Abstract
In neurons, synaptotagmin1 (Syt1) is thought to mediate the fusion of synaptic vesicles with the plasma membrane when presynaptic Ca2+ levels rise. However, in vitro reconstitution experiments have failed to recapitulate key characteristics of Ca2+-triggered membrane fusion. Using an in vitro single-vesicle fusion assay, we found that membrane-anchored Syt1 enhanced Ca2+-sensitivity and fusion speed. This stimulatory activity of membrane-anchored Syt1 dropped as the Ca2+ level rose beyond physiological levels. Thus, Syt1 requires the membrane anchor to stimulate vesicle fusion at physiological Ca2+ levels, and may function as a dynamic presynaptic Ca2+ sensor to control the probability of neurotransmitter release.
Intracellular membrane trafficking in eukaryotic cells involves fusion of membrane-bounded compartments (1) and is mediated by the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins found on both the vesicle (v-SNARE) and target membranes (t-SNARE)(2-4). In neurons, synaptotagmin 1 (Syt1) is a Ca2+ sensor that interacts with SNAREs and membranes to mediate synaptic vesicle fusion, triggering synchronous neurotransmission (3, 5, 6). Proteoliposome fusion mediated by the t- and v-SNARE proteins is a useful in vitro system (7) for dissecting the molecular functions of presynaptic fusion regulators including Syt1 (5, 6, 8). The soluble Syt1 variant, which has the two cytoplasmic C2 domains but lacks the transmembrane domain, has been the subject of extensive studies (9-14). This ‘soluble C2AB’ accelerates proteoliposome fusion to reach a time constant of about 10 s when 100 μM or 1 mM Ca2+ is added (13, 14). However, when the full-length, membrane-anchored Syt1 is used, addition of the same levels of Ca2+ instead inhibits fusion (12, 15, 16). This membrane anchor is preserved in all isoforms of the Syt family except Syt17 (17), suggesting that it plays an important role in regulating synaptic vesicle fusion. Understanding this currently hidden role of the membrane anchor requires the study of Ca2+-evoked vesicle fusion stimulated by membrane-anchored Syt1.
Here, we used a single-vesicle fluorescence fusion assay to track the time course of individual vesicle-vesicle fusion events (fig. S1)(18, 19). The assay involves one group of vesicles (called v-vesicles), containing v-SNARE, full-length Syt1 and lipidic acceptor dyes, immobilized on an imaging surface. A second group of vesicles (t-vesicles), with the precomplex of t-SNAREs and lipidic donor dyes, are mixed with a desired concentration of Ca2+ and introduced for reaction via microfluidic buffer exchange. When the t-vesicles react with the surface-immobilized v-vesicles, a single-vesicle complex is formed that can be detected by an increase in the efficiency of the fluorescence resonance energy transfer (FRET) between the donor and acceptor dyes. Docking between t- and v-vesicles generates a FRET efficiency of less than 0.2, while the fully fused state generates 0.75 or higher (18).
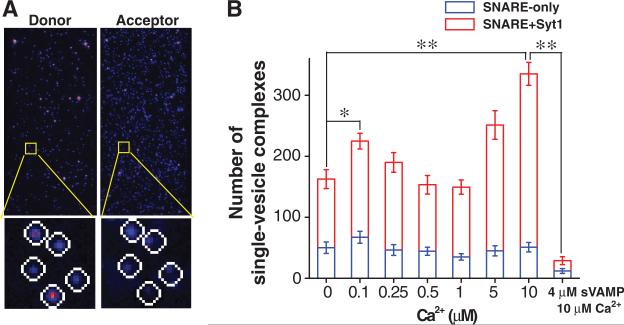
We first analyzed the ability of membrane-anchored Syt1 to stimulate SNARE-mediated single vesicle docking using μM Ca2+ levels. After a three-second reaction between the t- and v-vesicles, we counted the number of single-vesicle complexes formed per imaging area (Fig. 1A and figs. S2 to S5). In the absence of Ca2+, Syt1 increased the docking number by a factor of three (Fig. 1B). The stimulation of docking by Syt1 was Ca2+-dependent and there was another two-fold enhancement between 0 and 10 μM Ca2+ (Fig. 1B). We also tested whether this Syt1 effect on docking was strictly dependent on SNARE activity. We disabled the t-SNAREs using a SNARE-motif peptide of v-SNARE (sVAMP), which suppressed the docking number by as much as 90 % (Fig. 1B). This observation indicates that Syt1's stimulation essentially involves formation of the ternary SNARE complexes (20-22).
