Abstract
The nuclear factor κB transcription factor c-Rel is exclusively expressed in immune cells and plays a role in numerous cellular functions including proliferation, survival and production of chemokines and cytokines. c-Rel has also been implicated in the regulation of multiple genes involved in innate and adaptive immune responses to the intracellular protozoan parasite Toxoplasma gondii, in particular IL-12. To better understand how this transcription factor controls the CD8+ T-cell response to this organism, wild-type (WT) and c-Rel−/− mice were challenged with a replication-deficient strain of T. gondii that expresses the model antigen ovalbumin (OVA). These studies revealed that c-Rel was required for optimal primary expansion of OVA-specific CD8+ T cells and that immunized c-Rel-deficient mice were susceptible to challenge with a virulent strain of T. gondii. However, when c-Rel−/− cells specific for OVA were adoptively transferred into a WT recipient, or c-Rel−/− mice were treated with IL-12 at the time of immunization, there was no apparent proliferative defect. Surprisingly, upon secondary challenge, antigen-specific CD8+ T cells in c-Rel−/− mice expanded to a much greater degree in terms of frequency as well as numbers when compared with WT mice. Despite this, the cytokine responses of c-Rel−/− mice remained defective, consistent with their susceptibility to secondary challenge. Together, these results indicate that in this infection model, the major influence of c-Rel in generation of CD8+ T-cell responses is through its regulation of the inflammatory environment, rather than playing a substantial T-cell-intrinsic role.
Keywords: adaptive immune response, cytokines, inflammation, immunization, memory, parasite infection
Introduction
The nuclear factor-κB (NF-κB) family of transcription factors consists of five genes with similar structures and DNA recognition sequences: p50, p65 (Rel-A), p52, Rel-B and c-Rel [reviewed in ref. (1)]. The nuclear translocation of these transcription factors occurs in response to a wide variety of stimuli, ranging from T- and B-cell receptor and co-receptor signaling to activation via cytokines or microbial products. These events lead to the expression of a number of genes related to cell survival and proliferation as well as cytokine and chemokine production and co-ordinate innate and adaptive immune responses to infection (2, 3).
c-Rel expression is known to be restricted to hematopoietic cells (4–6) and was first identified due to its ability to transform B and T cells and induce lymphomas (7, 8). Early work documented that T cells lacking c-Rel proliferated poorly to polyclonal stimuli and displayed defects in the production of IL-2, granulocyte macrophage colony-stimulating factor and IL-3, thus providing critical insights into some potential targets of c-Rel (9, 10). The importance of c-Rel in the regulation of immunity is illustrated by multiple studies in which mice genetically deficient for this transcription factor were examined in a range of infectious and inflammatory settings. In murine systems, the development of autoimmune encephalomyelitis required expression of c-Rel in immune cells (11) and c-Rel has also been shown to be required for the development of diabetes (12) and colitis (13). In a model of influenza infection, c-Rel was necessary for optimal viral clearance during primary and secondary responses but was not required for cytotoxic T lymphocyte (CTL) induction and effector function (14). c-Rel is also dispensable for induction of CTL responses against herpes simplex virus-1 (15). However, mice lacking c-Rel are resistant to infection with a helminth parasite, Trichuris muris, and can generate sufficient T-cell-mediated immune responses to mediate parasite expulsion, indicating that c-Rel is not essential for the development of a protective Th2 response (16).
The intracellular protozoan parasite Toxoplasma gondii has been used as an experimental system to help understand the role of individual NF-κB family members in the regulation of innate and adaptive immunity (17–21). Infection with this pathogen induces a strongly polarized Th1 response, and resistance is dependent on the cytokines IL-12 and IFN-γ (22–24). Previous work from this laboratory demonstrated that c-Rel is critically required for resistance to infection with T. gondii, indicating that it possesses unique functions that cannot be compensated for by other family members (19). In those initial studies, c-Rel-deficient mice were able to mount a Th1-polarized immune response but the magnitude of the response was diminished, and the ability to produce IFN-γ was impaired. Further, the ability of c-Rel−/− mice to produce IL-12 acutely at the site of infection was also decreased compared with wild-type (WT) mice (25). Dissection of this phenotype was complicated by the fact that c-Rel−/− mice do not survive infection with a replicating strain of T. gondii, which is normally associated with high antigen loads and tissue damage; consequently, primary studies were limited to the analysis of the initial effector response. Questions still remain about the role of c-Rel in the development of CD8+ T-cell effectors and whether this transcription factor is required for the generation or maintenance of antigen-specific memory CD8+ T cells.
In order to better address the role of c-Rel in the immune response to T. gondii, a recently described replication-deficient strain of T. gondii that expresses the model antigen ovalbumin (OVA) was utilized (26, 27). This vaccination strategy induces protective immunity that is dependent on CD8+ T cells and allows the use of tetramer staining to identify endogenous antigen-specific CD8+ T cells. The use of this experimental system revealed that c-Rel was required for optimal expansion of these parasite-specific CD8+ T cells. Administration of IL-12p70 to immunized mice partially restored the CD8+ effector T-cell response to T. gondii, indicating that T-cell-extrinsic factors contribute to the initial expansion of the adaptive CD8+ T-cell response. When later time points following immunization were examined, c-Rel−/− mice maintained similar levels of tetramer+ cells in the blood, bone marrow and spleen, indicating that c-Rel is not required for maintenance of these cells. Further, c-Rel was not required for expression of markers associated with memory, such as IL-7Rα or Bcl-2, nor for the production of IL-2 at these time points. Surprisingly, upon secondary challenge, OVA-specific CD8+ T cells in c-Rel−/− mice expanded to a much greater degree in terms of frequency and numbers when compared with WT mice. Despite this, the cytokine responses of c-Rel−/− mice remained defective, consistent with their susceptibility to secondary challenge. Together, these results indicate that the major influence of c-Rel in the generation of a CD8+ T-cell response in this infection model is through its regulation of the inflammatory environment, rather than playing a substantial T-cell-intrinsic role.
