Abstract
Monodermal teratomas of the ovary can take the form of carcinoid tumors of which there are several types, mucinous carcinoid being the least common. Very few cases of primary mucinous carcinoid of the ovary have been reported in the literature and the behavior of these tumors over the long term is unclear. We describe a case of primary mucinous carcinoid of the ovary in a 39-year-old woman treated with unilateral salpingo-oophorectomy, where a metastasis occurred in the contralateral ovary ten years later. This case demonstrates that mucinous carcinoid of the ovary can metastasize even after a long interval, and careful follow-up of patients, particularly those treated conservatively, is appropriate.
Key words: mucinous carcinoid, metastasis, ovary
Introduction
Fewer than thirty cases of primary mucinous carcinoid of the ovary have been described in the literature and the clinical behavior of this tumor has not been well defined.1,2 We describe a patient treated by unilateral oophorectomy who presented with a metastasis in the contralateral ovary more than ten years later.
Case Report
A 39-year-old woman presented with weight loss and abdominal distention and was found to have a pelvic-abdominal mass. Her previous history included an appendectomy 27 years earlier and a negative laparoscopy four years previously. At laparotomy there was a 10 cm mass originating from the left ovary, and a left salpingo-oophorectomy was performed. The uterus and right ovary appeared normal at operation, as did the peritoneum, liver, diaphragm, and lymph nodes. A diagnosis of mucinous carcinoid of the left ovary was made after postoperative gastroscopy, computer tomography (CT) scan, mammography, ultrasound scan of the liver, and urine test for the serotonin metabolite 5-HIAA proved negative. In the light of these negative findings, and given a strong desire on the part of the patient for children, a decision was made to treat her conservatively.
The patient was followed up for ten years but shortly after, now 49 years old, she presented with constipation and abdominal discomfort. A CT scan showed a mass measuring 15×10 cm originating from the right ovary. A right salpingo-oophorectomy and partial omentectomy were performed; no abnormalities were seen on the peritoneum, liver, or diaphragm at operation and there was no obvious lymph node swelling. A histological diagnosis of metastatic mucinous carcinoid was made. Currently, five years after the second laparotomy, the patient remains well.
Pathological findings
Macroscopic appearances
The first tumor, from the left ovary, weighed 394 g and measured 9.0 cm in maximum diameter (sampled in nine blocks). The cut surface was partly solid and homogeneous with a light brown color and partly cystic. The right ovarian tumor, removed ten years later, was 19 cm in diameter and weighed 1900 g (sampled in 20 blocks). This lesion was predominantly cystic, with a solid area of 5 cm in diameter.
Microscopic appearances
The original tumor from the left ovary showed predominantly small, round glands lined by cells with globules of intracytoplasmic mucin, lying in a collagenous stroma (Figure 1A) or floating in lakes of mucin. In most of the tumor the epithelial cell nuclei were small and basal with not more than mild atypia (Figure 1B). In some areas, however, the glands were lined with more obviously atypical cells (Figure 1C). Here the glands lay closer together, sometimes abutting on one another. In one area there were smaller glands with some cell nests and individual signet ring cells. There was no severe cytological atypia, solid growth of tumor cells or necrosis. An occasional cystic space was present lined by goblet cells with some stratification. Mitotic activity in the more atypical areas reached 10 mitoses per 10 HPF. Elsewhere in the tumor the mitotic rate was lower but varied, with some areas showing 6 mitoses per 10 HPF.
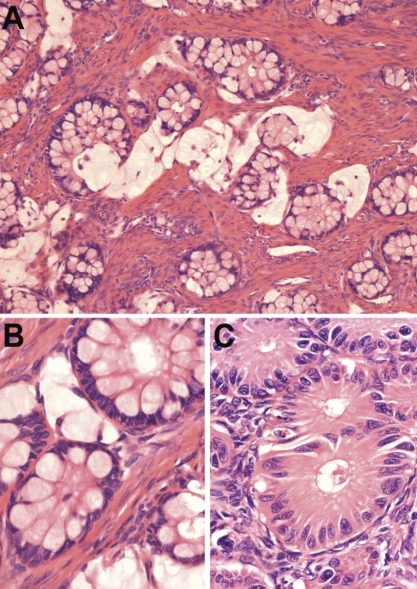
Figure 1.
(A)Primary tumor (left ovary): small round glands sometimes floating in lakes of mucin typified the primary tumor (hematoxylin and eosin, 100×). (B) Glands with small minimally atypical basal nuclei predominated in this tumor (hematoxylin and eosin, 400×). (C) In places there was moderate cytological atypia and some fusing of glands (hematoxylin and eosin, 400×).