Fig. 1.
Membrane-anchored syt1 stimulates single-vesicle docking using 10 μM Ca2+. (A) Exemplary images of single-vesicle FRET imaging. Fluorescence signals from single-vesicle complexes were separated with the threshold at 645 nm and detected as the donor and the acceptor channel signals respectively (Upper). Each single-vesicle complex appearing as a Gaussian peak is identified (Lower). (B) Number of single vesicle complexes formed between t- and v-vesicles under the conditions depicted. The surface-immobilized v-vesicles contain both v-SNARE and Syt1 (red bar) or only v-SNAREs (blue bar). *: p<0.05 and **: p<0.01 assessed using the paired t-test, and all the errors are SD unless otherwise specified.
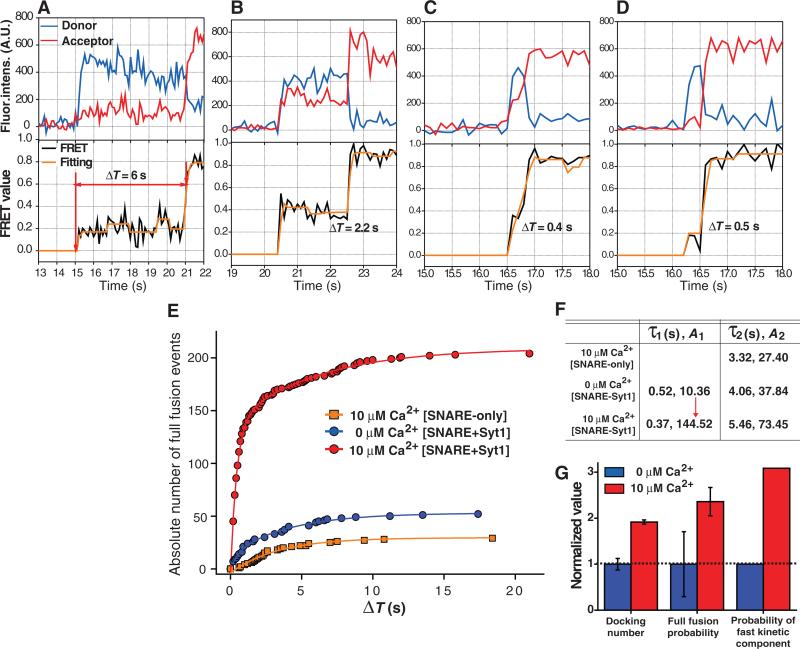
To control the late steps of neurotransmitter release, Syt1 should have the capacity to catalyze the transition to the full fusion state (3, 5, 6). To explore this possibility, we employed a real-time tracking approach, which followed the entire course of fusion events occurring in individual single-vesicle complexes (Fig. 2, A to D, fig. S6 and Movie S1). The time gap between the docking and full fusion, ΔT, exclusively measured the full fusion kinetics of an individual fusion event, separated from the docking kinetics (Fig. 2, A to D and SOM text 1). When the v-vesicle contained only v-SNAREs, the cumulative plot of ΔT shows a single exponential distribution (three independent movies used for each plot, Fig. 2, E and F). Inclusion of Syt1 without Ca2+ (Fig. 2E) created a small, fast-kinetic component (Fig. 2F, τ1 = 520 ms). When we added 10 μM Ca2+ to this reaction with membrane-anchored Syt1 (Fig. 2E), the absolute population of the fast-kinetic component increased fourteen-fold (Fig. 2F). This increase of the fast component was not simply due to docking enhancement, but rather the result of direct acceleration of the full fusion kinetics (Fig. 2G and SOM text 1). Although it was shown that lipid mixing could occur without proper mixing of contents (23), this fast fusion kinetics provides convincing evidences that we have observed actual fusion events rather than slow lipid rearrangements such as flip-flop transitions. Thus, in response to 10 μM Ca2+, the membrane-anchored Syt1 directly accelerates the full fusion reaction, in addition to the docking kinetics.