Methods
Mice and infections
C57BL/6, CD45.1 and Thy1.1 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) or National Cancer Institute (Frederick, MD, USA). c-Rel−/− mice were originally obtained from Tumang et al. (28) and were bred at the University of Pennsylvania. OT-I GFP mice were obtained from the laboratory of Wolfgang Weninger at the Wistar Institute (29). The following mice were obtained through the NIAID Exchange Program (Taconic line 4175): B6[TG]TCR-OT-I RAG1[KO] (30, 31). Mice were maintained under specific pathogen-free conditions and all animal work was done in accordance with the Institutional Animal Care and Use Guidelines of the University of Pennsylvania. For all immunization experiments using CPS-OVA, mice were injected intraperitoneally (i.p.) with 105 parasites. For challenge experiments, mice were given 103 RH-OVA tachyzoites (RH parental strain expressing secreted OVA) or 106 CPS-OVA, and for adoptive transfer experiments, mice received 105 Pru-OVA tachyzoites i.p. Tachyzoites were grown in human foreskin fibroblast monolayers in DMEM containing 1% FCS (CPS and RH parasites) or 10% FCS (Pru parasites) and 1% penicillin–streptomycin. CPS parasites were cultured in media containing 0.2 mM uracil, and OVA-transgenic parasites were maintained in media additionally containing 20 μM chloramphenicol.
For T-cell depletion experiments, mice received 0.5 mg of anti-CD4 (clone RM4.5) or anti-CD8 (clone 2.43) twice in the week prior to challenge. For depletion of IL-12, mice received two injections of anti-IL12p40 (clone C17.8) on day −1 and day 2 of immunization. For rescue of antigen-specific responses, c-Rel−/− mice received three i.p. injections containing 500 ng of recombinant IL-12p70 (eBioscience, San Diego, CA, USA) on days 0, 1 and 2 of infection. For adoptive transfer experiments, T cells were isolated via negative selection (R&D Systems, Minneapolis, MN, USA); mice received 5 × 105 WT or c-Rel−/− OT-I cells or 107 immune T cells (isolated 30 days following immunization) 1 day prior to infection or secondary challenge.
In vitro T-cell responses
Spleen, lymph node and peritoneal exudate cells (PECs) were harvested and dissociated into single-cell suspensions in complete RPMI 1640 (Gibco/Invitrogen, Carlsbad, CA, USA) containing 10% heat-inactivated FCS, 10 U ml−1 penicillin, 100 μg ml−1 streptomycin, 2 mM glutamine, 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 0.1 mM non-essential amino acids and 50 μM 2-beta mercaptoethanol. For analysis of PBMC, blood was collected and mixed with sodium citrate prior to lysis of erythrocytes with 0.86% ammonium chloride buffer. Spleen and lymph node cells (5 × 105 per well) and PECs (105 per well) were plated out in triplicate in 96-well round bottom plates (Costar, Carlsbad, CA) and cultured to assess for cytokine production. Cells were re-stimulated with anti-CD3 (1 μg ml−1) soluble Toxoplasma antigen (STAg; 10–25 μg ml−1), 500 μg ml−1 OVA (Worthington Biochemical Corporation, Lakewood, NJ, USA) or 1 μg ml−1 SIINFEKL peptide (CHI Scientific, Maynard, MA, USA). Supernatants were removed after ∼48 h and assayed for the production of IFN-γ by ELISA.
Flow cytometry
For identification of OVA-specific T-cell responses, single-cell suspensions were washed in FACS buffer (PBS + 2 mM EDTA + 2% BSA) and incubated for 15 min with Fc block (FACS Buffer containing 1 μg ml−1 2.4G2 from BD Pharmingen; rat and mouse IgG from Caltag/Invitrogen at 1 μg ml−1). Cells were stained with MHC class I/tetramer complexes of H-2Kb/SIINFEKL conjugated to PE or APC (Beckman Coulter Immunomics or generous gift from John Wherry at the Wistar Institute) for 25 min at room temperature, washed once and then stained for other surface markers for 15 min at 4°C. The following monoclonal antibodies were used: CD8 (conjugated to FITC, PerCP or APC), CD127 biotin, CD122 biotin, CD62L-APC or APC Alexa fluor 700 (BD Biosciences); tumor necrosis factor (TNF)-α FITC, IFN-γ-APC, IL-2 PE, killer cell lectin-like receptor G1 (KLRG1)-APC and Streptavidin–APC (eBioscience) and anti-human Granzyme B-APC (Caltag, Carlsbad, CA, USA).
For intracellular cytokine analysis, splenocytes or PECs were incubated (cell and antigen concentration as noted above) for 5.5 h total, with the addition of 10 μg ml−1 Brefeldin A (Sigma) for the final 4 h. Cells were first stained for surface markers, followed by fixation overnight with 2% PFA (Electron Microscopy Sciences, Hatfield, PA, USA). Cells were permeabilized with 0.1% saponin and then stained for intracellular cytokines for 1 h at 4°C. Flow cytometry samples were collected on a FACSCalibur or FACSCanto machine (BD Biosciences) and analyzed with FlowJo software (Tree Star Inc., Ashland, OR, USA).
Statistics
Statistical analysis was completed with Prism (Graphpad Software, La Jolla, CA, USA) using Student's t-test or one-way analysis of variance (ANOVA) where appropriate data are shown as mean ± standard deviation.
Results
c-Rel is required for optimal antigen-specific CD8+ T-cell expansion and resistance to secondary challenge
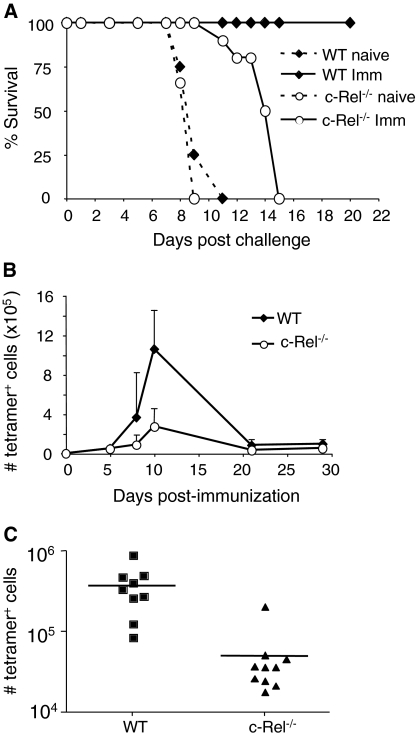
Because c-Rel−/− mice are susceptible to infection with replicating strains of T. gondii (25), a model was used in which a replication-deficient strain of T. gondii induces protective immunity mediated by CD8+ T cells (26). To first assess whether c-Rel was required for the ability of CPS-OVA to induce protective immunity, groups of WT and c-Rel−/− mice were immunized with 105 CPS-OVA parasites and challenged with virulent RH-OVA 30 days later. Naive WT and c-Rel−/− mice succumbed to infection between 8 and 9 days following infection (Fig. 1A). WT mice that had been immunized were able to control this challenge, whereas immunized c-Rel−/− mice were not able to survive challenge with RH-OVA. However, the immunized c-Rel−/− mice did display a modest but reproducible delay in susceptibility compared with naive mice (Fig. 1A, P = 0.0014 by one-way ANOVA). Similar to what was seen in previous work (26), the depletion of CD8+ T cells (but not CD4+ T cells) antagonized the ability of the immunized c-Rel−/− mice to survive challenge (data not shown).