The metastatic tumor from the right ovary showed round glands with an epithelial lining that included goblet cells. Between the goblet cells cylindrical cells with granular eosinophilic cytoplasm were present. Occasionally there were glands with small basal nuclei with mild atypia, but in most areas the glands showed a moderate epithelial atypia (Figure 2). In some areas the glands lay closer together, but nowhere was there a confluent growth pattern or cribriform growth. There were no solid areas. Individual signet ring cells in the stroma were not a feature of this tumor. The cysts seen macroscopically were a prominent feature and were lined by the same cell population as the small glands, often with multilayering (Figure 2). Fairly extensive areas of necrosis were present, involving glands and stroma and interpreted as infarction rather than true tumor necrosis. Mitotic activity was focally brisk, 17 per 10 HPF. In neither of the specimens was there any other tumor component (for example, struma or other carcinoid type or mature teratoma component) identified. No vascular or lymphatic invasion was identified.
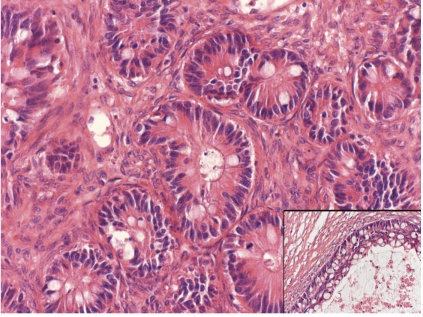
Figure 2.
Metastatic tumor (right ovary). Glands showing moderate cytological atypia predominated in this tumor. Note the cystic spaces lined by stratified atypical epithelium (inset) (hematoxylin and eosin, 200×).
Immunohistochemistry
Epitope retrieval was heat-induced and the buffer used was tris-EDTA, pH 9. Both tumors showed positivity for chromogranin (Biogenics LK2H10, 1;200). In the primary tumor, staining varied from sporadic cells in some areas to staining of several cells in every gland in other areas. In the metastasis, almost all glands showed staining, varying from a few cells to 50% of the cells in the gland (Figure 3A). Synaptophysin (Dako M0776, 1:400) staining was positive in occasional cells (<5%) in the primary tumor (Figure 3B), but no positive cells were found in the metastasis. NSE (Dako BBS/NC/V1H14, 1:50) and CD56 (Neomarker 123c3.D5, 1:25) staining were negative.
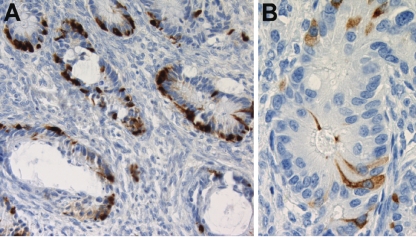
Figure 3.
Immunohistochemistry in primary tumor and metastatic tumor. (A) Metastatic tumor (right ovary): cells staining positively for chromogranin were present in most glands (200×). (B) Primary tumor (left ovary): scattered cells stained positively with synaptophysin, in a gland with cytological atypia (400×).
Molecular biology
To determine the relationship between the two tumors, loss of heterozygosity (LOH) analysis was performed. The area of the primary tumor showing the most cytological and architectural atypia was selected for analysis. Sixteen microsatellite markers on 12 different chromosomes were investigated for LOH. With four microsatellite markers on four different chromosomes, LOH was observed. An identical pattern of loss in both tumors was found with two microsatellite markers, D8S133 on chromosome 8p and AFMa086WG9 in intron1 of the PTEN gene on 10q. In addition, there was LOH identified only in the later tumor with markers D11S419 on chromosome 11p and D13S1307 on 13q.
Discussion
Mucinous carcinoid is an unusual tumor in the ovary and has been described more often as metastatic from the appendix than as a primary lesion.3–11 Distinguishing primary ovarian carcinoid from metastatic carcinoid can be very difficult. Primary carcinoids of the ovary are generally unilateral. Metastatic carcinoids are nearly always bilateral and scattered tumor deposits are present throughout both ovaries.3 In the case we describe, the appendix had been removed 27 years before the first ovarian tumor occurred, effectively excluding the appendix as the primary. After the initial surgery, extensive investigation failed to reveal any tumor elsewhere and no other primary has manifest itself in the following 15 years.
We regard the second ovarian tumor as a metastasis, and not as a second primary, on the grounds of the similar histological picture and the findings of the molecular biology analysis. Immunohistochemical staining for chromogranin was positive, and there was scanty staining for synaptophysin in the first tumor. These immunohistochemical stains can support neuroendocrine differentiation, but are not specific for mucinous carcinoid. The morphology is also of key importance. The fact that two identical markers were lost points to the tumors being the same entity. The new abnormalities in the metastatic tumor would represent further chromosomal abnormalities acquired during the progression of the tumor. Our patient did not receive adjuvant chemo-therapy after her second operation, because the literature suggests surgical resection of recurrent disease for ovarian carcinoid tumors,12,13 and to our knowledge there is no clear literature on management of metastatic mucinous carcinoid.
