of both cases showed a relatively well-circumscribed but not encapsulated dermal tumor without connection with the epidermis (Figure 1A). The features of malignancy include the presence of frank squamous carcinomatous changes, including high-grade cytological atypia, atypical mitoses, and focal tumor necrosis. Although the number of cases of pilomatrix carcinomas is very limited, these tumors can be classified as malignant by applying the simple criteria of disregarding the pilomatrix features and by the detection of obvious squamous cell carcinoma characteristics. Admixed with the epithelial cells there were pigmented dendritic melanocytes in both tumors, highlighted by immunohistochemical positivity with melanocyte markers S-100 (Figure 1B), Melan-A, and HMB45 (not shown). The tumors were composed of peripheral basaloid cells admixed with larger keratinocytes and focal full keratinization admixed with pigmented melanocytes, and formation of shadow cells with loss of the nucleus (Figure 1C). Highgrade cytological atypia was present and atypical mitoses were frequent (Figure 1D). Calcification or foreign body type giant cell reaction was not present. Staining with E-cadherin (Figure 2A) and P-cadherin (Figure 2B) showed a cell membrane distribution. In contrast to E- and P-cadherin, β-catenin was expressed in the nucleus and cytoplasm of all the tumor cells (Figures 2C and D), consistent with a role as a transcriptional regulator rather than a cell-cell adhesion protein.
Figure 1.
A, a routine hematoxylin and eosin (H&E) section from the tumor of patient 1, showing a dermal nodule composed of peripheral basaloid cells with central squamous differentiation (bar = 500 µm). B, S-100 staining demonstrating numerous dendritic melanocytes admixed with the epithelial tumor cells (bar = 500 µm). C and D, routine H&E sections showing melanin-pigmented cells (short arrows in C) admixed with the epithelial tumor cells with ghost terminally differentiated squamous cells (long arrow in C). There is marked epithelial cytological atypia and atypical mitoses (short arrows in D) (bar = 125 µm).
Figure 2.
A, plasma membrane distribution of E-cadherin (bar = 50 µm) and B, P-cadherin (bar = 500 µm). The overlying epidermis serves as the internal positive control. In contrast, β-catenin is in the nucleus and cytoplasm of all epithelial tumor cells (C and D). Note the plasma membrane distribution of β-catenin in the epidermis in C, which serves as the internal positive control. (Bar = 500 µm in C, and 50 µm in D.)
Discussion
Matrical tumors of the hair follicle constitute a spectrum that includes those from the common benign pilomatricomas to the exceptional melanocytic matricomas.1–8 Melanocytic matricomas contain dendritic melanocytes admixed with the epithelial tumor cells, and the malignant variant called melanocytic pilomatrix carcinoma is the rarest of the group.9
Hair matrical tumors have features that resemble the stages of hair development. During the development of the hair follicle, primitive mesenchymal cells form the dermal papilla and release soluble Wnt signals that prevent the degradation of β-catenin in follicu lar epithelial cells. Stable intracytoplasmic β-catenin translocates to the nucleus in the inner hair matrix cells and acts as a transcription factor activating Lef1 and down-regulating the cell-cell adhesion protein E-cadherin. In contrast, β-catenin is distributed at the cell membranes in the outer and inner follicular root sheaths.15 Therefore, β-catenin has a dual role in hair development. It acts as a transcription factor in matrix cells and is part of the cadherin/catenin cell-cell adhesion complex in the differentiated follicular cells, where it is co-expressed with E (epithelial)-cadherin and P (placental)-cadherin.13,16,17 Mutation and consequent aberrant transcriptional activation of β-catenin and up-regulation of Wnt signals was demonstrated in benign pilomatricomas.18,19 By immunohistochemistry, pilomatricomas are seen to express nuclear β-catenin in the basaloid and transitional cells.20,21 In contrast, the fully differentiated shadow cells are negative for β-catenin and are not under the control of Wnt signals.22 Similarly to benign pilomatricomas, malignant pilomatricomas have frequent mutations in the exon 3 of CTNNB1, the gene encoding for β-catenin,23 and the β-catenin expression can be decreased, probably reflecting low E-cadherin21 as in other epithelial tumors with aggressive behavior. In our previous work24 we showed that melanocytic matricoma exhibits β-catenin in the nucleus and cytoplasm of basaloid cells. In contrast β-catenin was expressed in the plasma membrane of the more differentiated cells. In this paper we show that, in contrast to the plasma membrane distribution of E- and P-cadherin, β-catenin is expressed diffusely in the nucleus and cytoplasm of all tumor cells in two cases of melanocytic pilomatrix carcinoma. Comparative results of β-catenin mutation and immunohistochemical distribution in benign and malignant pilomatrix tumors are shown in Table 1. These results are the first to show expression of cadherins and β-catenin in melanocytic pilomatrix carcinoma, and are consistent with a widespread transcriptional role of β-catenin throughout the tumor cells.
Table 1. List of reports and studies of benign and malignant variants of pilomatrical tumors and their β-catenin mutations and immunohistochemical distribution.
| Hair matrical tumor | β-catenin mutations | Nuclear β-cat (IHC) | Source |
|---|---|---|---|
| Pilomatricoma | Frequent | In basaloid cells | Gat et al., 199818 |
| Park et al., 200120 | |||
| Hassanein and Glanz, 200421 | |||
| Xia et al., 200619 | |||
| Matricoma | Not known | Not known | Ackerman et al., 19931 |
| Melanocytic matricoma | Not known | In basaloid cells | Peralta Soler et al., 200724 |
| Pilomatrix carcinoma | Frequent | In basaloid cells | Hassanein and Glanz, 200421 |
| Lazar et al., 200523 | |||
| Melanocytic pilomatrix carcinoma | Not known | In all cells | This paper |
Acknowledgments
Acknowledgements: we thank Rachel Tierny of the Richfield Laboratory of Dermatopathology for helping with the photography work.