Fig. 1.
c-Rel−/− mice generate sub-optimal CD8+ T-cell responses to immunization. (A) WT and c-Rel mice were immunized with 105 CPS-OVA parasites and challenged 30 days later with 103 RH-OVA (results combined from two similar experiments with four to five mice per group). (B) Kinetics of CD8+ antigen-specific response in the spleen (results from one of two similar experiments with three mice per time point). (C) Number of antigen-specific CD8+ T cells in the spleen 8 days following immunization (combined results from three similar experiments, each with three to four mice per group).
Since CD8+ T cells are required for protection against challenge in this infection model, studies were next performed to assess if the absence of c-Rel affected the generation of antigen-specific CD8+ T cells. Thus, WT and c-Rel−/− mice were immunized with CPS-OVA and the antigen-specific response was tracked using a tetramer reagent that recognizes T-cell receptors specific for the immunodominant SIINFEKL epitope derived from OVA, in the context of MHC class I H2Kb. Analysis of the primary OVA-specific response revealed that WT mice displayed a robust primary expansion (Fig. 1B). The kinetics of expansion of this population was similar in the spleen and PECs of WT and c-Rel−/− mice; however, consistent with their increased susceptibility to rechallenge, c-Rel−/− mice exhibited a decrease in frequency as well as number of OVA-specific CD8+ T cells at all time points examined in the first 30 days of infection (Fig. 1B and data not shown). The combined results from three experiments analyzed 8 days following immunization demonstrated that both the frequency (7.175 ± 0.76% versus 1.8 ± 0.35%, P < 0.0001) as well as absolute number (3.76 × 105 ± 8.0 × 104 versus 5.04 × 104 ± 1.76 × 104; P = 0.0006) of antigen-specific cells generated in the spleens of c-Rel−/− mice were significantly reduced compared with WT control mice (Fig. 1C). Similar results were seen at this time point in the PECs as well as the blood (data not shown). Thus, during the primary response to CPS-OVA immunization, c-Rel is required for the optimal development of antigen-specific CD8+ T cells, and this correlates with insufficient protection during secondary challenge.
c-Rel is not required for effector function but is required to up-regulate the activation marker KLRG1
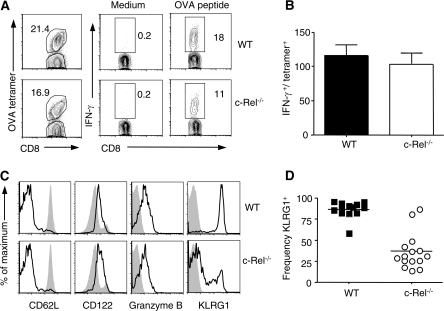
The data shown in Fig. 1 indicate that c-Rel influences the magnitude of the CD8+ T-cell response; however, subsequent studies were performed to determine if the antigen-specific cells generated in these mice were functionally competent. Therefore, PECs from immunized WT and c-Rel−/− mice taken 8 days post-immunization were stimulated ex vivo with OVA peptide. As T cell receptor (TCR) stimulation leads to down-regulation of tetramer binding (32, 33) dual staining for cytokines and OVA tetramer was not successful. However, in response to anti-CD3 (data not shown) or OVA peptide stimulation (Fig. 2A and B), both WT and c-Rel−/− cells were capable of making IFN-γ and TNF-α. This conclusion was drawn by comparing the frequency of cells that produced IFN-γ ex vivo in response to peptide stimulation to the frequency of OVA-specific tetramer+ cells in the tissues of individual mice (Fig. 2A and B). Of the cells that were making IFN-γ in response to OVA peptide, there was no difference in the mean fluorescence intensity (MFI) of this cytokine (535 ± 21 versus 491.7 ± 23, P = 0.3; n = 3 per group from one representative experiment). Similar frequencies of cells that made IFN-γ could also produce TNF-α ex vivo (32.7 ± 5.4 versus 32.6 ± 7.7; n = 3 per group from one representative experiment), and the MFI of TNF-α from double producers did not differ between WT and c-Rel−/− mice (468.7 ± 2.9 versus 472 ± 4.2; n = 3 per group from one representative experiment). Furthermore, cells from the PECs or spleen were also stimulated polyclonally with anti-CD3 and analyzed for ex vivo cytokine production as well as by ELISA. At all time points examined, a smaller percentage of CD8+ T cells from c-Rel−/− mice were able to produce IFN-γ. Nevertheless, as with OVA peptide stimulation, a similar frequency of the cells that produced IFN-γ could also make TNF-α (data not shown). Similarly, when splenocytes and PECs were stimulated with STAg, less IFN-γ was found in supernatants from c-Rel−/− mice as compared with WT mice (data not shown); however, this is consistent with the reduced numbers of antigen-specific cells following immunization. Moreover, when an overnight in vivo cytolysis assay was used to determine if c-Rel−/− OVA-specific CD8+ T cells could mediate cytotoxicity of SIINFEKL-pulsed targets, killing of target cells was comparable to that seen in WT mice (data not shown). Taken together, these data are consistent with the idea that although c-Rel−/− mice generate reduced numbers of antigen-specific CD8+ T cells following immunization, those that do expand are functionally competent.
Fig. 2.
Function and phenotype of antigen-specific CD8+ T cells. (A) Representative example used to calculate ability of antigen-specific cells to produce IFN-γ (top row, WT and bottom row, c-Rel−/−). Cells from the peritoneal exudate were stimulated ex vivo with SIINFEKL peptide and gated on CD8+ in right-hand panels. The frequency of IFN-γ-producing cells was divided by the frequency of tetramer+ CD8+ T cells as shown in the left-hand panels. Summary of these data are shown in (B). (C) Representative WT (top row, black line) and c-Rel−/− (bottom row, black line) tetramer+ splenocytes were compared with CD8+ T cells from naive mice (filled gray histogram). (D) Frequency of KLRG1+ subpopulation of tetramer+ splenocytes in WT and c-Rel−/− mice (P < 0.0001). All experiments were repeated at least three times with three to four mice per group.
