Abstract
Many preclinical experiments have attested to the critical role of CD4+ T cell help in CD8+ cytotoxic T lymphocyte (CTL)-mediated immunity. Recent clinical trials have demonstrated that reinfusion of CD4+ T cells can induce responses in infectious diseases and cancer. However, few standardized and versatile systems exist to expand antigen-specific CD4+ Th for clinical use. K562 is a human erythroleukemic cell line, which lacks expression of HLA class I and class II, invariant chain and HLA-DM but expresses adhesion molecules such as intercellular adhesion molecule-1 and leukocyte function-associated antigen-3. With this unique immunologic phenotype, K562 has been tested in clinical trials of cancer immunotherapy. Previously, we created a K562-based artificial antigen-presenting cell (aAPC) that generates ex vivo long-lived HLA-A2-restricted CD8+ CTL with a central/effector memory phenotype armed with potent effector function. We successfully generated a clinical version of this aAPC and conducted a clinical trial where large numbers of anti-tumor CTL are reinfused to cancer patients. In this article, we shifted focus to CD4+ T cells and developed a panel of novel K562-derived aAPC, where each expresses a different single HLA-DR allele, invariant chain, HLA-DM, CD80, CD83 and CD64; takes up soluble protein by endocytosis and processes and presents CD4+ T-cell peptides. Using this aAPC, we were able to determine novel DR-restricted CD4+ T-cell epitopes and expand long-lived CD4+ T-cells specific for multiple antigens without growing bystander Foxp3+ regulatory T cells. Our results suggest that K562-based aAPC may serve as a translatable platform to generate both antigen-specific CD8+ CTL and CD4+ Th.
Keywords: adoptive therapy, artificial APC, CD4, Foxp3, HLA-DR, K562
Introduction
Adoptive cell therapy is an emerging treatment modality, which exploits unique mechanisms of action to overcome refractoriness to conventional therapies (1–4). Results from recent clinical trials suggest that adoptive cell therapy may be effective in treating infectious diseases and cancer (5–10). In adoptive cell therapy, cytotoxic CD8+ T lymphocytes are believed to serve as the major effector cells since they possess potent antigen-specific effector functions, namely cytotoxicity and proinflammatory cytokine secretion. In fact, anti-tumor activity has been observed in patients who received infusions of CD8+ cytotoxic T lymphocyte (CTL) or of ex vivo expanded tumor-infiltrating lymphocytes which had a high proportion of CD8+ T cells.
However, the role of antigen-specific CD4+ T cells in T-cell immunity cannot be underestimated. In humans receiving adoptively transferred cytomegalovirus (CMV)-specific CD8+ T cells, long-term in vivo persistence was achieved only when CMV-specific CD4+ T cells co-existed in vivo (11). Recently, Yee et al. reported that reinfusion of a NY-ESO-1-specific CD4+ T-cell clone was able to induce a complete response in a patient with metastatic melanoma, suggesting that adoptively transferred anti-tumor CD4+ T cells functioned as effector T cells and/or provided help to other effector cells most likely CD8+ anti-tumor CTL (7). These results suggest that adoptive cell therapy for cancer can be made more effective by including both antigen-specific CD4+ T cells as well as CD8+ CTL in the graft (12). Unfortunately, however, there have been few versatile systems available to generate large numbers of antigen-specific human CD4+ T cells for the purpose of adoptive therapy.
Previously, we reported the generation of a K562-based artificial antigen-presenting cell (aAPC), which expresses HLA-A2, CD80 and CD83 (13). We have shown that this aAPC can uniquely support the priming and prolonged expansion of large numbers of antigen-specific CD8+ CTL that display a central/effector memory phenotype, possess potent effector function and can be maintained in vitro for >1 year without any feeder cells or cloning (14). aAPC is equipped with constitutive proteasome and inducible immunoproteasome machinery and can naturally process and present CD8+ T-cell peptides via transduced A2 molecules (15, 16). We have successfully generated clinical grade aAPC33 under cGMP conditions and translated our findings to the clinic (14). We are currently conducting a clinical trial where large numbers of MART1-specific CTL generated ex vivo using aAPC33, IL-2 and IL-15 are reinfused to patients with advanced melanoma (17).
Based on our experience with aAPC33 and CD8+ T cells, we have generated a novel aAPC to stimulate HLA-DR-restricted antigen-specific CD4+ T cells. K562 has been engineered to express HLA-DR as a single HLA allele in addition to invariant chain (Ii), HLA-DM, CD80 and CD83. CD83 delivers a CD80-dependent T-cell stimulatory signal that allows T cells to be long lived (13, 18). In addition to its endogenous pinocytic activity, aAPC was made capable of Fcγ receptor-mediated endocytosis by transduction of CD64. Using this standardized and renewable aAPC that can take up soluble protein and process and present DR-restricted peptides, we have determined novel DR-restricted CD4+ T-cell epitopes and expanded antigen-specific CD4+ T cells without growing bystander Foxp3+ regulatory T cells (Treg).
Methods
Cells
Peripheral blood cells were obtained from healthy donors. Appropriate informed consent and institutional review board approval were obtained. All protocols were in accordance with the Declaration of Helsinki. K562-derived aAPC and EBV-transformed lymphoblastoid cells (LG2, Preiss, DBB and T1) were cultured in RPMI 1640 supplemented with 10% FCS and gentamycin (Invitrogen, Carlsbad, CA, USA). Immature and mature dendritic cells (DC) were generated ex vivo using peripheral blood CD14+ monocytes as published previously (14, 19). Where indicated, bovine IgG-depleted FCS (Invitrogen) was used instead of regular FCS. LG2, Preiss and DBB are homozygous for DR1, DR4 and DR7, respectively. T1 cells are hemizygous for DR7. T2 cells are defective for HLA class II. K562, T1 and T2 cells were obtained from American Type Culture Collection. LG2 and Preiss cells were a gift from Kai Wucherpfennig (Dana-Farber Cancer Institute). DBB cells were obtained from Gordon Freeman (Dana-Farber Cancer Institute).
cDNAs
Invariant chain (Ii), HLA-DRA1*0101 and DRB1*0701 cDNA were a gift from Gordon Freeman. DRB1*0101 and DRB1*0401 cDNA were cloned from LG2 and Preiss, respectively, by reverse transcription (RT)-PCR. cDNA encoding HLA-DMα and β chains, human CD64 and common Fcγ receptor were all cloned from normal PBMC using RT-PCR based upon the published sequence. All cDNA were cloned into the pMX vector, and the sequence was verified (20, 21).