The paucity of cases described in the literature means that the behavior of ovarian mucin ous carcinoid is not clearly defined. Baker et al. 1 described 17 cases that they subdivided into three groups, initially characterized by them as grade 1, 2, and 3 but ultimately classified as well-differentiated, atypical, and carcinoma arising in mucinous carcinoid, in an attempt to conform to the terminology used in appendiceal carcinoid. They then attempted to correlate the histological type with the behavior. These authors observed a gradation in mitotic activity, cytological atypia, and architectural abnormality along the spectrum of lesions they describe. They commented that although they found no metastases in their “well-differentiated” or “atypical” categories they nevertheless suspect that these lesions have a low malignant potential. Two cases were categorized as carcinoma arising in mucinous carcinoid, both more than stage I, and were fatal within one year. All other patients, even within this category, were stage I and remained well. No cases of late metastasis were observed in this series.
Our case showed areas in the primary tumor that probably can best be classified as atypical according to the categorization of Baker et al.,1 although the bulk of the tumor was well differentiated. The metastasis showed much more widespread cytological atypia; however, the severe cytological atypia, solid growth pattern, and tumor-type necrosis described by Baker et al1 in their examples of carcinoma arising in mucinous carcinoid were not apparent. The tumors we describe are difficult to place exactly in the categories described by Baker et al.,1 but the atypical histological features coupled with the prolonged clinical course seem to match their suggestion of a spectrum of histological abnormality and clinical behavior in these lesions. On the basis of the case described here we suggest that any atypical architecture or increased mitotic activity, even focally, signals a need for caution in assessing prognosis.
Take home messages:
1. Primary mucinous carcinoid in the ovary is a rare tumor and should be diagnosed only after rigorous exclusion of a primary elsewhere.
2. The course of the disease may be prolonged with metastases after many years.
3. Atypical architecture or increased mitotic activity, even focally, signals a need for caution in assessing prognosis.
References
- 1.Baker PM, Oliva E, Young RH, et al. Ovarian mucinous carcinoids including some with a carcinomatous component. A report of 17 cases. Am J Surg Pathol. 2001;25:557–68. doi: 10.1097/00000478-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Alenghat E, Okagaki T, Talerman A. Primary mucinous carcinoid tumor of the ovary. Cancer. 1986;58:777–82. doi: 10.1002/1097-0142(19860801)58:3<777::aid-cncr2820580327>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Robboy SJ, Scully RE, Norris HJ. Carcinoid metastatic to the ovary: a clinicopathologic analysis of 35 cases. Cancer. 1974;33:798–811. doi: 10.1002/1097-0142(197403)33:3<798::aid-cncr2820330327>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Hristov AC, Young RH, Vang R, et al. Ovarian metastases of appendiceal tumours with goblet cell carcinoid-like and signet ring cell patterns; a report of 30 cases. Am J Surg Pathol. 2007;31:1502–11. doi: 10.1097/PAS.0b013e31804f7aa1. [DOI] [PubMed] [Google Scholar]
- 5.Ronnett BM, Kurman RJ, Shmookler BM, et al. The morphologic spectrum of ovarian metastases of appendiceal adenocarcinomas. A clinicopathologic and immunohistochemical analysis of tumors often misinterpreted as primary ovarian tumors or metastatic tumors from other gastrointestinal sites. Am J Surg Pathol. 1997;21:1144–55. doi: 10.1097/00000478-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Haqqani MT, Williams G. Mucin producing carcinoid tumors of the vermiform appendix. J Clin Pathol. 1977;30:473–80. doi: 10.1136/jcp.30.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hood IC, Jones BA, Watts JC. Mucinous carcinoid tumor of the appendix presenting as bilateral ovarian tumors. Arch Pathol Lab Med. 1986;110:336–40. [PubMed] [Google Scholar]
- 8.Heisterberg L, Wåhlin A, Nielsen KS. Two cases of goblet cell carcinoid tumor of the appendix with bilateral ovarian metastases. Acta Obstet Gynecol Scand. 1982;61:153–6. doi: 10.3109/00016348209156547. [DOI] [PubMed] [Google Scholar]
- 9.Thomas R, Barnhill D, Worsham F, et al. Krukenberg tumor of the ovary from an occult appendiceal primary: case report and literature review. Obstet Gynecol. 1985;65:95–8s. [PubMed] [Google Scholar]
- 10.Zirkin RM, Brown S, Hertz M. Adenocarcinoid of appendix presenting as bilateral ovarian tumors. A case report with histochemical and ultrastructural studies. Diagn Gynecol Obstet. 1980;2(3):269–74. [PubMed] [Google Scholar]
- 11.Paone JF, Bixler TJ, Imbembo AL. Primary mucinous adenocarcinoma of the appendix with bilateral Krukenberg ovarian tumors. Johns Hopkins Med J. 1978;143:43–7. [PubMed] [Google Scholar]
- 12.Timmins PF, Yi-Shin Kuo D, Anderson PS, et al. Ovarian carcinoid: management of primary and recurrent tumors. Gynecol Oncol. 2000;76:112–4. doi: 10.1006/gyno.1999.5608. [DOI] [PubMed] [Google Scholar]
- 13.Davis KP, Hartmann LK, Keeney GL, et al. Primary ovarian carcinoid tumors. Gynecol Oncol. 1996;61:259–65. doi: 10.1006/gyno.1996.0136. [DOI] [PubMed] [Google Scholar]