WT and c-Rel−/− splenocytes were next subjected to tetramer staining and compared with CD8+ T cells from naive WT mice (Fig. 2C). Extensive phenotypic characterization of splenocytes revealed no observable differences between tetramer+ subpopulations of WT and c-Rel−/− mice in their expression of CD62L, CD122 or Granzyme B (representative histograms shown in Fig. 2C). A significant phenotypic difference at the day 8 time point was the expression of the NK cell activation marker KLRG1, and significantly fewer tetramer+ splenocytes (Fig. 2D; 86.6 ± 2.7 versus 36.6 ± 5.6) or PECs (75.9 ± 2.8 versus 35.5 ± 5.7; P < 0.0001) from c-Rel−/− mice expressed this marker. Though originally described as an NK cell activation marker, it has since been shown that KLRG1 is up-regulated on CD4+ and CD8+ T cells following infection (34, 35) and further that its expression is linked to responsiveness to IL-12 (36–38). Overall, these data indicate that c-Rel−/− OVA-specific CD8+ T cells are functionally competent and suggest that differences in phenotype could be due to an IL-12-deficient environment.
A T-cell-extrinsic function for c-Rel in the regulation of CD8+ T-cell responses
In parallel, studies using a replication competent strain of T. gondii expressing OVA (Pru-OVA) were used to distinguish T-cell-intrinsic versus T-cell-extrinsic requirements for c-Rel. c-Rel−/− mice were crossed with OT-I transgenic mice to generate TCR transgenic CD8+ T cells lacking c-Rel. WT and c-Rel−/− OT-I cells were transferred into Thy-disparate recipient mice that were infected 1 day later with Pru-OVA. When mice were analyzed between 7 and 9 days following infection, WT and c-Rel−/− OT-I cells expanded similarly in terms of frequency (Fig. 3A) as well as number (Fig. 3B). This finding indicates that, in this system of antigen-induced proliferation, CD8+ T cells themselves do not intrinsically require c-Rel. Further, it suggests that other factors in the c-Rel−/−environment, such as pro-inflammatory cytokines or CD4+ T-cell help, might contribute to the decreased generation of tetramer+ cells in c-Rel−/− mice. The WT host environment also impacted the phenotype of the transferred antigen-specific OT-I cells because the c-Rel−/− OT-I cells expressed similar levels of KLRG1 compared with WT cells (Fig. 3C).
Fig. 3.
WT and c-Rel−/− OT-I cells expand to a similar degree in WT recipients. 5 × 105 naive WT and c-Rel−/− OT-I cells (expressing Thy1.2) were purified and transferred into naive Thy1.1 recipients. One day later, mice were infected with 104 Pru-OVA. At various time points following infection, mice were analyzed and donor cells were identified by Thy1.2 and CD8 staining. Donor cell frequency (A) and number (B) are shown, with each point representing an individual mouse. (C) WT (left plot, black line) and c-Rel−/− (right plot, black line) donor cell expression of KLRG1 compared with CD3+CD8+ T cells from naive mice (shaded gray histogram). Representative data from one of two similar experiments with at least three mice per time point.
Reciprocal adoptive transfer experiments were also completed, in which WT OT-I cells that express GFP (29) were transferred into WT and c-Rel−/− recipients. Consistent with the results from Fig. 2(D), c-Rel was required within the host environment for proper activation of donor CD8+ T cells. Phenotypic analysis revealed that expression of KLRG1 on WT OT-I donor cells was decreased in c-Rel−/− recipient mice compared with WT recipient mice (44.8 ± 1.3% KLRG1+ versus 26.1 ± 0.8%, P = 0.002, see representative plots in Supplementary Figure 1 is available at International Immunology Online). In contrast, the donor WT OT-I cells expressed similar levels of CD44, CD122 and CD127, regardless of whether they were transferred into WT or c-Rel−/− recipients. Together with the data from Fig. 3, these findings suggest that the inability of cells to specifically up-regulate KLRG1 in c-Rel−/− mice is not intrinsic to the T cell but rather is dependent on the environment in which the cell expands in response to infection.
IL-12 can rescue initial CD8+ T-cell expansion
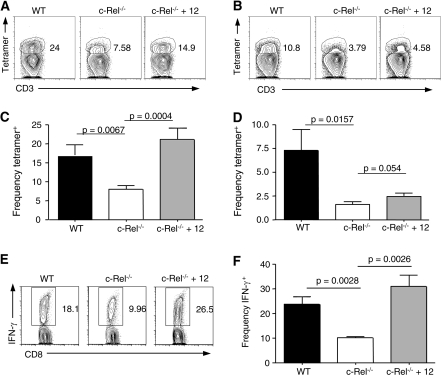
The pro-inflammatory cytokine IL-12 has been shown to contribute to the primary expansion of CD8+ T cells in response to infection with a number of organisms, serving as a ‘signal 3’ in addition to the obligatory TCR/MHC plus co-stimulation required to generate an antigen-specific response and has been linked to KLRG1 expression (38, 39). Indeed, when WT mice were treated with anti-IL-12p40 antibody during CPS-OVA immunization, there was a marked decrease in the generation of tetramer+ cells in the spleen (Supplementary Figure 2A is available at International Immunology Online). IL-12 depletion also caused a significant decrease in the frequency of tetramer+ splenocytes expressing KLRG1 (Supplementary Figure 2B is available at International Immunology Online). c-Rel−/− mice have previously been shown to exhibit defects in acute IL-12 responses to T. gondii at the site of infection (25); thus, it was pertinent to determine if this cytokine was impacting the development of the CD8+ T-cell response. Indeed, treatment with exogenous IL-12p70 restored OVA-specific CD8+ T-cell responses in the PECs (Fig. 4A and C) but did not consistently restore CD8+ responses in the spleen (Fig. 4B and D). Moreover, treated c-Rel−/− mice generated increased numbers and frequencies of antigen-specific cells in the PECs that could produce IFN-γ in response to OVA peptide stimulation (Fig. 4E and F). Together, these data establish that in the CPS-OVA immunization model, IL-12 dictates both the initial expansion as well as the ability to generate a KLRG1+ population of antigen-specific CD8+ T cells.
Fig. 4.