Generation of K562-based aAPC
Retrovirus system was employed to establish K562-based HLA-DR-aAPC as described previously (13). K562, which is deficient in HLA class I and class II expression, was transduced with CD80 and CD83 to generate HLA-null aAPC. HLA null aAPC was successively transduced with DRA1*0101 and DRB1*0701 and DR7-positive cells were isolated by mAb staining and flow cytometry-guided sorting. DR7 transduced aAPC (DR7-aAPC) was then transduced with Ii, and CLIP-positive cells were collected by flow cytometry-guided sorting to generate DR7-aAPC/Ii. DR7-aAPC/Ii was then transduced with HLA-DMα and β chain and CLIP-negative cells were collected to generate DR7-aAPC/Ii/DM. Polyclonal cell lines consisting of at least 104 independent clones were used to prevent cloning induced variations. CD64 and common Fcγ receptor were introduced to produce DR7-aAPC/Ii/DM/CD64. The expression of transduced molecules on K562 was stable after continuous culture at least for 2 months. DR1-aAPC/Ii/DM/CD64 and DR4-aAPC/Ii/DM/CD64 were similarly established except that magnetic bead-guided sorting was employed to purify transduced cells.
Flow cytometry analysis
mAbs recognizing the following surface antigens were used: HLA-DR, DP, DQ, CD4, CD25, CD45RO and CD62L from Beckman Coulter, Fullerton, CA, USA; CD45RA and CD64 from Invitrogen; HLA-DR, invariant chain (Ii), CLIP, CD80, CD83, HLA-DM, IL-2, IL-4, IFN-γ and mouse isotype controls from BD Biosciences, San Diego, CA, USA. Surface molecule staining was performed as described elsewhere (13). To detect intracellular Ii, cells were first incubated with saturating amount of unconjugated anti-Ii mAb to block extracellular invariant chain. Following fixation and permeabilization, the cells was stained with FITC-conjugated anti-Ii mAb. For intracellular staining of Foxp3 (BioLegend, San Diego, CA, USA), CD4+ CD25+ T cells were first stained with PC5-conjugated anti-CD4 and PE-conjugated anti-CD25 mAbs. The cells were then fixed, permeabilized and counterstained with Alexa Fluor 488-conjugated anti-Foxp3 mAb according to the manufacturer’s instruction (14). Intracellular cytokine staining was performed using FITC-conjugated anti-IL-2, PE-conjugated anti-IL-4 and PC7-conjugated anti-IFN-γ mAbs as described previously (19). HLA-DR-transduced T2 cells were pulsed with peptide and used as a stimulator.
Production of HLA-DR-restricted CD4+ T cells
DR7-specific genotyping was conducted by sequence-specific PCR (22). High resolution HLA class II genotyping was performed at the American Red Cross, Dedham, MA, USA. CD4+ T cells were purified using a positive or negative isolation kit (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer’s instruction.
Purified HLA-DR-matched CD4+ T cells were plated in 24-well plates at 2 × 106 cells per well in serum-free X-Vivo 20 (Lonza, Walkersville, MD, USA). An aAPC was pulsed with peptide or protein antigen overnight at 37°C unless otherwise noted. These cells were then irradiated (200 Gy) and added to the responder cells at a responder to stimulator ratio of 20:1 (day 0). When only small numbers of CD4+ T cells were available, 96-well plates were utilized and input numbers of cells were reduced accordingly. On day 3 following stimulation, heat inactivated human AB serum was added to a final concentration of 2%. Starting on day 3, 10 IU ml−1 IL-2 (Chiron, Emeryville, CA, USA) and 10 ng ml−1 IL-15 (Peprotech, Rocky Hill, NJ, USA) were added to the cultures every 3 days. Unless otherwise noted, on day 9, CD4+ T cells were harvested, washed and restimulated similarly for a next cycle of T-cell culture.
Tetanus toxoid was obtained from EMD Chemicals (Gibbstown, NJ, USA). Recombinant full-length CMV pp65 protein was purchased from Miltenyi Biotec. Anti-CMV pp65 mAbs were purchased from Millipore, Billerica, MA, USA, East Coast Bio; North Berwick, ME, USA and Fitzgerald Industries Intl, Concord, MA, USA. pp65 protein and pp65 mAb were coincubated in serum-free RPMI to form an immune complex (IC).
CMV Ab titer was measured using a CMV IgG ELISA purchased from Immuno-Biological Laboratories, Minneapolis, MN, USA, according to the manufacturer’s manual. Peptides used were pp65 peptide mix (BD Biosciences), 57 20-mer pp65 peptides overlapping by 10 amino acids (JPT Peptide Technologies, Acton, MA, USA) (Supplementary Table S1 is available at International Immunology Online), tetanus toxin TT830 peptide (830QYIKANSKFIGITELKK846), influenza virus HA306 peptide (306PKYVKQNTLKLAT318) and control pan-DR PADRE peptide (AKFVAAWTLKAA (23)).
Tetramer assays, ELISPOT assays, proliferation assay and pinocytic activity assay
PE-conjugated HLA-DR1-restricted TT830-specific tetramer and CLIP-specific control tetramer were purchased from Beckman Coulter and used according to the manufacturer’s instruction. Where indicated, tetramer-positive cells were costained with FITC-conjugated anti-CD45RA, ECD-conjugated anti-CD45RO and PC5-conjugated anti-CD62L mAbs. IFN-γ and IL-2 ELISPOT assays were performed as described before (13). Proliferation assay was performed as described before (19). Pinocytic activity was measured as the cellular uptake of FITC–dextran (1 mg ml−1; molecular weight 70 000; Invitrogen) and was quantified by flow cytometry as described previously (19).
Fcγ receptor-mediated endocytic activity assay
Cells were incubated with PE-conjugated mouse IgG1, 2a or 2b at indicated concentrations for 24 h at 37°C to allow Fcγ receptor-mediated endocytosis. The cells were trypsinized and washed intensively to remove membrane-bound mIgG. Endocytosed PE–mIgG was measured in the FL2 channel by flow cytometry analysis.
Statistical analysis
Statistical analysis was performed using unpaired, two-tailed Student's t-test. P values of <0.05 were considered significant.