Fig. 2.
Membrane-anchored Syt1 catalyzes full fusion on the hundreds of ms scale in response to 10 μM Ca2+. (A-D) Exemplary real-time traces of single-vesicle fusion events. Upper, the changes in the donor (blue) and acceptor (red) fluorescence intensities. Lower, the corresponding changes in FRET (black) and stepwise increases in the FRET signals identified by Schwarz information criterion (orange). (E) Cumulative distributions of ΔT for different molecular conditions. (F) Fitting of the ΔT distributions shown in (E) using two exponential functions: A1[1–exp(–t/τ1)]+A2[1–exp(–t/τ2)]. (G) Acceleration of different fusion steps by membrane-anchored Syt1 in response to 10 μM Ca2+ (SOM text 1). Each parameter was normalized by that of 0 μM Ca2+ reaction.
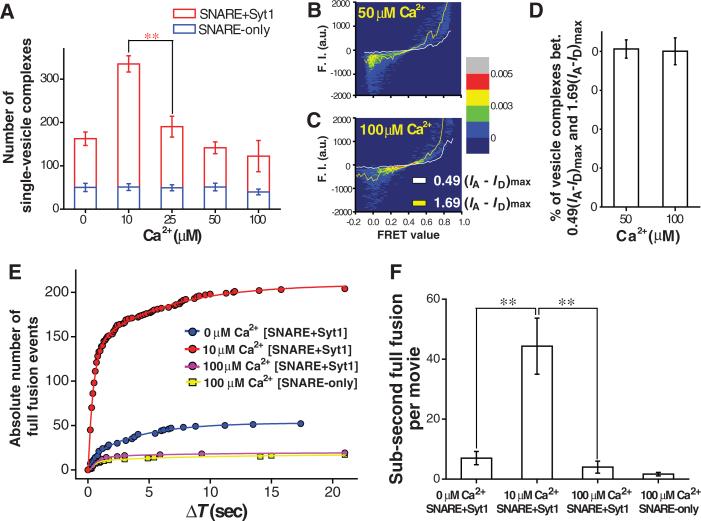
To determine what happens when the Ca2+ concentration increases beyond 10 μM, we repeated the docking-number analysis while increasing Ca2+ to 100 μM (Fig. 3, A to D). Unexpectedly, the population of single-vesicle complexes that peaked at 10 μM Ca2+ dropped as the Ca2+ level rose further, which was visible at 25 μM Ca2+ (Fig. 3A). At 100 μM Ca2+, the docking number became comparable to that obtained with no Ca2+ (Fig. 3A). In addition, the fluorescence intensity analyses excluded the possibility of multiple vesicle aggregation at high Ca2+ concentrations (Fig. 3, B to D and SOM text 2). We also performed real-time tracking measurements with higher Ca2+ and found that 100 μM Ca2+ indeed cut down the catalysis of full fusion states (Fig. 3E); the absolute population showing full fusion event was smaller than that of the 0 μM Ca2+ reaction, and similar to that observed for the SNARE-only reaction without Syt1 (Fig. 3E). Thus, at 100 μM Ca2+, membrane-anchored Syt1 is largely deactivated and loses its capacity to catalyze the full-fusion state (Fig. 3F).
Fig. 3.