IL-12 can rescue antigen-specific responses in c-Rel−/− mice. Representative plot of antigen-specific responses by tetramer staining in the PECs (A) and spleen (B), gated on CD3+CD8+ cells. (C and D) c-Rel−/− mice that received exogenous IL-12p70 generated higher levels of tetramer+ cells in the PECs (C) but not the spleen (D). (E and F) Representative (E) or summarized (F) ex vivo IFN-γ production by PECs in response to SIINFEKL peptide from c-Rel−/− mice treated with IL-12. IL-12 treatment of c-Rel−/− mice was repeated four times with three to four mice per group; data from one representative experiment are shown.
c-Rel and the formation of memory CD8+ T cells
Attempts to clarify the role of c-Rel in the generation or maintenance of CD8+ T-cell memory have been limited, but the approach of using replication-deficient parasites allowed for investigation of these responses at late time points following infection. Because c-Rel has previously been linked not only with proliferation but also with the expression of pro-survival factors such as Bcl-xL and Bcl-2, it was not clear that immunization-induced antigen-specific cells would be able to survive (40). While 30 days following immunization, c-Rel−/− mice had reduced levels of OVA-specific cells; at time points 2–3 months after immunization, the levels of OVA-specific cells were not substantially different in WT and c-Rel−/− mice (Fig. 5A and B). Similar frequencies of OVA-specific cells were also identified in the blood and bone marrow, indicating that c-Rel−/− mice were able to maintain a pool of memory CD8+ T cells 2–3 months following immunization (data not shown). When splenocytes were stained at these time points for intracellular expression of Bcl-2, tetramer+ cells from c-Rel−/− mice actually expressed higher levels of this survival protein compared with WT mice (33 ± 0.97% versus 59.2 ± 13.7%, P = 0.04, with an n = 5 for WT and n = 3 for c-Rel−/− mice). Additionally, cytokine production in response to OVA protein was assessed ex vivo 60 days following immunization, and the ability of cells to co-produce IFN-γ and IL-2 was measured. In these studies, c-Rel−/− OVA-specific IFN-γ-producing CD8+ T cells demonstrated no differences in their ability to also produce IL-2 (18.93 ± 5.3% versus 14.7 ± 0.36%, P = .4685 and n = 3 for both groups from one representative experiment).
Fig. 5.
c-Rel is not required for maintenance of tetramer+ cells. (A) Representative plots of splenocytes from WT (top row) and c-Rel−/− mice (bottom row) immunized 3 months prior with 105 CPS-OVA. (B) Frequency of tetramer+ cells in the spleens of WT and c-Rel−/− mice showing combined results from four separate experiments and time points ranging from 60 to 90 days after immunization. (C) Overlays showing CD8+ T cells from the spleen of naive mice (shaded gray histogram) compared with WT (black line, top row) or c-Rel−/− (black line, bottom row) OVA-tetramer+ cells. Representative staining from one mouse per group 75 days following immunization is shown; experiment was repeated at least three times with similar results. (D) CD127 expression on WT and c-Rel−/− tetramer+ cells.
Similar to data previously published by this laboratory (26), in WT mice antigen-specific cells even at late time points following immunization were primarily KLRG1+ and expressed low levels of CD62L. Representative staining of splenocytes from WT and c-Rel−/− mice are shown at 60 days post-immunization (Fig. 5C). KLRG1, which was significantly lower on c-Rel−/− tetramer+ cells at acute time points, was now expressed at a similar frequency on OVA-specific cells. Moreover, CD27 and CD127 (the IL-7Rα chain) are surface molecules expressed at high levels on naive T cells that become down-regulated on recently activated T cells, before being preferentially expressed on memory CD8+ T cells (41). In naive WT and c-Rel−/− mice, the CD8+ T cells expressed comparable levels of these markers, but analysis of the memory populations at day 60 revealed that tetramer+ cells from the c-Rel−/− mice expressed increased levels of CD27 as compared with WT tetramer+ cells (MFI of 491 ± 9 versus 589 ± 4.7; P = 0.0006). Similarly, CD127 was more highly expressed on c-Rel−/− tetramer+ cells both in terms of frequency of IL-7Rα+ cells (Fig. 5D; 67.7 ± 2.9% versus 88.5 ± 0.8%) as well as MFI of this cytokine receptor (WT 236 ± 10.5 versus c-Rel−/− 305 ± 15; P = 0.0028) (Fig. 5C, far right panels).
Another property associated with memory CD8+ T cells is their ability to expand more rapidly compared with naive cells following pathogen exposure (42). Because these mice were not able to survive challenge with RH-OVA, one way to test the memory response was to examine the expansion of antigen-specific cells following secondary challenge with CPS-OVA. Therefore, 30 days following primary immunization with 105 CPS-OVA, mice were challenged with a 10-fold higher dose of CPS-OVA (106) and immune responses were then analyzed 5 days later. As expected, there was an expansion of tetramer-specific cells in WT mice following challenge (Fig. 6). However, despite the requirement for c-Rel in the induction of the primary CD8+ T-cell response, OVA-specific cells from the PECs or spleen of c-Rel−/− mice expanded to a much greater degree in terms of frequency and total number as compared with WT mice (Fig. 6).
Fig. 6.
c-Rel is not required for secondary expansion following rechallenge. Mice were immunized with 105 CPS-OVA, and 30 days later, rechallenged with 106 CPS-OVA. Tetramer responses were then analyzed 5 days later. Frequency (A) and number (B) of tetramer+ cells in the PECs. Frequency (C) and number (D) of cells in the spleen. Data from one of three similar experiments are shown.
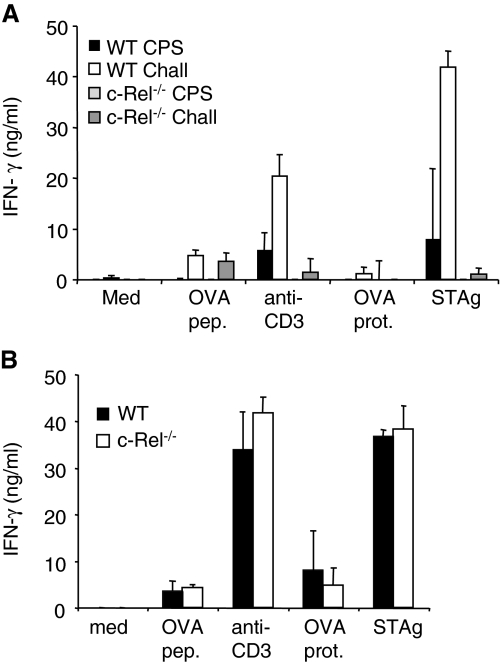
Despite this capacity to greatly expand the CD8+ T-cell population, these cells were defective in their ability to produce effector cytokines in response to STAg or OVA protein as detected by ELISA (Fig. 7A). The secondary responses were also tested using an adoptive transfer system whereby immune T cells (30 days post-immunization) were isolated from WT and c-Rel mice and transferred into Thy-disparate mice. These mice were then challenged with 106 CPS-OVA and 1 week later, expansion of the OVA-specific donor cells was assessed. The ability of the transferred OVA-specific cells to expand following secondary challenge compared with PBS-treated controls was similar in WT and c-Rel−/− mice (Supplementary Figure 3 is available at International Immunology Online). However, the WT environment restored the ability of these memory T cells to produce IFN-γ protein in response to a variety of stimuli (Fig. 7B).