Results
Generation of K562-based aAPC expressing a single HLA-DR allele, Ii and HLA-DM
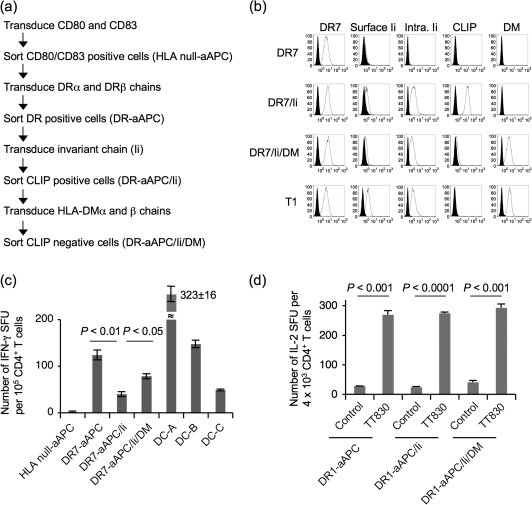
We and others previously demonstrated that K562 expresses a very low or undetectable level of HLA class I and II, invariant chain (Ii) and HLA-DM (24, 25). A schematic protocol to generate a K562-based aAPC that expresses a single HLA-DR allele is depicted in Fig. 1(a). HLA null-aAPC was established by transducing immunoaccessory molecules, CD80 and CD83 to HLA-negative K562 as described previously (Supplementary Figure S1 available at International Immunology Online) (13). Using this cell line as a backbone, DR-aAPC, DR-aAPC/Ii and DR-aAPC/Ii/DM were generated by sequential transduction of DRαβ heterodimers, Ii and HLA-DMαβ heterodimers, respectively.
Fig. 1.
Generation of K562-based aAPC that expresses DR7 as a single HLA allele. (a) The experimental protocol to generate K562-based aAPC that expresses DR7 as a single HLA allele is shown. K562 was sequentially transduced with CD80, CD83, HLA-DR7αβ heterodimers, Ii and HLA-DMαβ heterodimers. Each molecule was retrovirally infected, and transduced cells were isolated using specific mAb and flow cytometry-guided sorting. (b) HLA null aAPC was serially transduced with DR7, Ii and HLA-DM to generate stable cell lines, DR7-aAPC (DR7), DR7-aAPC/Ii (DR7/Ii) and DR7-aAPC/Ii/DM (DR7/Ii/DM). T1 cells that hemizygously express DR7 served as a control. The expression of cell surface DR7, cell surface and intracellular Ii, cell surface CLIP and intracellular HLA-DM was studied by flow cytometric analysis using specific mAb. (c) DR7-transduced aAPC were immunogenic and able to induce allogeneic responses. CD4+ T cells purified from DR7− healthy donors were stimulated with DR7-aAPC, DR7-aAPC/Ii, DR7-aAPC/Ii/DM and monocyte-derived mature DC derived from three different allogeneic donors (DC-A, B and C). IFN-γ secretion by allogeneic CD4+ T cells was measured by IFN-γ ELISPOT. Data shown represent means ± SDs of quadricates. (d) DR1-restricted tetanus TT830 peptide-specific CD4+ T cells were stimulated with DR1-aAPC, DR1-aAPC/Ii and DR1-aAPC/Ii/DM pulsed with pan-DR PADRE (control) or TT830 peptide. TT830-specific IL-2 secretion by CD4+ T cells were evaluated by IL-2 ELISPOT on day 28. Data shown represent means ± SDs of triplicates.
Figure 1(b) demonstrates the expression of cell surface DR7, cell surface and intracellular Ii, cell surface CLIP and intracellular HLA-DM by HLA null-aAPC, DR7-aAPC, DR7-aAPC/Ii and DR7-aAPC/Ii/DM. Transduction of DRα or β alone did not induce HLA-DR surface expression, suggesting that K562 expresses neither DRβ nor α endogenously (data not shown). Forced expression of either DR or Ii alone was unable to bring the expression of the Ii fragment, CLIP to the cell surface, confirming that no or very low level of endogenous expression of Ii or DR (Fig. 1b and data not shown). Following the transduction of Ii, however, CLIP appeared on the cell surface of DR7-aAPC/Ii. Furthermore, the introduction of HLA-DM almost completely abrogated CLIP expression on DR7-aAPC/Ii/DM. This result is in accordance with previous studies showing that HLA-DM catalyses the removal of CLIP from DR thus enabling exogenous peptides to bind empty DR molecules in late endosomes. The expression profile of T1 cells, which are EBV-transformed lymphoblastoid cells hemizygous for HLA-DR7, is demonstrated as a control (Fig. 1b). In T1 cells, endogenous HLA-DM expression completely abrogated CLIP expression. We have successfully established DR1 and DR4-aAPC expressing Ii and/or DM according to the same strategy (Supplementary Figure S1 is available at International Immunology Online). It should be noted that cell surface but not intracellular Ii expression was almost undetectable on DR1-aAPC/Ii/DM and DR4-aAPC/Ii/DM, suggesting that the majority of Ii molecules was retained intracellularly and were not exported to the cell surface in these aAPC.
To evaluate the immunogenicity of these aAPC expressing a single class II HLA allele, we measured IFN-γ production by ELISPOT in one-way mixed lymphocyte reactions using HLA-DR7-negative CD4+ T cells as alloresponders (Fig. 1c). DR7-aAPC/Ii was less immunogenic compared with DR7-aAPC and DR7-aAPC/Ii/DM. Monocyte-derived mature DC generated from three different allogeneic donors were employed as control stimulators. It should be noted that, while mature DC express all HLA class II alleles that can evoke allogeneic responses, these K562-based aAPC express the single HLA class II allele, DR7, and therefore induce allogeneic responses only against DR7. Considering that DR7 expression levels on these three aAPC are comparable, this result supports previous reports that CLIP peptides derived from Ii reduces the density of peptides other than CLIP in the cleft of DR, thereby decreasing the immunogenicity of Ii positive, HLA-DM negative APC (26, 27).
We also evaluated the peptide-specific immunogenicity of HLA-DR expressing aAPC by using a DR1-restricted TT830-specific CD4+ T-cell line (Fig. 1d). When antigenic peptide was exogenously pulsed, the magnitude of the T-cell responses induced by all three aAPC was similar, confirming that DR expression levels on DR1-aAPC, DR1-aAPC/Ii and DR1-aAPC/Ii/DM are comparable. These results suggest that similar amounts of immunogenic peptide were pulsed regardless of Ii or HLA-DM expression and that K562-derived aAPC were sufficiently immunogenic to induce an antigen-specific CD4+ T-cell effector response. Similar results were observed with aAPC expressing DR7. An HA306-specific T-cell line equally recognized peptide-pulsed DR7-aAPC, DR7-aAPC/Ii and DR7-aAPC/Ii/DM in an IFN-γ ELISPOT (data not shown).