Membrane-anchored Syt1 is inactivated at sub-mM Ca2+ levels. (A) Docking-number analysis for the Ca2+ range between 10 and 100 μM Ca2+. (B-D) Fluorescence intensity analysis of the single-vesicle complexes. Single-vesicle complexes are placed on the graph plane of (FRET efficiency, IA-ID) to make a density plot (SOM text 2). The percentages of single-vesicle complexes between white and yellow lines, 0.49(IA-ID)max and 1.69(IA-ID)max (rough estimates for 30% difference in size), are 81.2 % (50 μM) and 80.5 % (100 μM Ca2+) (D). (E) Cumulative distributions of ΔT of single-vesicle fusion events for different molecular conditions (each based on three independent real-time movies). The total docking numbers are 1,166 (blue), 2,232 (red), 1,010 (purple) and 1,272 (yellow), respectively. (F) The absolute number of sub-second full fusion events (ΔT<1 s) per imaging area, for different protein compositions and Ca2+ concentrations.
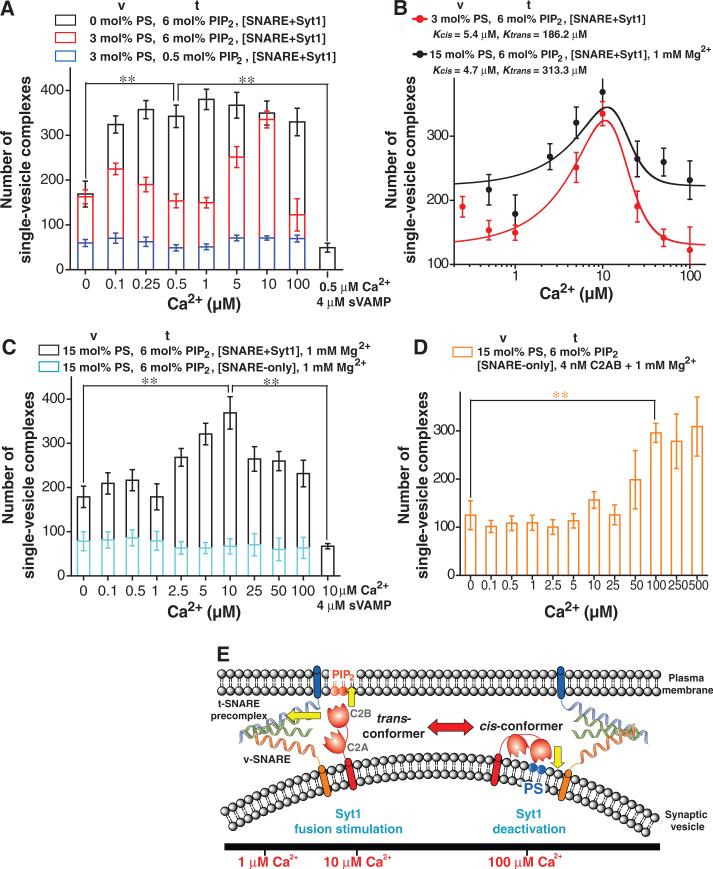
To study the physical mechanism of Syt1 activity (Fig. 4), we weakened the interaction between Syt1 and the cis-membrane by removing negatively-charged phosphatidyl serine (PS) lipids from the v-vesicle membrane (9, 12, 24). Without the PS lipids in the cis-membrane, the docking stimulation by Syt1 lost the tendency to decrease up to 100 μM Ca2+ (Fig. 4A). On the other hand, when we decreased the phosphatidylinositol 4,5-bisphosphate (PIP2) lipids in the t-vesicle membrane from 6 (25) to 0.5 mol%, the stimulatory effect of Syt1 was abolished in the entire Ca2+ range studied (Fig. 4A). Thus, Syt1 needs to interact with both the t-SNARE precomplex (Fig, 1B and 4A, sVAMP treatment) and PIP2 on the trans-membrane for its stimulatory effect while the folding back of Syt1 to the cis-membrane causes its inactivation (Fig. 4E)(12, 24).
Fig. 4.