Fig. 7.
c-Rel−/− mice have impaired cytokine responses that can be rescued in a WT environment. (A) Cytokine responses of immunized (CPS, day 35 post-immunization) or immunized and challenged (Chall, day 5 post-challenge with 106 CPS-OVA) WT and c-Rel−/− splenocytes were assessed by ELISA. Data represent average ± standard deviation with three to four mice per group from one of two similar experiments. (B) Cytokine responses from splenocytes as described in Fig. 7(B) were measured by ELISA. Data represent average ± standard deviation for five mice per group from one representative experiment.
Discussion
The NF-κB family of transcription factors plays a key role in the development of innate and adaptive immunity. c-Rel has been shown to play key roles in development, survival and function of CD4+ and CD8+ T cells (11, 19, 43, 44), macrophages (45), dendritic cells (46, 47), NK cells (18, 48) and more recently, thymically derived regulatory CD4+ T cells (49). Previous studies demonstrated the importance of one member, c-Rel, in the development of a potent Th1 immune response to the parasite T. gondii and have also implicated c-Rel in the development of T-cell responses in a variety of other settings (11, 13, 19, 50). In contrast, other reports have indicated that c-Rel was not required for the activation and effector function of CD8+ T cells in the context of influenza infection (14). Attempts to assess the role of c-Rel in the generation of CD8+ T-cell responses to T. gondii have been hindered by the susceptibility of c-Rel−/− mice to this organism. This difficulty was overcome by utilizing a non-replicating strain of T. gondii engineered to secrete the model antigen OVA, thus allowing the primary and secondary antigen-specific CD8+ T-cell response to be tracked with improved specificity (26).
Previous studies have established that B-cell responses are defective in c-Rel−/− mice (28). A single round of immunization with CPS-OVA does not induce circulating levels of Toxoplasma-specific antibodies (data not shown), and previous work from this laboratory has demonstrated that in this model, resistance to secondary challenge is mediated primarily by CD8+ T cells (26). Although B cells and antibody are known to be important during infection with T. gondii (51, 52), and have been shown to produce cytokines that modulate T-cell responses (53), B cells are not required for protection in the system utilized for these studies. Further, other infection models have demonstrated that while CD4+ T-cell memory responses were severely limited in B-cell-deficient systems, CD8+ T-cell responses were unaffected (54). Use of the CPS-OVA experimental system revealed that while c-Rel is required for optimal generation of CD8+ T cells in response to T. gondii, it is not intrinsically needed by CD8+ T cells for their expansion or effector function. This is in contrast to recent work from our laboratory which indicates that NF-κB1 is intrinsically required by CD8+ T cells for expansion and effector function during T. gondii infection (55).
There are several possible explanations for the role of c-Rel in the development of an optimal primary CD8+ T-cell response. IL-12 is essential for protective CD8+ T-cell responses to T. gondii (38) and previous studies have identified a key role for c-Rel in the production of IL-12. Although there are also c-Rel-independent pathways for IL-12 production (17, 45, 56), there is a defect in early IL-12 in c-Rel−/− mice infected with T. gondii (25). Moreover, studies by Gerondakis et al. highlighted a role for c-Rel in regulating the differentiation and survival programs of plasmacytoid dendritic cells (57), which are an important source of IL-12 during toxoplasmosis (58). Additionally, recent work has associated the expression of KLRG1 by CD8+ T cells with exposure to IL-12 (38, 39, 59), and while antigen-specific CD8+ T cells from c-Rel−/− mice were for the most part phenotypically similar to WT antigen-specific CD8+ T cells, the only major difference was reduced expression of KLRG1. In addition to regulating the effector phenotype of CD8+ T cells, IL-12 also impacts the initial expansion of these cells (60, 61), which might explain the impaired expansion of CD8+ T cells in c-Rel−/− mice. Indeed, providing exogenous IL-12 to c-Rel−/− mice resulted in a partial rescue of CD8+ effector T-cell expansion following immunization. The observation that tetramer-specific responses in the c-Rel−/− mice were not fully rescued in the spleen might indicate either that insufficient doses of IL-12 were given to the mice or that other c-Rel-dependent mechanisms are required for these effects. For example, several groups have reported reduced ability of c-Rel−/− CD4+ T cells to produce IL-2 (10, 43), and the ability of the CPS parasites to promote CD8+ T-cell responses is dependent on CD4+ T cells (26). Thus, defects in the CD4+ T cell compartment, in terms of either numbers or functionality of these cells, would also impact the CD8+ T-cell response. Indeed, while the ability of c-Rel−/− OTI cells transferred into WT mice to expand normally indicates that c-Rel is not intrinsically required for optimal CD8+ T-cell responses, these experiments do not distinguish between the role of c-Rel in antigen-presenting cell populations and CD4+ T cells.
Perhaps the most surprising element of these studies was that although memory was functionally compromised in terms of the ability of c-Rel−/− mice to survive a secondary challenge with virulent T. gondii, there were comparable frequencies of memory CD8+ T cells in the WT and c-Rel−/− mice and tetramer+ cells from c-Rel−/− mice also expressed higher levels of the ‘memory’ markers CD27 and CD127. Further, upon rechallenge, c-Rel−/− mice exhibited increased expansion of the CD8+ T-cell subset compared with WT mice. This phenotype may also be a function of lower production of IL-12 as several studies indicate that although IL-12 is important for CD8+ effector T-cell generation and function, in its absence CD8+ T cells form better memory (39, 62). This conclusion also seems to depend on the system used, as IL-12 has not been shown to induce similar effects in all infection models (63); IL-12 seems to be important in the expansion of effector CD8+ T cells in response to Listeria monocytogenes and T. gondii (38, 39, 62). However, IL-12 is not as critical in some viral systems including lymphocytic choriomeningitis virus (LCMV) and vesicular stomatitis virus, while there are contradictory reports about its role in CD8+ T-cell responses to vaccinia virus (59, 63).