Generation of long-lived HLA-DR-restricted antigen-specific CD4+ T cells using DR-aAPC/Ii/DM
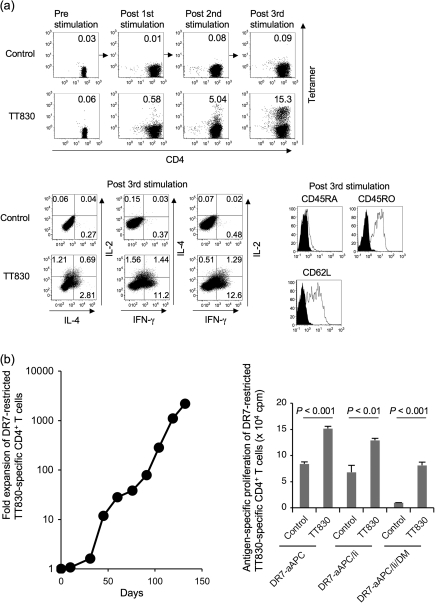
Since, in vivo, APC such as DC and monocytes physiologically express Ii and HLA-DM in conjunction with HLA-DR, we chose DR-aAPC/Ii/DM to stimulate and expand DR-restricted antigen-specific CD4+ T cells in the studies below. Purified CD4+ T cells derived from the DR1+ healthy donors were repeatedly stimulated with TT830 peptide-pulsed and -irradiated DR1-aAPC/Ii/DM. Using HLA-DR1/TT830 peptide tetramer, we quantitated the enrichment of DR1-restricted TT830-specific CD4+ T cells (Fig. 2a, top). Prior to stimulation, there were no DR1-restricted TT830 T cells detectable in freshly isolated CD4+ T cells. However, stimulation with peptide-pulsed DR1-aAPC/Ii/DM increased the percentage of antigen-specific CD4+ T cells to 5.04% following the second stimulation and to 15.3% following the third stimulation. Expanded TT830-specific CD4+ T cells secreted more IFN-γ than IL-4 in an antigen-specific manner, suggesting a Th1-biased phenotype (Fig. 2a, bottom left) and displayed a CD45RA− CD45RO+ CD62L+ central memory phenotype (Fig. 2a, bottom right).
Fig. 2.
DR-aAPC/Ii/DM can generate long-lived antigen-specific CD4+ T cells. (a) DR1-aAPC/Ii/DM can expand DR1-restricted antigen-specific Th1-biased CD4+ T cells with a central memory phenotype in the presence of low dose IL-2 and IL-15. Purified DR1+ CD4+ T cells were stimulated every 10 days with TT830 peptide-pulsed and -irradiated DR1-aAPC/Ii/DM. Following each stimulation, IL-2 (10 IU ml−1) and IL-15 (10 ng ml−1) were added to the T-cell cultures every 3 days. The percentage of DR1-restricted TT830-specific CD4+ T cells was determined by TT830 tetramer staining (top). DR1-restricted CLIP-specific tetramer was employed as a negative control. Following three stimulations (day 28), IL-2, IL-4 and IFN-γ secretion was also analyzed by intracellular cytokine staining (bottom left). DR1-transduced T2 cells were pulsed with TT830 or pan-DR PADRE (control) peptide and were used for stimulation. Cells were co-stained with anti-CD45RA, CD45RO and CD62L mAbs along with TT830-tetramer (bottom right). Expression on gated tetramer-staining cells is depicted. Isotype staining was used as a control. (b) DR-aAPC/Ii/DM was able to generate long-lived antigen-specific CD4+ T cells. Purified CD4+ T cells isolated from DR7-positive healthy donors were repeatedly stimulated with TT830-pulsed and -irradiated DR7-aAPC/Ii/DM every 14 days. Between stimulations, 10 IU ml−1 IL-2 and 10 ng ml−1 IL-15 were added to the T-cell cultures. To confirm the antigen specificity of the long-lived DR7-restricted TT830-specific T cells, antigen-specific proliferation was measured on day 103 using pan-DR PADRE (control) or TT830 peptide-pulsed and mitomycin-C treated DR7-aAPC, DR7-aAPC/Ii or DR7-aAPC/II/DM as a stimulator. Data shown represents means ± SDs of triplicates. A TT830-specific proliferation assay was also performed on days 77, 91 and 110 with similar results (data not shown). In addition, TT830 specificity was confirmed by IFN-γ ELISPOT on day 150 (data not shown).
As shown in Fig. 2(b), left, repeated stimulations using peptide-pulsed and -irradiated DR7-aAPC/Ii/DM in the absence of any feeder cells or cloning were able to expand TT830-specific CD4+ T cells for prolonged culture periods, up to 150 days. The antigen specificity of the long-lived CD4+ T-cell line was confirmed by its TT830-specific proliferation on days 77, 91, 103 and 110 (Fig. 2b, right and data not shown). The observed T-cell responses to DR7-aAPC and DR7-aAPC/Ii pulsed with control peptide were most likely directed to self peptides that were largely absent on DR7-aAPC/Ii/DM because of the ectopic expression of HLA-DM. We were also able to maintain DR7-restricted HA306-specific CD4+ T-cell line using peptide-pulsed DR7-aAPC/Ii/DM for up to 150 days (data not shown). These results suggest that K562-derived DR-aAPC/Ii/DM is also capable of generating long-lived antigen-specific CD4+ T cells without any feeder cells or T-cell cloning.
Endogenous pinocytic activity allows DR-aAPC/Ii/DM to take up soluble protein and process and present DR-restricted CD4+ T-cell epitopes
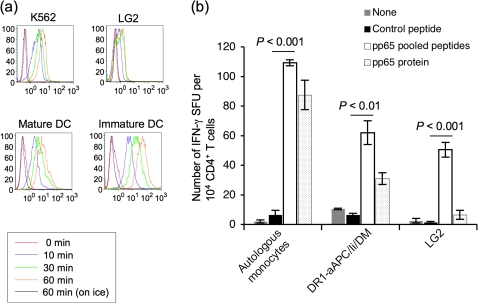
Monocytes and DC can process and present HLA class II-restricted peptides derived from exogenous protein taken up by endocytosis, i.e. pinocytosis, receptor-mediated endocytosis or phagocytosis. As has previously been observed, the EBV-transformed lymphoblastoid cell line, LG2, demonstrated impaired pinocytic activity (Fig. 3a). In contrast, the time-dependent pinocytic activity of the DR-aAPC/Ii/DM backbone cell, K562 and monocyte-derived mature DC was comparable.
Fig. 3.