Dynamic Ca2+-dependent stimulation of vesicle fusion by membrane-anchored Syt1. (A-D) Single-vesicle docking data under the molecular conditions depicted for the cis (vvesicle) and trans (t-vesicle) membranes. Reactions are carried out with the membrane-anchored Syt1 in the cis-membrane (A-C) or soluble C2AB in the fusion buffer (D, see SOM text 4). Theoretical fitting of the docking-number data using a modified the MWC model (B, SOM text 3). 15 mol% PS lipids are included for the trans-membrane in every case. (E) Molecular model for the activity of the membrane-anchored Syt1.
The data also suggest that the balance of Syt1's cis- and trans-membrane interactions shifts dynamically as a function of Ca2+ concentration. From the single-vesicle data (Fig. 4A), we presume two conformations of Syt1, cis and trans-conformers, where only the transconformer stimulates fusion (SOM text 3 and fig. S7). The Ca2+-dependent power shift between these two conformers could be explained by assigning different Ca2+ dissociation constants to the cis- (Kcis) and trans-conformers (Ktrans)(Fig. 4B), reminiscent of the classical Monod-Wyman-Changeux (MWC) model for protein allostery. Our model predicts a large anisotropy in the two dissociation constants; Ktrans/Kcis≈34 (Fig. 4B), indicating that at high Ca2+ levels where [Ca2+]/Kcis>>1, Syt1 predominantly partitions into the cis-conformer and becomes inactivated (Fig. 4E). Because the C2B domain exhibits a much larger Ca2+-binding dissociation constant (5, 10, 26), it is tempting to speculate that the trans-conformer, critical for fusion stimulation, is predominantly mediated by the C2B domain.
Finally, we observed that the presence of another divalent ion, Mg2+, affected the overall shape of the Ca2+-dependent stimulation pattern. Simply increasing the PS lipids in the cis-membrane to the physiological level of 15 mol% (27) pushed the stimulation peak to sub-μM Ca2+ levels, followed by a quick deactivation of Syt1 that began at 1 μM Ca2+ (fig. S8A). However, in the presence of physiological 1 mM Mg2+, the stimulation peak at 10 μM Ca2+ was restored (Fig. 4, B and C) probably due to the enhanced screening of electrostatic interactions by Mg2+ ions. Furthermore, this non-monotonic docking pattern correlated well with up- and down-regulation of the ability of Syt1 to accelerate the full-fusion kinetics (fig. S8, B and C). Thus, the essential molecular activities of membrane-anchored Syt1 can be reproduced under physiological charge conditions in the cis- and trans-membranes. Synaptic vesicles directly isolated from rat brain, which have native Syt1 and lipids, also show a deactivation behaviour at 100 μM and 1 mM Ca2+ (16). These observations collectively suggest that the dynamic Ca2+-dependent activity of Syt1 would occur in physiological contexts.
We have demonstrated that anchoring Syt1 to a lipid membrane fundamentally changes its molecular activity. In the presynaptic active zone, tens of μM or even several μM of presynaptic Ca2+ is sufficient to trigger strong neurotransmitter release (28, 29). The membrane-anchored Syt1 reconstituted in our assay has a remarkable Ca2+- sensitivity, responding to those Ca2+ levels operational at the presynaptic termini. In comparison, at the same effective concentration, we found the soluble C2AB required one order of magnitude higher Ca2+ for docking stimulation and did not show any deactivation pattern up to 500 μM Ca2+ (SOM text 4). Thus, in addition to the enhanced Ca2+-sensitivity, Syt1's membrane anchor seems to give it the Ca2+-dependent, non-monotonic activity. A simple use of sub-mM Ca2+ only inactivates membrane-anchored Syt1, which may explain why previous in vitro studies failed to observe stimulatory effects with full-length Syt1. Our work suggests that Syt1 modulates the probability of neurotransmitter release in response to the presynaptic Ca2+ levels and molecular composition of membranes, which may contribute to the dynamic plasticity of neuronal communication.
Supplementary Material
Footnotes
One-sentence summary: Membrane-anchoring of a synaptic vesicle protein, synaptotagmin 1, is critical for stimulating fusion in vitro at physiological Ca2+ concentrations.