Several viral and bacterial systems have been used extensively to define the factors that influence the development of CD8+ T-cell effector and memory populations (37, 39, 64). Perhaps not surprisingly, in other infectious settings, there are some differences emerging; for instance, the acquisition of a CD62L+ memory phenotype seems to take longer in bacterial and parasite infection as compared with LCMV (64–66). Additionally, while KLRG1 expression is maintained long term on antigen-specific cells that develop following CPS-OVA immunization (26), this marker is down-regulated over time after LCMV infection (36). Certainly, much work remains to be done in the study of memory CD8+ T cells that develop in distinct inflammatory environments and the contributions made by different cytokines, including IL-12. However, our results are in agreement with a model supporting a role for IL-12 in the promotion of primary responses while its absence enhances development of memory CD8+ T cells (36, 39). While some transcription factors associated with effector or memory CD8+ T-cell differentiation have been described, including T-bet and Eomesodermin (36, 62, 67), it is likely that more will be found. Determination of the factors regulating CD8+ T-cell effector and memory development has strong implications for rational vaccine design or control of immune system dysfunction (68). Overall, it seems likely that c-Rel is not required for the function of mature CD8+ T cells, in agreement with previous work that showed normal function and proliferation of effector CD8+ T cells induced following infection (14). Rather, the work presented here suggests that c-Rel plays a significant role in creating the inflammatory environment that influences the development and function of the CD8+ T-cell response and memory formation.
Supplementary data
Supplementary Figures 1–3 are available at International Immunology Online.
Supplementary Material
Acknowledgments
National Institutes of Health (AI 071302 to C.A.H., T32-AI 007532 to E.D.T.) and the State of Pennsylvania. Cancer Research Institute training grant ‘Predoctoral Emphasis Pathways in Tumor Immunology’ to K.A.J.
References
- 1.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994;12:141. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 4.Moore BE, Bose HR., Jr. Expression of the c-rel and c-myc proto-oncogenes in avian tissues. Oncogene. 1989;4:845. [PubMed] [Google Scholar]
- 5.Grumont RJ, Gerondakis S. The murine c-rel proto-oncogene encodes two mRNAs the expression of which is modulated by lymphoid stimuli. Oncogene Res. 1990;5:245. [PubMed] [Google Scholar]
- 6.Brownell E, Mathieson B, Young HA, Keller J, Ihle JN, Rice NR. Detection of c-rel-related transcripts in mouse hematopoietic tissues, fractionated lymphocyte populations, and cell lines. Mol. Cell. Biol. 1987;7:1304. doi: 10.1128/mcb.7.3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmore TD. Role of rel family genes in normal and malignant lymphoid cell growth. Cancer Surv. 1992;15:69. [PubMed] [Google Scholar]
- 8.Davis JN, Bargmann W, Bose HR., Jr. Identification of protein complexes containing the c-rel proto-oncogene product in avian hematopoietic cells. Oncogene. 1990;5:1109. [PubMed] [Google Scholar]
- 9.Bunting K, Rao S, Hardy K, et al. Genome-wide analysis of gene expression in T cells to identify targets of the NF-kappa B transcription factor c-Rel. J. Immunol. 2007;178:7097. doi: 10.4049/jimmunol.178.11.7097. [DOI] [PubMed] [Google Scholar]
- 10.Kontgen F, Grumont RJ, Strasser A, et al. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 11.Hilliard BA, Mason N, Xu L, et al. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J. Clin. Invest. 2002;110:843. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamhamedi-Cherradi SE, Zheng S, Hilliard BA, et al. Transcriptional regulation of type I diabetes by NF-kappa B. J. Immunol. 2003;171:4886. doi: 10.4049/jimmunol.171.9.4886. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Rickman BH, Poutahidis T, et al. c-Rel is essential for the development of innate and T cell-induced colitis. J. Immunol. 2008;180:8118. doi: 10.4049/jimmunol.180.12.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harling-McNabb L, Deliyannis G, Jackson DC, Gerondakis S, Grigoriadis G, Brown LE. Mice lacking the transcription factor subunit Rel can clear an influenza infection and have functional anti-viral cytotoxic T cells but do not develop an optimal antibody response. Int. Immunol. 1999;11:1431. doi: 10.1093/intimm/11.9.1431. [DOI] [PubMed] [Google Scholar]
- 15.Mintern JD, Belz G, Gerondakis S, Carbone FR, Heath WR. The cross-priming APC requires a Rel-dependent signal to induce CTL. J. Immunol. 2002;168:3283. doi: 10.4049/jimmunol.168.7.3283. [DOI] [PubMed] [Google Scholar]
- 16.Artis D, Shapira S, Mason N, et al. Differential requirement for NF-kappa B family members in control of helminth infection and intestinal inflammation. J. Immunol. 2002;169:4481. doi: 10.4049/jimmunol.169.8.4481. [DOI] [PubMed] [Google Scholar]
- 17.Mason N, Aliberti J, Caamano JC, Liou HC, Hunter CA. Cutting edge: identification of c-Rel-dependent and -independent pathways of IL-12 production during infectious and inflammatory stimuli. J. Immunol. 2002;168:2590. doi: 10.4049/jimmunol.168.6.2590. [DOI] [PubMed] [Google Scholar]
- 18.Tato CM, Villarino A, Caamano JH, Boothby M, Hunter CA. Inhibition of NF-kappa B activity in T and NK cells results in defective effector cell expansion and production of IFN-gamma required for resistance to Toxoplasma gondii. J. Immunol. 2003;170:3139. doi: 10.4049/jimmunol.170.6.3139. [DOI] [PubMed] [Google Scholar]
- 19.Mason NJ, Liou HC, Hunter CA. T cell-intrinsic expression of c-Rel regulates Th1 cell responses essential for resistance to Toxoplasma gondii. J. Immunol. 2004;172:3704. doi: 10.4049/jimmunol.172.6.3704. [DOI] [PubMed] [Google Scholar]
- 20.Caamano J, Alexander J, Craig L, Bravo R, Hunter CA. The NF-kappa B family member RelB is required for innate and adaptive immunity to Toxoplasma gondii. J. Immunol. 1999;163:4453. [PubMed] [Google Scholar]
- 21.Caamano J, Tato C, Cai G, et al. Identification of a role for NF-kappa B2 in the regulation of apoptosis and in maintenance of T cell-mediated immunity to Toxoplasma gondii. J. Immunol. 2000;165:5720. doi: 10.4049/jimmunol.165.10.5720. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 23.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl Acad. Sci. USA. 1993;90:6115. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 1991;146:286. [PubMed] [Google Scholar]
- 25.Mason NJ, Artis D, Hunter CA. New lessons from old pathogens: what parasitic infections have taught us about the role of nuclear factor-kappaB in the regulation of immunity. Immunol. Rev. 2004;201:48. doi: 10.1111/j.0105-2896.2004.00189.x. [DOI] [PubMed] [Google Scholar]