References
- 1.Ackerman AB, DeViragh PA, Chongchtnant N. Pilomatricoma and matricoma. In: Spahr JF, editor. Neoplasms with Follicular Differentiation. Philadelphia, PA: Lea and Febiger; 1993. pp. 477–506. [Google Scholar]
- 2.Kaddu S, Soyer HP, Cerroni L, et al. Clinical and histopathologic spectrum of pilomatricomas in adults. Int J Dermatol. 1994;33:705–8. doi: 10.1111/j.1365-4362.1994.tb01515.x. [DOI] [PubMed] [Google Scholar]
- 3.Spitz D, Fisher D, Friedman RJ, Kopf AW. Pigmented pilomatricoma. A clinical simulator of malignant melanoma. J Dermatol Surg Oncol. 1981;7:903–6. doi: 10.1111/j.1524-4725.1981.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 4.Weedon D, Strutton G. In: Skin Pathology. 2nd edn. Houston MJ, editor. New York, NY:: Churchill Livingstone (Elsevier Science Ltd); 2002. pp. 869–71. [Google Scholar]
- 5.Kaddu S, Soyer HP, Wolf IH, Kerl H. Proliferating pilomatricoma. A histopathologic simulator of matrical carcinoma. J Cutan Pathol. 1997;24:228–34. doi: 10.1111/j.1600-0560.1997.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 6.Dutta R, Boadle R, Ng T. Pilomatrix carcinoma: case report and review of literature. Pathology. 2001;33:248–51. [PubMed] [Google Scholar]
- 7.Hardisson D, Linares MD, Cuevas-Santos J, Contreras F. Pilomatrix carcinoma: a clinicopathologic study of six cases and review of the literature. Am J Dermatopathol. 2001;23:394–401. doi: 10.1097/00000372-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Monteagudo C, Fernández-Figueras MT, San Juan J, et al. Matrical carcinoma with prominent melanocytic hyperplasia (malignant melanocytic matricoma?) A report of two cases. Am J Dermatopathol. 2003;25:485–9. doi: 10.1097/00000372-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174–7. doi: 10.1097/DAD.0b013e318165b8fe. [DOI] [PubMed] [Google Scholar]
- 10.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–52. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 11.Grunwald GB. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993;5:797–805. doi: 10.1016/0955-0674(93)90028-o. [DOI] [PubMed] [Google Scholar]
- 12.Hirai Y, Nose A, Kobayashi S, Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. II. Skin morphogenesis. Development. 1989;105:271–7. doi: 10.1242/dev.105.2.271. [DOI] [PubMed] [Google Scholar]
- 13.Müller-Röver S, Tokura Y, Welker P, et al. Pcadherin expression during murine hair follicle morphogenesis and cycling. Exp Dermatol. 1999;8:237–46. doi: 10.1111/j.1600-0625.1999.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 14.Sprecher E, Bergman R, Richard G, et al. Hypotrichosis with juvenile macular dystrophy is caused by a mutation in CDH3, encoding P-cadherin. Nat Genet. 2001;29:134–6. doi: 10.1038/ng716. [DOI] [PubMed] [Google Scholar]
- 15.Ridanpää M, Fodde R, Kielman M. Dynamic expression and nuclear accumulation of beta-catenin during the development of hair follicle-derived structures. Mech Dev. 2001;109:173–81. doi: 10.1016/s0925-4773(01)00563-9. [DOI] [PubMed] [Google Scholar]
- 16.Hardy MH, Vielkind U. Changing patterns of cell adhesion molecules during mouse pelage hair follicle development. 1. Follicle morphogenesis in wild-type mice. Acta Anat (Basel) 1996;157:169–82. doi: 10.1159/000147879. [DOI] [PubMed] [Google Scholar]
- 17.Behrens J, von Kries JP, Kühl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 18.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 19.Xia J, Urabe K, Moroi Y, et al. Beta-catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci. 2006;41:67–75. doi: 10.1016/j.jdermsci.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Park SW, Suh KS, Wang HY, et al. Beta-catenin expression in the transitional cell zone of pilomatricoma. Br J Dermatol. 2001;145:624–9. doi: 10.1046/j.1365-2133.2001.04433.x. [DOI] [PubMed] [Google Scholar]
- 21.Hassanein AM, Glanz SM. Beta-catenin expression in benign and malignant pilomatrix neoplasms. Br J Dermatol. 2004;150:511–6. doi: 10.1046/j.1365-2133.2004.05811.x. [DOI] [PubMed] [Google Scholar]
- 22.Cribier B, Worret WI, Braun-Falco M, et al. Expression patterns of hair and epithelial keratins and transcription factors HOXC13, LEF1, and beta-catenin in a malignant pilomatricoma: a histological and immunohistochemical study. J Cutan Pathol. 2006;33:1–9. doi: 10.1111/j.0303-6987.2006.00308.x. [DOI] [PubMed] [Google Scholar]
- 23.Lazar AJ, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148–57. doi: 10.1111/j.0303-6987.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 24.Soler AP, Burchette JL, Bellet JS, Olson JA., Jr Cell adhesion protein expression in melanocytic matricoma. J Cutan Pathol. 2007;34:456–60. doi: 10.1111/j.1600-0560.2006.00649.x. [DOI] [PubMed] [Google Scholar]