K562-based aAPC possesses pinocytic activity and processes protein to present DR-restricted peptides to CD4+ T cells. (a) K562 cells, the aAPC backbone, possess pinocytic activity. K562, LG2 (EBV-transformed lymphoblastoid cells) and immature and mature DC ex vivo generated using peripheral blood monocytes were incubated with FITC–dextran (1 mg ml−1; molecular weight 70 000; Invitrogen) at 37°C for 0, 10, 30 and 60 min. As a control experiment, cells were similarly incubated with FITC–dextran on ice for 60 min. After incubation, the cells were washed twice with cold PBS to stop pinocytosis and to remove excess dextran. Pinocytosis was measured as the cellular uptake of FITC–dextran and was quantified by flow cytometry. Data shown are representative of three similar experiments. (b) DR-aAPC/Ii/DM can process and present CD4+ T-cell peptides derived from pinocytosed protein. CD4+ T cells isolated from DR1+ and CMV Ab (+) healthy donors were stimulated once with autologous DC pulsed with recombinant full-length pp65 protein. IFN-γ ELISPOT was conducted using autologous monocytes, DR1-aAPC/Ii/DM and LG2 mixed with 10 nM control pan-DR PADRE peptide, pp65 peptide mix or full-length pp65 protein as a stimulator. Data shown represent means ± SDs of triplicates.
We examined whether K562-derived aAPC can process and present HLA class II-restricted peptides derived from exogenous protein taken up by pinocytosis. As shown in Fig. 3(b), DR1-aAPC/Ii/DM was able to process and present DR1-restricted CD4+ T-cell pp65 epitopes derived from pinocytosed pp65 protein. In contrast, LG2, which is homozygous for DR1, failed to process pp65 protein-derived CD4+ T-cell peptides reflecting its poor pinocytic activity as described above. It should be noted that autologous monocytes induced a greater number of IFN-γ spot forming units than DR1-aAPC/Ii/DM. This is probably because autologous monocytes express all HLA class II alleles, while DR1-aAPC/Ii/DM only expresses the single HLA class II allele, DR1, and therefore induces only DR1-restricted responses.
Ectopic expression of CD64 enables DR-aAPC/Ii/DM receptor-mediated endocytosis
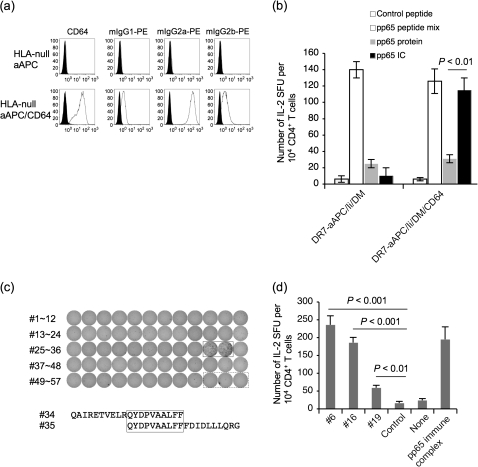
K562 endogenously expresses CD32 (Fcγ receptor II) but not CD16 (Fcγ receptor III) nor CD64 (Fcγ receptor I) (data not shown and Fig. 4a) (28). Previously, Suhoski et al. (29) demonstrated that CD64 ectopically expressed on K562 can be loaded with mouse mAbs against immunostimulatory molecules to stimulate human T cells. We investigated whether CD64 transduced to K562 enables Fcγ receptor-mediated endocytic activity. As shown in Supplementary Figure S2 (available at International Immunology Online), parental K562 was unable to endocytose PE-conjugated mouse Ig of any class whose molecular weight is >400 kDa. However, when CD64 was introduced, CD64+ K562 acquired Fcγ receptor-mediated endocytic activity (Fig. 4a). The observed Fcγ receptor-mediated endocytic activity was dose dependent (Supplementary Figure S2 is available at International Immunology Online). The endocytosis of PE-conjugated mIgG1, which is known to possess the least binding affinity to human CD64, was substantially augmented by the depletion of bovine IgG from the culture media (Supplementary Figure S2 is available at International Immunology Online) (30). mIgG2a was most effectively endocytosed since it possesses the highest binding affinity to human CD64.
Fig. 4.
Transduction of CD64 enables K562-based aAPC to effectively endocytose via Fcγ receptor and process protein for presentation of DR-restricted peptides to CD4+ T cells. (a) HLA-null aAPC (K562/CD80/CD83) was transduced with CD64 and common Fcγ receptor to generate HLA-null aAPC/CD64. The surface expression of CD64 was confirmed by specific mAb staining. HLA-null aAPC and HLA-null aAPC/CD64 cultured in the presence of 10% bovine IgG-depleted FCS were incubated with PE-conjugated mouse IgG1, 2a or 2b at 1 μg ml−1 for 24 h at 37°C. Cells were trypsinized and washed intensively to remove membrane-bound mIgG. Endocytosed PE–mIgG was measured in the FL2 channel by flow cytometry analysis. (b) DR7-restricted pp65-specific CD4+ T cells were generated using autologous monocytes pulsed with pp65 protein from DR7+ CMV Ab (+) healthy donors. On day 19, cells were subjected to an IL-2 ELISPOT assay using DR7-aAPC/Ii/DM or DR7-aAPC/Ii/DM/CD64 as an APC. Unlike in Fig. 3(b), the APC was pulsed for only 30 min with 10 nM pp65 protein or pp65 IC, washed and then used. Pan-DR PADRE peptide and pp65 peptide mix were used as negative and positive controls, respectively. Data shown represent means ± SDs of triplicates. (c) CD4+ T cells purified from DR7-positive CMV Ab (+) healthy donors were stimulated with DR7-aAPC/Ii/DM/CD64 mixed with 10 nM pp65 IC. IL-2 ELISPOT was conducted on day 48 using DBB (EBV-transformed lymphoblastoid cells homozygous for DR7) pulsed with each of 57 20-mer pp65 peptides overlapping by 10 amino acids (#1∼#57). Two adjacent wells (#34 and #35) that were positive were boxed in a solid line. Three wells boxed in a dotted line served as a control. The overlapping sequence, QYDPVAALFP, between #34 and #35 peptides is boxed. (d) CD4+ T cells purified from DR4-positive CMV Ab (+) healthy donors were stimulated with DR4-aAPC/Ii/DM/CD64 mixed with 10 nM pp65 IC. On day 28, IL-2 ELISPOT was conducted using Preiss (EBV-transformed lymphoblastoid cells homozygous for DR4) pulsed with each of 57 20-mer pp65 peptides overlapping by 10 amino acids (#1∼#57) or IC. Data for three positive peptides, #6, #16 and 19, and IC is depicted. Pan-DR PADRE peptide was used as a negative control.