- 26.Jordan KA, Wilson EH, Tait ED, et al. Kinetics and phenotype of vaccine-induced CD8+ T cell responses to Toxoplasma gondii. Infect. Immun. 2009;77:3894. doi: 10.1128/IAI.00024-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzierszinski F, Pepper M, Stumhofer JS, et al. Presentation of Toxoplasma gondii antigens via the endogenous Mhc class I pathway in non-professional and professional antigen presenting cells. Infect Immun. 2007;75:5200. doi: 10.1128/IAI.00954-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumang JR, Owyang A, Andjelic S, et al. c-Rel is essential for B lymphocyte survival and cell cycle progression. Eur. J. Immunol. 1998;28:4299. doi: 10.1002/(SICI)1521-4141(199812)28:12<4299::AID-IMMU4299>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Mrass P, Takano H, Ng LG, et al. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J. Exp. Med. 2006;203:2749. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 31.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 32.Kao C, Daniels MA, Jameson SC. Loss of CD8 and TCR binding to Class I MHC ligands following T cell activation. Int. Immunol. 2005;17:1607. doi: 10.1093/intimm/dxh340. [DOI] [PubMed] [Google Scholar]
- 33.Drake DR, 3rd, Ream RM, Lawrence CW, Braciale TJ. Transient loss of MHC class I tetramer binding after CD8+ T cell activation reflects altered T cell effector function. J. Immunol. 2005;175:1507. doi: 10.4049/jimmunol.175.3.1507. [DOI] [PubMed] [Google Scholar]
- 34.Beyersdorf NB, Ding X, Karp K, Hanke T. Expression of inhibitory “killer cell lectin-like receptor G1” identifies unique subpopulations of effector and memory CD8 T cells. Eur. J. Immunol. 2001;31:3443. doi: 10.1002/1521-4141(200112)31:12<3443::aid-immu3443>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 35.Robbins SH, Terrizzi SC, Sydora BC, Mikayama T, Brossay L. Differential regulation of killer cell lectin-like receptor G1 expression on T cells. J. Immunol. 2003;170:5876. doi: 10.4049/jimmunol.170.12.5876. [DOI] [PubMed] [Google Scholar]
- 36.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 38.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii infection. J. Immunol. 2008;180:5935. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 39.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J. Immunol. 2007;179:2074. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 40.Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol. Cell Biol. 2000;20:2687. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matter MS, Claus C, Ochsenbein AF. CD4+ T cell help improves CD8+ T cell memory by retained CD27 expression. Eur. J. Immunol. 2008;38:1847. doi: 10.1002/eji.200737824. [DOI] [PubMed] [Google Scholar]
- 42.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2002;2:251. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 43.Liou HC, Jin Z, Tumang J, Andjelic S, Smith KA, Liou ML. c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int. Immunol. 1999;11:361. doi: 10.1093/intimm/11.3.361. [DOI] [PubMed] [Google Scholar]
- 44.Saibil SD, Jones RG, Deenick EK, et al. CD4+ and CD8+ T cell survival is regulated differentially by protein kinase Ctheta, c-Rel, and protein kinase B. J. Immunol. 2007;178:2932. doi: 10.4049/jimmunol.178.5.2932. [DOI] [PubMed] [Google Scholar]
- 45.Sanjabi S, Hoffmann A, Liou HC, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc. Natl Acad. Sci. USA. 2000;97:12705. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boffa DJ, Feng B, Sharma V, et al. Selective loss of c-Rel compromises dendritic cell activation of T lymphocytes. Cell. Immunol. 2003;222:105. doi: 10.1016/s0008-8749(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Wang X, Hussain S, et al. Distinct roles of different NF-kappa B subunits in regulating inflammatory and T cell stimulatory gene expression in dendritic cells. J. Immunol. 2007;178:6777. doi: 10.4049/jimmunol.178.11.6777. [DOI] [PubMed] [Google Scholar]
- 48.Tato CM, Mason N, Artis D, et al. Opposing roles of NF-kappaB family members in the regulation of NK cell proliferation and production of IFN-gamma. Int. Immunol. 2006;18:505. doi: 10.1093/intimm/dxh391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isomura I, Palmer S, Grumont RJ, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J. Exp. Med. 2009;206:3001. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell IK, Gerondakis S, O′Donnell K, Wicks IP. Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J. Clin. Invest. 2000;105:1799. doi: 10.1172/JCI8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J. Immunol. 2000;164:2629. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 52.Sayles PC, Gibson GW, Johnson LL. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun. 2000;68:1026. doi: 10.1128/iai.68.3.1026-1033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menard LC, Minns LA, Darche S, et al. B cells amplify IFN-gamma production by T cells via a TNF-alpha-mediated mechanism. J. Immunol. 2007;179:4857. doi: 10.4049/jimmunol.179.7.4857. [DOI] [PubMed] [Google Scholar]
- 54.Whitmire JK, Asano MS, Kaech SM, et al. Requirement of B cells for generating CD4+ T cell memory. J. Immunol. 2009;182:1868. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris TH, Wilson EH, Tait ED. NF-kappaB1 contributes to T cell-mediated control of Toxoplasma gondii in the CNS. J. Neuroimmunol. 2010;222:19. doi: 10.1016/j.jneuroim.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grumont R, Hochrein H, O′Keeffe M, et al. c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J. Exp. Med. 2001;194:1021. doi: 10.1084/jem.194.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O′Keeffe M, Grumont RJ, Hochrein H, et al. Distinct roles for the NF-kappaB1 and c-Rel transcription factors in the differentiation and survival of plasmacytoid and conventional dendritic cells activated by TLR-9 signals. Blood. 2005;106:3457. doi: 10.1182/blood-2004-12-4965. [DOI] [PubMed] [Google Scholar]
- 58.Pepper M, Dzierszinski F, Wilson E, et al. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J. Immunol. 2008;180:6229. doi: 10.4049/jimmunol.180.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J. Immunol. 2009;182:2786. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 2002;169:6842. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 61.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 62.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 2006;177:7515. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 63.Keppler SJ, Theil K, Vucikuja S, Aichele P. Effector T-cell differentiation during viral and bacterial infections: role of direct IL-12 signals for cell fate decision of CD8(+) T cells. Eur. J. Immunol. 2009;39:1774. doi: 10.1002/eji.200839093. [DOI] [PubMed] [Google Scholar]
- 64.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 2004;78:5535. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat. Med. 2008;14:542. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bixby LM, Tarleton RL. Stable CD8+ T cell memory during persistent Trypanosoma cruzi infection. J. Immunol. 2008;181:2644. doi: 10.4049/jimmunol.181.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 68.Tian W, Liou HC. RNAi-mediated c-Rel silencing leads to apoptosis of B cell tumor cells and suppresses antigenic immune response in vivo. PLoS One. 2009;4:e5028. doi: 10.1371/journal.pone.0005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.