Reference and Notes
- 1.Bonifacino JS, Glick BS. Cell. 2004;116:153. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 2.Rothman JE. Nature. 1994;372:55. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 3.Sudhof TC. Annu Rev Neurosci. 2004;27:509. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 4.Jahn R, Scheller RH. Nat Rev Mol Cell Biol. 2006;7:631. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 5.Chapman ER. Annu Rev Biochem. 2008;77:615. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 6.Rizo J, Rosenmund C. Nat Struct Mol Biol. 2008;15:665. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber T, et al. Cell. 1998;92:759. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 8.Brunger AT, Weninger K, Bowen M, Chu S. Annu Rev Biochem. 2009;78:903. doi: 10.1146/annurev.biochem.77.070306.103621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tucker WC, Weber T, Chapman ER. Science. 2004;304:435. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- 10.Arac D, et al. Nat Struct Mol Biol. 2006;13:209. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 11.Martens S, Kozlov MM, McMahon HT. Science. 2007;316:1205. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 12.Stein A, Radhakrishnan A, Riedel D, Fasshauer D, Jahn R. Nat Struct Mol Biol. 2007;14:904. doi: 10.1038/nsmb1305. [DOI] [PubMed] [Google Scholar]
- 13.Xue M, Ma C, Craig TK, Rosenmund C, Rizo J. Nat Struct Mol Biol. 2008;15:1160. doi: 10.1038/nsmb.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Cell. 2009;138:709. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahal LK, Sequeira SM, Gureasko JM, Sollner TH. J Cell Biol. 2002;158:273. doi: 10.1083/jcb.200203135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt M, Riedel D, Stein A, Schuette C, Jahn R. Curr Biol. 2008;18:715. doi: 10.1016/j.cub.2008.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craxton M. BMC Genomics. 2007;8:259. doi: 10.1186/1471-2164-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon TY, Okumus B, Zhang F, Shin YK, Ha T. Proc Natl Acad Sci U S A. 2006;103:19731. doi: 10.1073/pnas.0606032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon TY, et al. Nat Struct Mol Biol. 2008;15:707. doi: 10.1038/nsmb.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF. Neuron. 2002;34:599. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]
- 21.Bhalla A, Chicka MC, Tucker WC, Chapman ER. Nat Struct Mol Biol. 2006;13:323. doi: 10.1038/nsmb1076. [DOI] [PubMed] [Google Scholar]
- 22.de Wit H, et al. Cell. 2009;138:935. doi: 10.1016/j.cell.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Chan YH, van Lengerich B, Boxer SG. Proc Natl Acad Sci U S A. 2009;106:979. doi: 10.1073/pnas.0812356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai J, Tucker WC, Chapman ER. Nat Struct Mol Biol. 2004;11:36. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 25.James DJ, Khodthong C, Kowalchyk JA, Martin TF. J Cell Biol. 2008;182:355. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui E, Bai J, Chapman ER. Biophys J. 2006;91:1767. doi: 10.1529/biophysj.105.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takamori S, et al. Cell. 2006;127:831. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 28.Schneggenburger R, Neher E. Curr Opin Neurobiol. 2005;15:266. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, et al. Nature. 2007;450:676. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.T.-Y.Y. thanks W. Bae and J. Ryu for writing analysis programs, K. Kim for help with supporting movie, and T. Ha and W. Jones for critical reading of the manuscript. This work was supported by Korea Research Foundation Grants (KRF-2008-313-C00365 to T.-Y.Y.; KRF-C00142 and KRF-C00180 to C.H.) and National Research Foundation of Korea Grants funded by the Korean government (2009-0087691 to T.-Y.Y.; R01-2008-000-10920-0 to C.H.; 2007-D00243 to D.-H.K.). This work was also supported by National Institutes of Health Grant (R01 GM051290 to Y.-K.S.) and the World Class University program in Korea (Y.-K.S. and T.-Y.Y.).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.