We confirmed that CD4+ T-cell epitopes can be processed and presented when whole protein antigen is delivered to aAPC via Fcγ receptor-mediated endocytosis of antigen:antibody IC. DR7-restricted pp65-specific CD4+ T cells generated using pp65 protein-pulsed autologous monocytes were subjected to an IL-2 ELISPOT assay (Fig. 4b). DR7-aAPC/Ii/DM or DR7-aAPC/Ii/DM/CD64 were used as an APC after they were pulsed with 10 pM pp65 protein or pp65 IC for 30 min. Under these conditions, Fcγ receptor-mediated endocytosis was more efficient than pinocytosis in taking up exogenous protein antigen and processing and presenting CD4+ T-cell epitopes.
CD64+ DR-aAPC/Ii/DM was also useful for the induction phase of antigen-specific CD4+ T cells. Purified CD4+ T cells from DR7+ CMV Ab (+) healthy donors were repeatedly stimulated with DR7-aAPC/Ii/DM/CD64 pulsed with pp65 IC. As shown in Fig. 4(c), generated pp65-specific CD4+ T cells demonstrated IL-2 secretion in response to peptides #34 and #35. The overlapping sequence of these two peptides is QYDPVAALFF, which appears to be a novel, newly identified DR7-restricted pp65-derived CD4+ T-cell epitope (31–38). Interestingly, this epitope appears to overlap with some HLA class I epitopes previously reported by others (39, 40).
We have also successfully generated DR4-restricted pp65-specific CD4+ T cells using DR4-aAPC/Ii/DM/CD64 pulsed with pp65 IC (Fig. 4d). This CD4+ T-cell line produced IL-2 in response to peptides #6, #16 and #19 in a DR4-specific manner. These results suggest that CD64-transduced DR-aAPC/Ii/DM can take up antigenic protein IC by CD64-mediated endocytosis, process and present DR-restricted epitopes derived from the antigen and then stimulate CD4+ T cells specific for these epitopes. However, we have yet to study whether the quality and quality of peptides processed and presented by K562-derived aAPC are similar to those processed and presented by DC.
DR-aAPC/Ii/DM does not expand bystander Foxp3+ Treg
Thymus-derived naturally occurring CD4+ CD25+ Foxp3+ Treg can be expanded ex vivo by delivering strong T-cell receptor (TCR) engagement, costimulation and IL-2 (41, 42). Furthermore, it has been demonstrated that Foxp3-negative peripheral T cells can be converted into Foxp3-positive Treg in vivo and in vitro (43–45). We were therefore interested in whether our K562-based DR-aAPC/Ii/DM stimulates bystander Foxp3+ Treg while expanding antigen-specific CD4+ T cells.
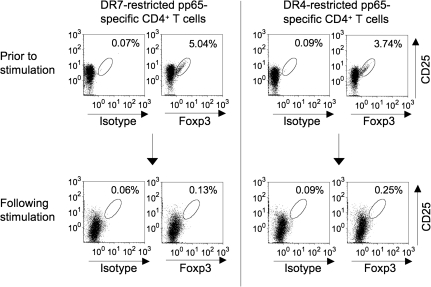
DR7-restricted pp65-specific CD4+ T cells (day 48) were analyzed for intracellular expression of Foxp3 (Fig. 5, left). Before stimulation, freshly isolated CD4+ CD25+ T cells demonstrated 5.04% Foxp3 positivity. After stimulation, the pp65-specific CD4+ T-cell line was only 0.13% positive for Foxp3. Similar results were obtained with DR4-restricted pp65-specific CD4+ T cells on day 38 (Fig. 5, right). While Foxp3 staining was 3.74% positive prior to stimulation, it was merely 0.25% positive following stimulation with DR4-aAPC/Ii/DM. These results suggest that under the conditions tested, K562-based DR-aAPC/Ii/DM does not support the growth of bystander Foxp3+ Treg.
Fig. 5.
K562-based DR-aAPC/Ii/DM does not grow Foxp3+ CD4+ T cells as a bystander. DR7-restricted pp65-specific CD4+ T cells (day 48) and DR4-restricted pp65-specific CD4+ T cells (day 38) were analyzed for the intracellular expression of Foxp3. Total CD4+ T cells in culture were gated. Purified CD4+ CD25+ T cells from the same donors prior to stimulation were also studied.
Discussion
In many mouse models, it has been shown that CD4+ T-cell help is required to generate effective CD8+ T-cell memory. The absence of CD4+ T-cell help results in short-lived antigen-specific CD8+ T cells and defective secondary CD8+ T-cell responses. Furthermore, recent data suggest that CD4+ T-cell help is also critical for the mobilization of the effector phase of CD8+ T cells (46). It has been shown that tumor-reactive CD4+ T cells can also mediate anti-tumor effects via many mechanisms such as direct cytotoxicity of tumor cells, enhancement of the immune environment through cytokine secretion, recruitment of CTL and activation of innate immunity (47, 48). These observations suggest that CD4+ T cells can function directly as effector T cells and/or provide help to other effector cells such as CD8+ CTL. Furthermore, they underline the potential for reinfusion of antigen-specific CD4+ T cells as a treatment modality for infectious diseases and cancer (12). This has prompted us to generate a standardized and renewable aAPC that aides the efficient identification of CD4+ T-cell epitopes and enables the ex vivo generation of large numbers of antigen-specific CD4+ T cells.
Previously, we generated K562-based aAPC by transducing HLA-A2, CD80 and CD83 (13, 14). This HLA-A2-aAPC can generate large numbers of antigen-specific CD8+ CTL that display a central/effector memory phenotype and possess potent effector function (13). Interestingly, these CTL are surprisingly long lived and can be maintained in vitro for >1 year without any feeder cells or cloning (14). We have successfully created clinical grade aAPC33 and are currently conducting a clinical trial where large numbers (2 × 109 m−2) of MART1-specific CD8+ CTL generated ex vivo using aAPC33 are adoptively transferred to patients with metastatic melanoma (14). We have found that it is feasible to generate CTL grafts within 3 weeks and that it is safe to reinfuse these CTL to patients (17). Furthermore, early results demonstrate that adoptively transferred MART1-specific CTL can expand and persist as memory T cells for >16 months without lymphodepletion or cytokine administration. These in vivo results suggest that the K562 backbone might be ideal for aAPC used to generate antigen-specific T cells for clinical use.
In this study, we have successfully developed K562-based HLA-DR-aAPC that can take up soluble protein, process and present DR-restricted peptides and stimulate long-lived peptide-specific CD4+ T cells able to secrete cytokines. While we previously demonstrated that K562-derived A2-aAPC is capable of priming naive CD8+ T cells, it has yet to be determined whether DR-aAPC can prime naive CD4+ T cells (13). Since DR-aAPC expresses transduced HLA-DR as a single HLA allele but not any other class I or II allele, it can be used to stimulate CD4+ T cells from any DR-matched donors. Furthermore, it can be employed to identify CD4+ T-cell epitopes restricted by the transduced single DR allele and unhindered by reactivity to epitopes presented by other HLA alleles (Fig. 4c and d). In addition to DR1, DR4 and DR7-aAPC/Ii/DM/CD64 described in this study, we have already generated and are now validating CD64+ aAPC/Ii/DM expressing DR3 (DRB1*0301), DR10 (DRB1*1001), DR11 (DRB1*1101) and DR15 (DRB1*1501). With all these aAPC available, we will be able to generate DR-restricted antigen-specific CD4+ T cells from 74% of European Americans and 55% of African Americans (49, 50).
In future clinical trials, we would like to compare the efficacy of reinfusing antigen-specific CD4+ T cells alone, CD8+ T cells alone and both CD4+ and CD8+ T cells generated ex vivo using our K562-based aAPC. Whether reinfused CD4+ T cells can provide help to CD8+ T cells is an important question to address. Reinfused CD8+ T cells might acquire properties that promote their persistence and function in the presence of ‘help’, leading to improved eradication of tumor cells. It is also possible that reinfused CD4+ T cells themselves could also function as effector T cells. We will longitudinally measure and compare the phenotype, persistence and function of reinfused T cells to address this critical question.
It is critical that CD4+ T-cell grafts for the treatment of infectious diseases and cancer are not contaminated with expanded Foxp3+ Treg. Under the experimental conditions studied, the expansion of bystander Foxp3+ Treg was not observed in the CD4+ T-cell cultures generated using our DR-aAPC/Ii/DM. While K562 cells secrete transforming growth factor-β, which is known to promote the generation of Foxp3+ Treg, it also produces IL-6, which counteracts the generation of Foxp3+ Treg (14, 51, 52). It is also conceivable that TCR engagement provided by DR molecules on aAPC might not be sufficiently potent for the expansion of Foxp3+ Treg, which require strong TCR engagement for proliferation. Also, only low dose (10 IU ml−1) IL-2 was added to our T cells, possibly retarding Foxp3+ Treg growth that requires a higher IL-2 concentration. It should be noted that CD4+ T cells repeatedly stimulated by DR-aAPC/Ii/DM and expanded in the presence of IL-2/IL-15 did not secrete IL-10 (data not shown).
In summary, we have molecularly engineered K562-derived aAPC that can take up soluble protein, process and present HLA-DR-restricted peptides and stimulate peptide-specific CD4+ T cells. This platform can serve as a very useful tool in the identification of CD4+-restricted epitopes. Considering previous success in clinical translation of K562-based aAPC for HLA-A2-restricted CD8+ CTL, K562-based aAPC may also be a promising platform to expand CD4+ Th for clinical use.
Supplementary data
Supplementary Table S1 and Figures S1 and S2 are available at International Immunology Online.
Funding
Cancer Research Institute to M.O.B., M.T., O.I. and L.M.N.; National Institutes of Health (CA87720 to M.O.B, K22CA129240 and R01CA148673 to N.H.; American Society of Hematology Scholar Award.
Supplementary Material
Acknowledgments
We thank Gordon Freeman and Kai Wucherpfennig (Dana-Farber Cancer Institute) for providing us with valuable reagents. The authors have no conflict of interest to declare.
References
- 1.June CH. Adoptive T cell therapy for cancer in the clinic. J. Clin. Invest. 2007;117:1466. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu. Rev. Immunol. 2007;25:243. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 2009;21:233. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger C, Turtle CJ, Jensen MC, Riddell SR. Adoptive transfer of virus-specific and tumor-specific T cell immunity. Curr. Opin. Immunol. 2009;21:224. doi: 10.1016/j.coi.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl J. Med. 2008;358:2698. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J. Clin. Oncol. 2006;24:5060. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 9.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 1995;333:1038. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 12.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4+ T cells. Curr. Opin. Immunol. 2009;21:200. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirano N, Butler MO, Xia Z, et al. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood. 2006;107:1528. doi: 10.1182/blood-2005-05-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler MO, Lee JS, Ansen S, et al. Long-lived antitumor CD8+ lymphocytes for adoptive therapy generated using an artificial antigen-presenting cell. Clin. Cancer Res. 2007;13:1857. doi: 10.1158/1078-0432.CCR-06-1905. [DOI] [PubMed] [Google Scholar]
- 15.Hirano N, Butler MO, Xia Z, et al. Identification of an immunogenic CD8+ T-cell epitope derived from gamma-globin, a putative tumor-associated antigen for juvenile myelomonocytic leukemia. Blood. 2006;108:2662. doi: 10.1182/blood-2006-04-017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano N, Butler MO, Xia Z, et al. Efficient presentation of naturally processed HLA class I peptides by artificial antigen-presenting cells for the generation of effective antitumor responses. Clin. Cancer Res. 2006;12:2967. doi: 10.1158/1078-0432.CCR-05-2791. [DOI] [PubMed] [Google Scholar]
- 17.Butler M, Friedlander P, Mooney M, et al. Establishing CD8(+) T cell immunity by adoptive transfer of autologous, IL-15 expanded, anti-tumor CTL with a central/effector memory phenotype can induce objective clinical responses. Blood. 2009;114:323. [Google Scholar]
- 18.Prazma CM, Yazawa N, Fujimoto Y, Fujimoto M, Tedder TF. CD83 expression is a sensitive marker of activation required for B cell and CD4+ T cell longevity in vivo. J. Immunol. 2007;179:4550. doi: 10.4049/jimmunol.179.7.4550. [DOI] [PubMed] [Google Scholar]
- 19.Ansen S, Butler MO, Berezovskaya A, et al. Dissociation of its opposing immunologic effects is critical for the optimization of antitumor CD8+ T-cell responses induced by interleukin 21. Clin. Cancer Res. 2008;14:6125. doi: 10.1158/1078-0432.CCR-08-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano N, Butler MO, Von Bergwelt-Baildon MS, et al. Autoantibodies frequently detected in patients with aplastic anemia. Blood. 2003;102:4567. doi: 10.1182/blood-2002-11-3409. [DOI] [PubMed] [Google Scholar]
- 21.Hirano N, Butler MO, Guinan EC, Nadler LM, Kojima S. Presence of anti-kinectin and anti-PMS1 antibodies in Japanese aplastic anaemia patients. Br. J. Haematol. 2005;128:221. doi: 10.1111/j.1365-2141.2004.05317.x. [DOI] [PubMed] [Google Scholar]
- 22.Bunce M, O'Neill CM, Barnardo MC, et al. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 23.Alexander J, Sidney J, Southwood S, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 24.Hirano N, Takahashi T, Ohtake S, et al. Expression of costimulatory molecules in human leukemias. Leukemia. 1996;10:1168. [PubMed] [Google Scholar]
- 25.Papadimitriou L, Morianos I, Michailidou V, Dionyssopoulou E, Vassiliadis S, Athanassakis I. Characterization of intracellular HLA-DR, DM and DO profile in K562 and HL-60 leukemic cells. Mol. Immunol. 2008;45:3965. doi: 10.1016/j.molimm.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Bryant P, Ploegh H. Class II MHC peptide loading by the professionals. Curr. Opin. Immunol. 2004;16:96. doi: 10.1016/j.coi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson L. DM and DO shape the repertoire of peptide-MHC-class-II complexes. Curr. Opin. Immunol. 2005;17:65. doi: 10.1016/j.coi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Warmerdam PA, van de Winkel JG, Gosselin EJ, Capel PJ. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32) J. Exp. Med. 1990;172:19. doi: 10.1084/jem.172.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol. Ther. 2007;15:981. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daëron M. Fc RECEPTOR BIOLOGY. Ann. Rev. Immunol. 1997;15:203. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 31.Khattab BA, Lindenmaier W, Frank R, Link H. Three T-cell epitopes within the C-terminal 265 amino acids of the matrix protein pp65 of human cytomegalovirus recognized by human lymphocytes. J. Med. Virol. 1997;52:68. doi: 10.1002/(sici)1096-9071(199705)52:1<68::aid-jmv11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 32.Li Pira G, Bottone L, Ivaldi F, et al. Identification of new Th peptides from the cytomegalovirus protein pp65 to design a peptide library for generation of CD4 T cell lines for cellular immunoreconstitution. Int. Immunol. 2004;16:635. doi: 10.1093/intimm/dxh065. [DOI] [PubMed] [Google Scholar]
- 33.Trivedi D, Williams RY, O'Reilly RJ, Koehne G. Generation of CMV-specific T lymphocytes using protein-spanning pools of pp65-derived overlapping pentadecapeptides for adoptive immunotherapy. Blood. 2005;105:2793. doi: 10.1182/blood-2003-05-1433. [DOI] [PubMed] [Google Scholar]
- 34.Bronke C, Palmer NM, Westerlaken GHA, et al. Direct ex vivo detection of HLA-DR3-restricted cytomegalovirus- and mycobacterium tuberculosis-specific CD4+ T cells. Human Immunol. 2005;66:950. doi: 10.1016/j.humimm.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Kern F, Bunde T, Faulhaber N, et al. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J. Infect. Dis. 2002;185:1709. doi: 10.1086/340637. [DOI] [PubMed] [Google Scholar]
- 36.Weekes MP, Wills MR, Sissons JGP, Carmichael AJ. Long-term stable expanded human CD4+ T cell clones specific for human cytomegalovirus are distributed in both CD45RAhigh and CD45ROhigh populations. J. Immunol. 2004;173:5843. doi: 10.4049/jimmunol.173.9.5843. [DOI] [PubMed] [Google Scholar]
- 37.Gallot G, Vivien R, Ibisch C, et al. Purification of Ag-specific T lymphocytes after direct peripheral blood mononuclear cell stimulation followed by CD25 selection. I. Application to CD4+ or CD8+ cytomegalovirus phosphoprotein pp65 epitope determination. J. Immunol. 2001;167:4196. doi: 10.4049/jimmunol.167.8.4196. [DOI] [PubMed] [Google Scholar]
- 38.Harcourt GC, Scriba TJ, Semmo N, Bounds S, Taylor E, Klenerman P. Identification of key peptide-specific CD4+ T cell responses to human cytomegalovirus: implications for tracking antiviral populations. Clin. Exp. Immunol. 2006;146:203. doi: 10.1111/j.1365-2249.2006.03193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghei M, Stroncek DF, Provenzano M. Analysis of memory T lymphocyte activity following stimulation with overlapping HLA-A*2402, A*0101 and Cw*0402 restricted CMV pp65 peptides. J. Transl. Med. 2005;3:23. doi: 10.1186/1479-5876-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provenzano M, Lim JB, Mocellin S, et al. The matrix protein pp65(341-350): a peptide that induces ex vivo stimulation and in vitro expansion of CMV-specific CD8+ T cells in subjects bearing either HLA-A*2402 or A*0101 allele. Transfusion. 2003;43:1567. doi: 10.1046/j.1537-2995.2003.00564.x. [DOI] [PubMed] [Google Scholar]
- 41.June CH, Blazar BR. Clinical application of expanded CD4(+)25(+) cells. Semin. Immunol. 2006;18:78. doi: 10.1016/j.smim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells-ex vivo expansion and therapeutic potential. Semin. Immunol. 2006;18:103. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Wahl SM. TGF-beta: the missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 2003;14:85. doi: 10.1016/s1359-6101(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 44.Hoglund P. Induced peripheral regulatory T cells: the family grows larger. Eur. J. Immunol. 2006;36:264. doi: 10.1002/eji.200535797. [DOI] [PubMed] [Google Scholar]
- 45.Walker MR, Kasprowicz DJ, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25-T cells. J. Clin. Invest. 2003;112:1437. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005;54:721. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy R, Celis E. Multiple roles for CD4+T cells in anti-tumor immune responses. Immunol. Rev. 2008;222:129. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 49.Klitz W, Maiers M, Spellman S, et al. New HLA haplotype frequency reference standards: high-resolution and large sample typing of HLA DR-DQ haplotypes in a sample of European Americans. Tissue Antigens. 2003;62:296. doi: 10.1034/j.1399-0039.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 50.Zachary AA, Bias WB, Johnson A, Rose SM, Leffell MS. Antigen, allele, and haplotype frequencies report of the ASHI minority antigens workshops: part 1, African-Americans. Hum. Immunol. 2001;62:1127. doi: 10.1016/s0198-8859(01)00305-6. [DOI] [PubMed] [Google Scholar]
- 51.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Ann. Rev. Immunol. 2009;27:485. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.